Facile Formation of Multifunctional Biomimetic Hydrogel Fibers for Sensing Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Preparation of Multifunctional Biomimetic Hydrogel Fibers
2.1.1. Design and Preparation of Linear Hydrogel Fibers
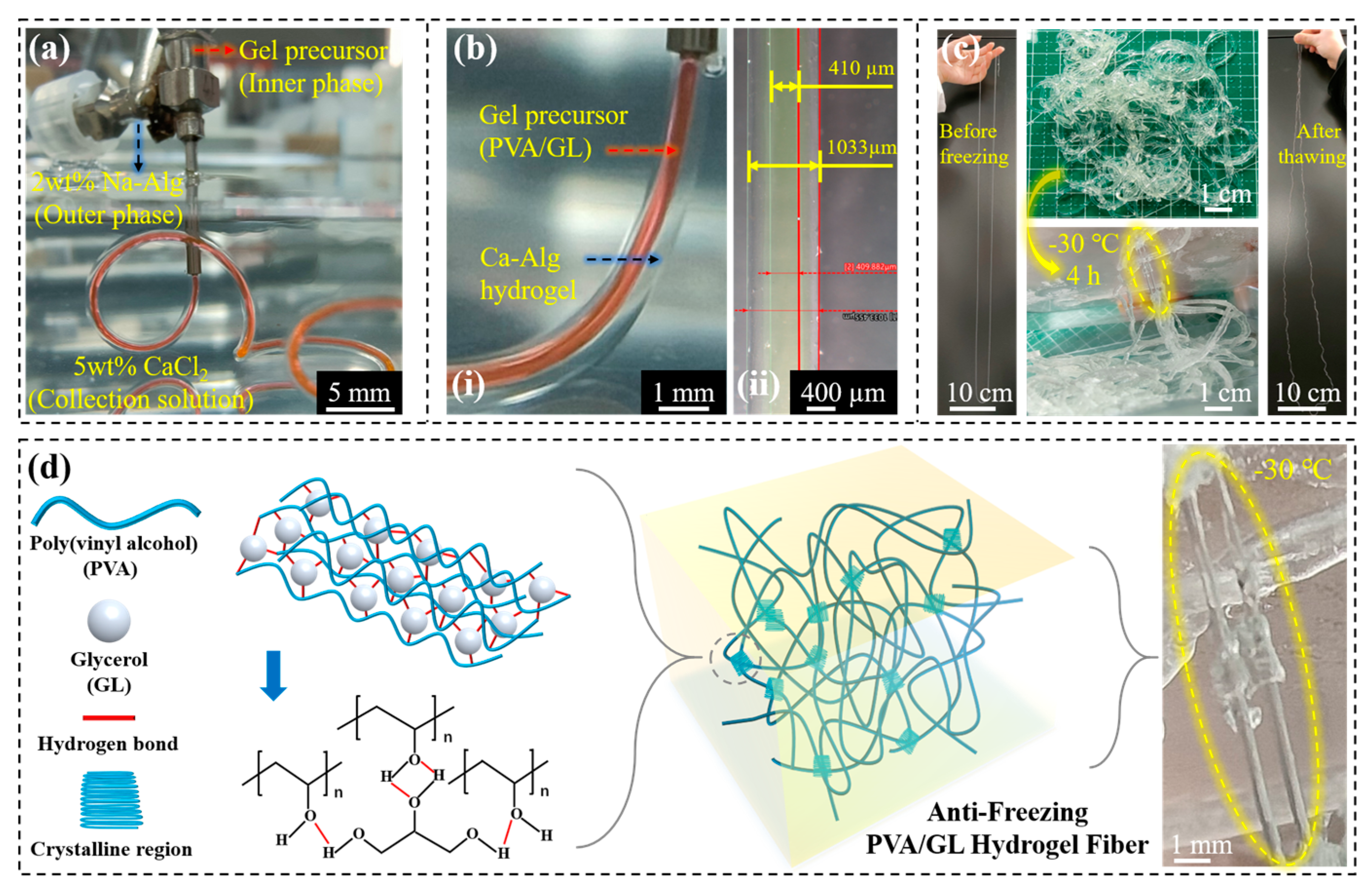
2.1.2. Design and Preparation of Core–Shell and Anti-Freeze Hydrogel Fibers
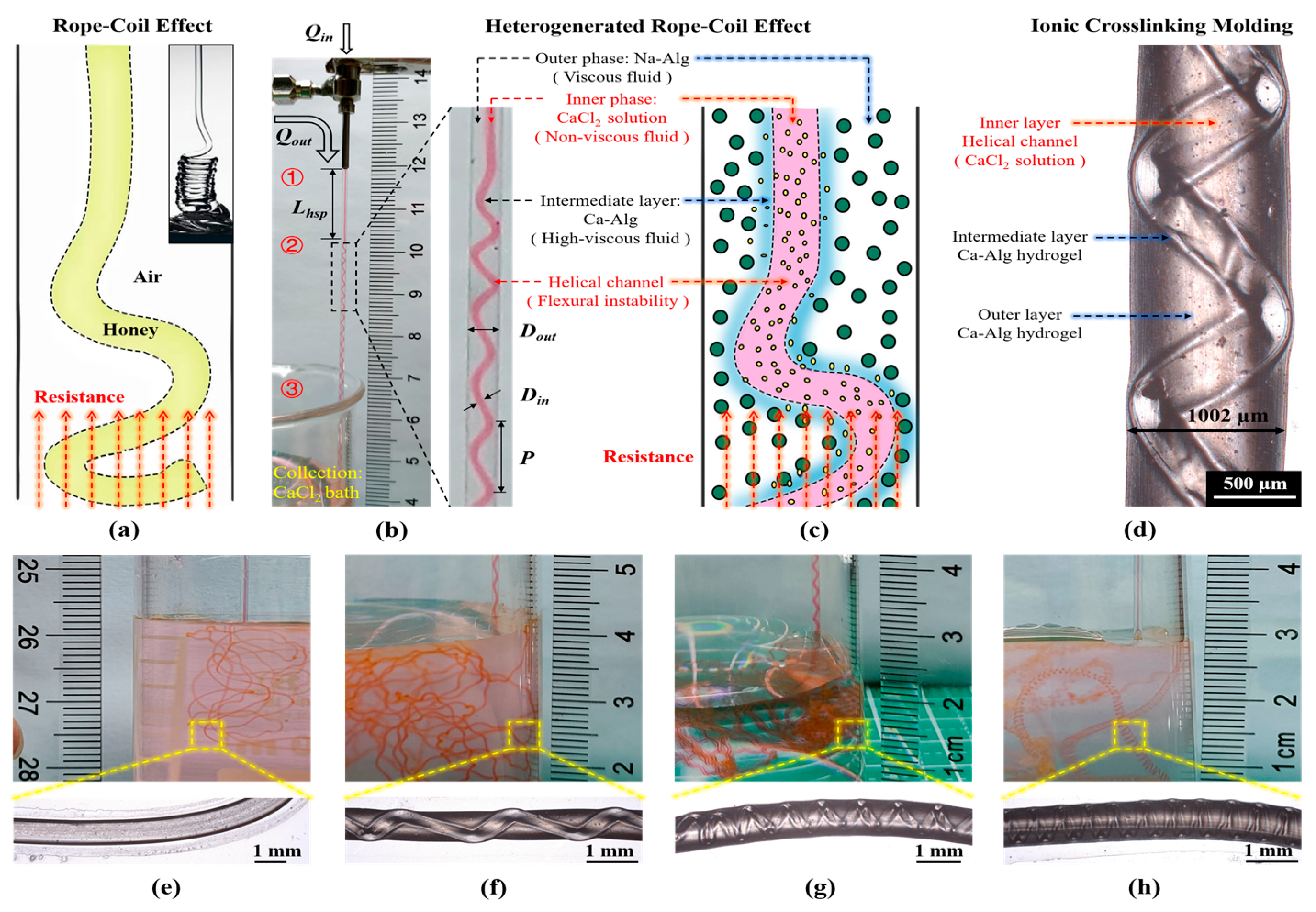
2.1.3. Design and Preparation of Hydrogel Fibers with Embedded Helical Channels
2.1.4. Design and Preparation of Hollow Tubular Hydrogel Fibers
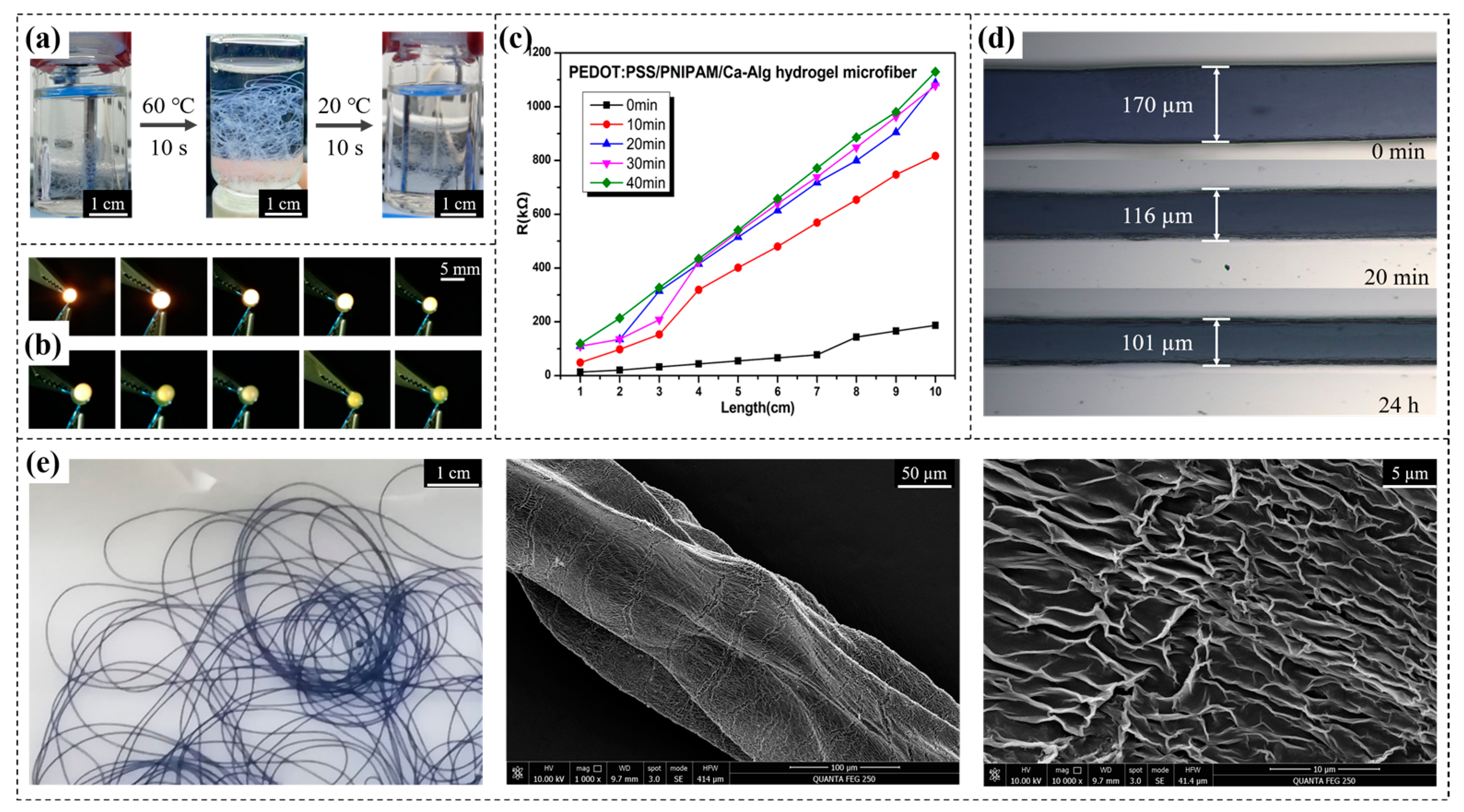
2.1.5. Design and Preparation of Necklace-Shaped Hydrogel Fibers
2.2. Characterization of Multifunctional Biomimetic Hydrogel Fibers
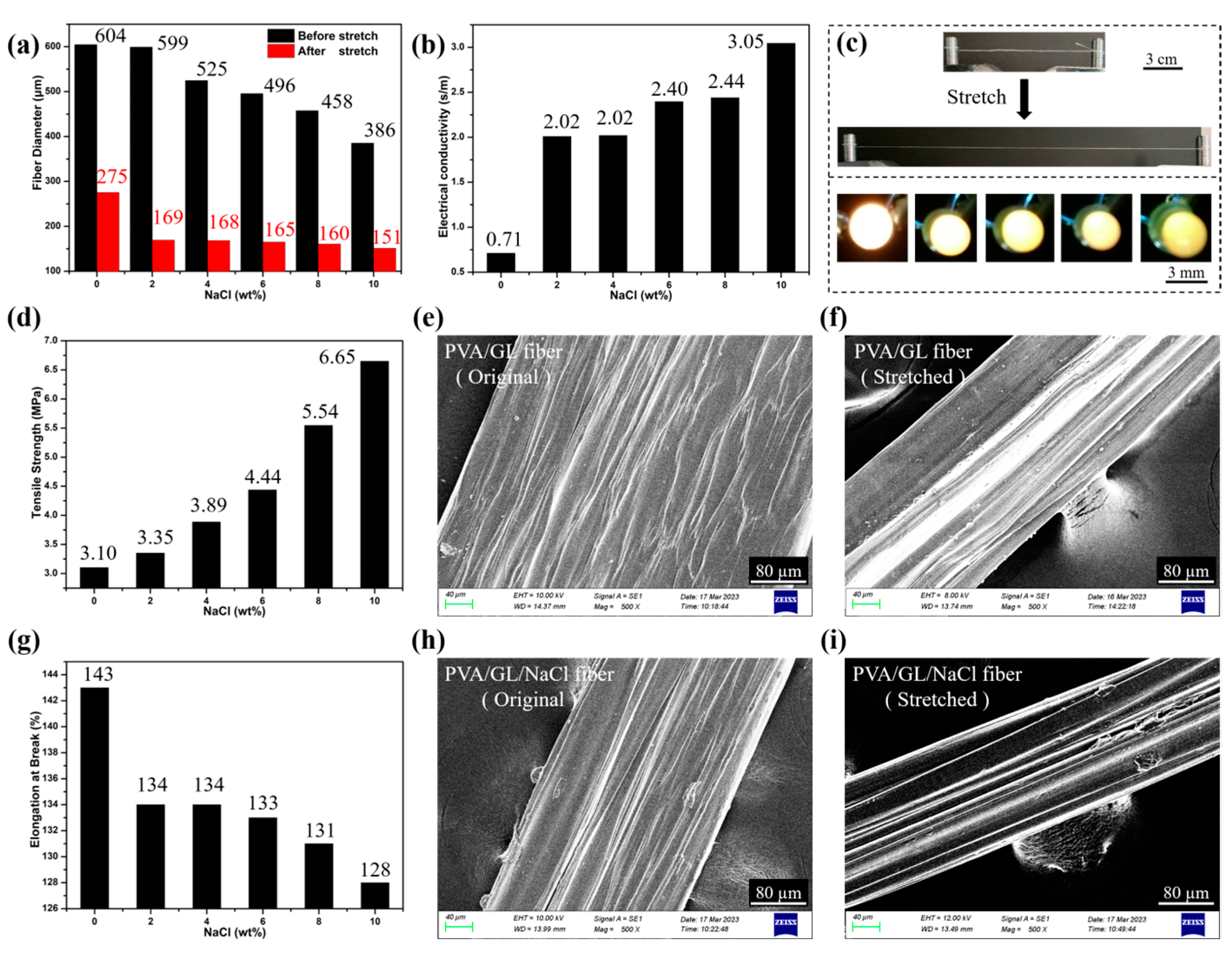
2.2.1. Characterization of Linear Hydrogel Fibers
2.2.2. Characterization of Anti-Freeze Hydrogel Fibers
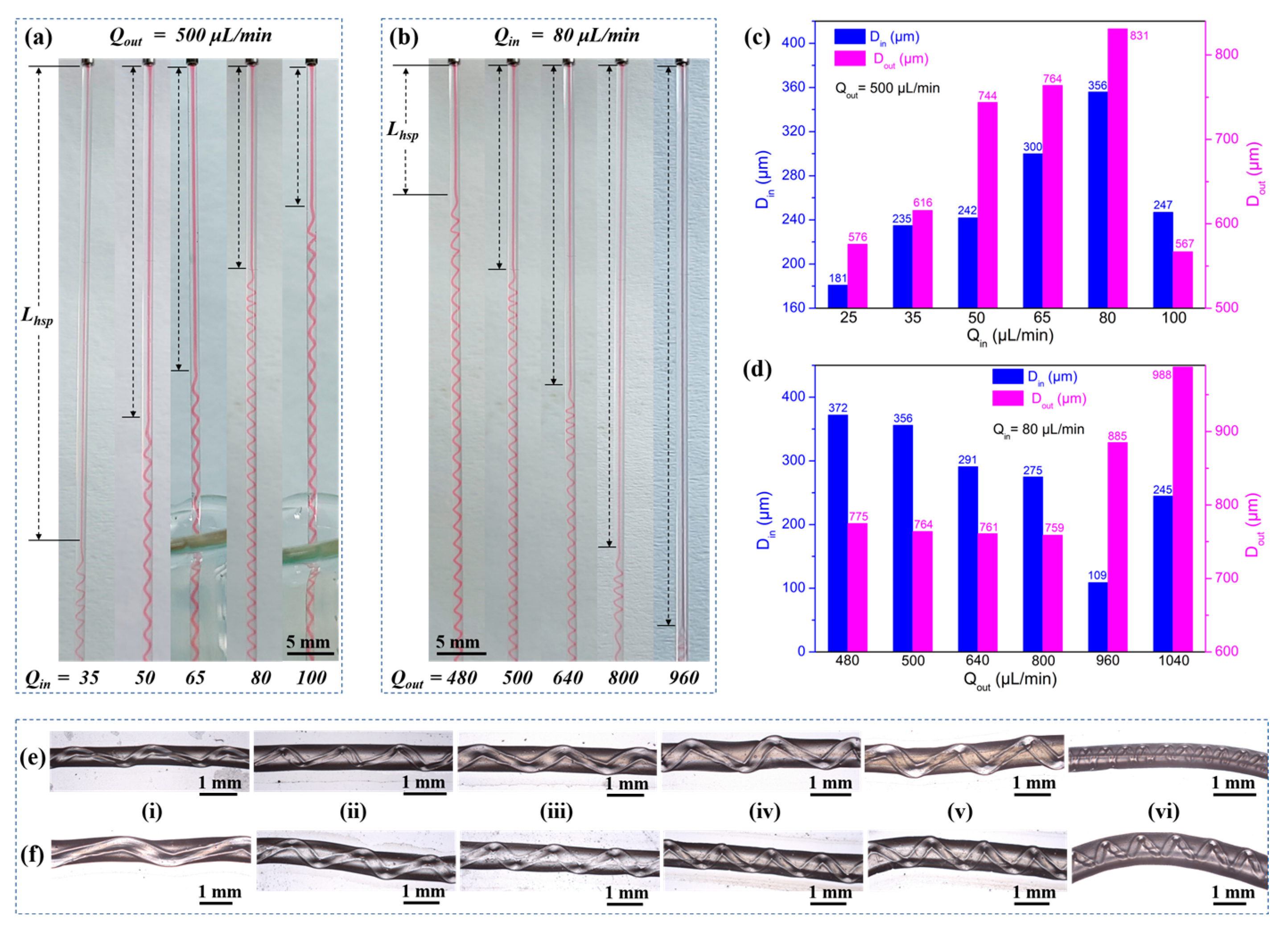
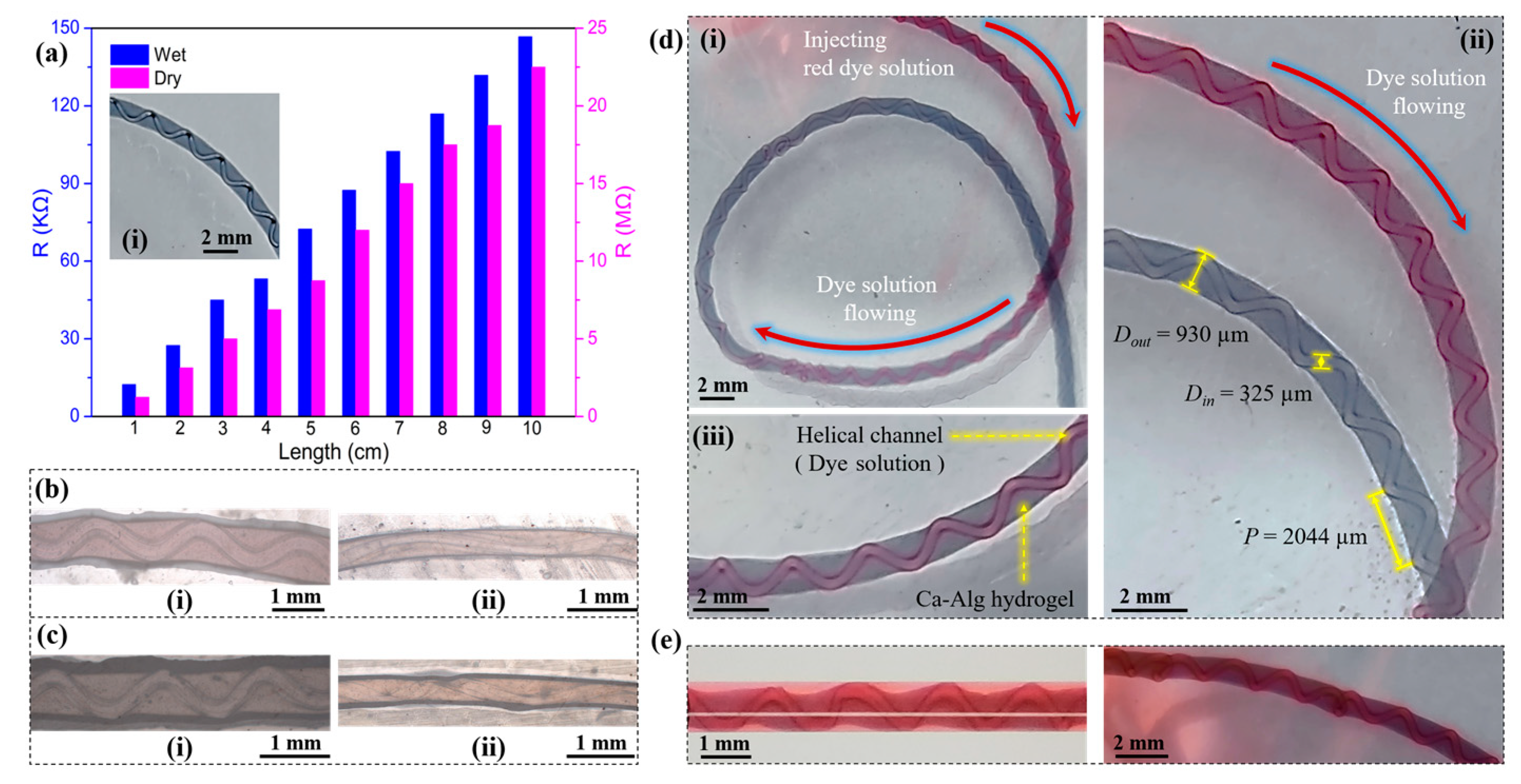
2.2.3. Characterization of Hydrogel Fibers with Embedded Helical Channels
- Lhsp decreased with a higher Qin but increased with a higher Qout (Figure 10a,b). The important factor to consider was the fluid interface where the high-viscosity Ca-Alg layer formed. For a constant Qout, increasing Qin led to faster intermediate layer formation and a shorter Lhsp. However, with constant Qin, the time required for the ionic crosslinking reaction was constant. As Qout increased, helix formation began farther away from the exit of the coaxial needle.
- Both Din and Dout increased as Qin increased, with the exception of Qin = 100 µL/min (Figure 10c). However, when Qout was varied with a constant Qin, Din and Dout behaved differently (Figure 10d). As Qout was increased, Din remained relatively constant before increasing at Qout = 960 µL/min. Dout generally decreased as Qout increased.
- The pitches of the helical channels varied without a clear pattern. This might be due to the fact that in addition to Qout and Qin, weak factors like the crosslinking reaction conditions, gravity, the force generated by the inner wall of the capillary glass tube, and the axial resistance generated by entry into the solidification receiving bath all had an influence on p. The current experimental conditions did not allow us to conduct control variable comparison tests for these weak factors.
2.3. Application Summaries of Hydrogel Fibers in Sensing for Healthcare
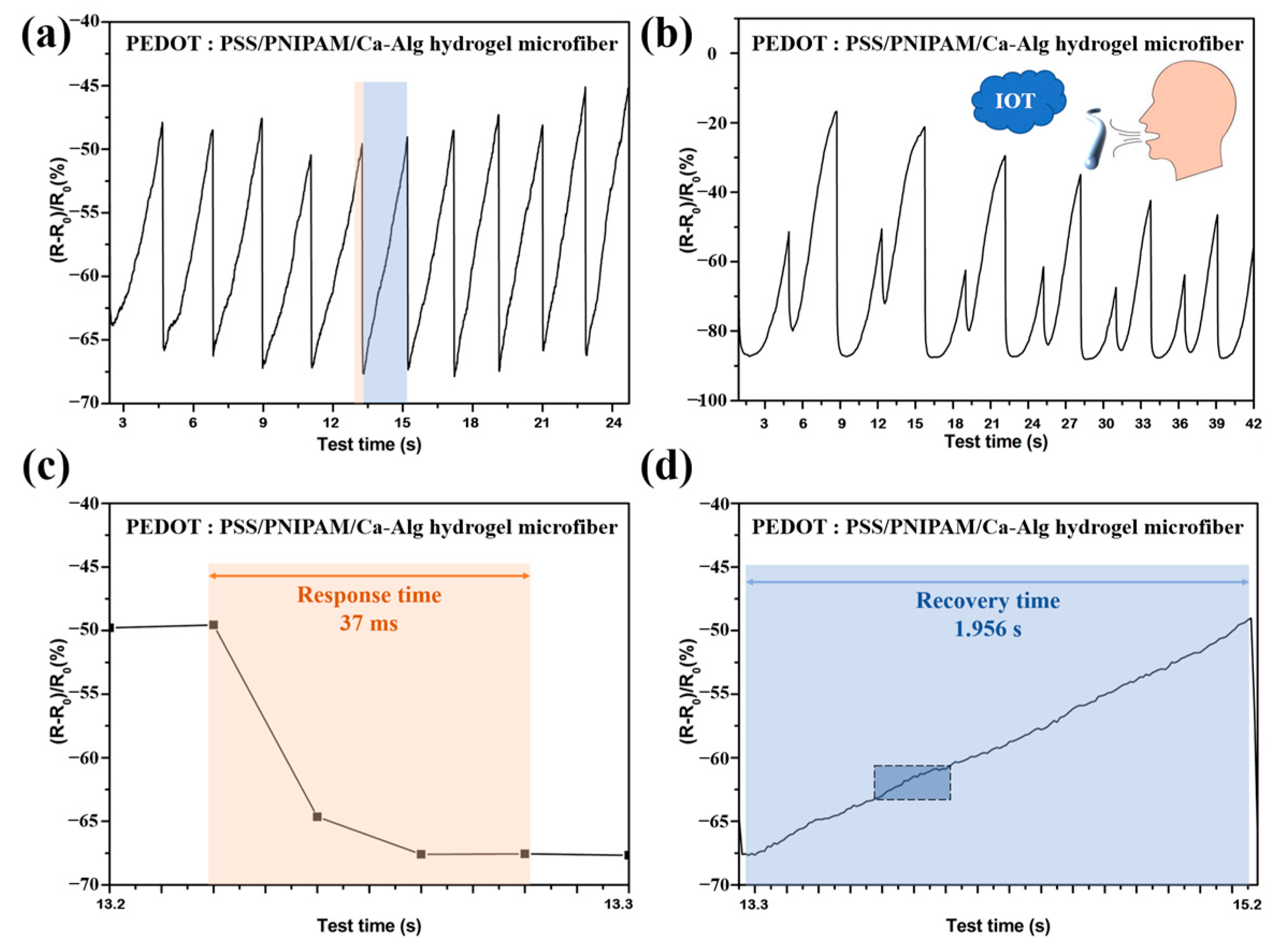
2.3.1. Thermosensitive Electrically Conductive Linear Hydrogel Fibers Used for Human Respiratory Monitoring
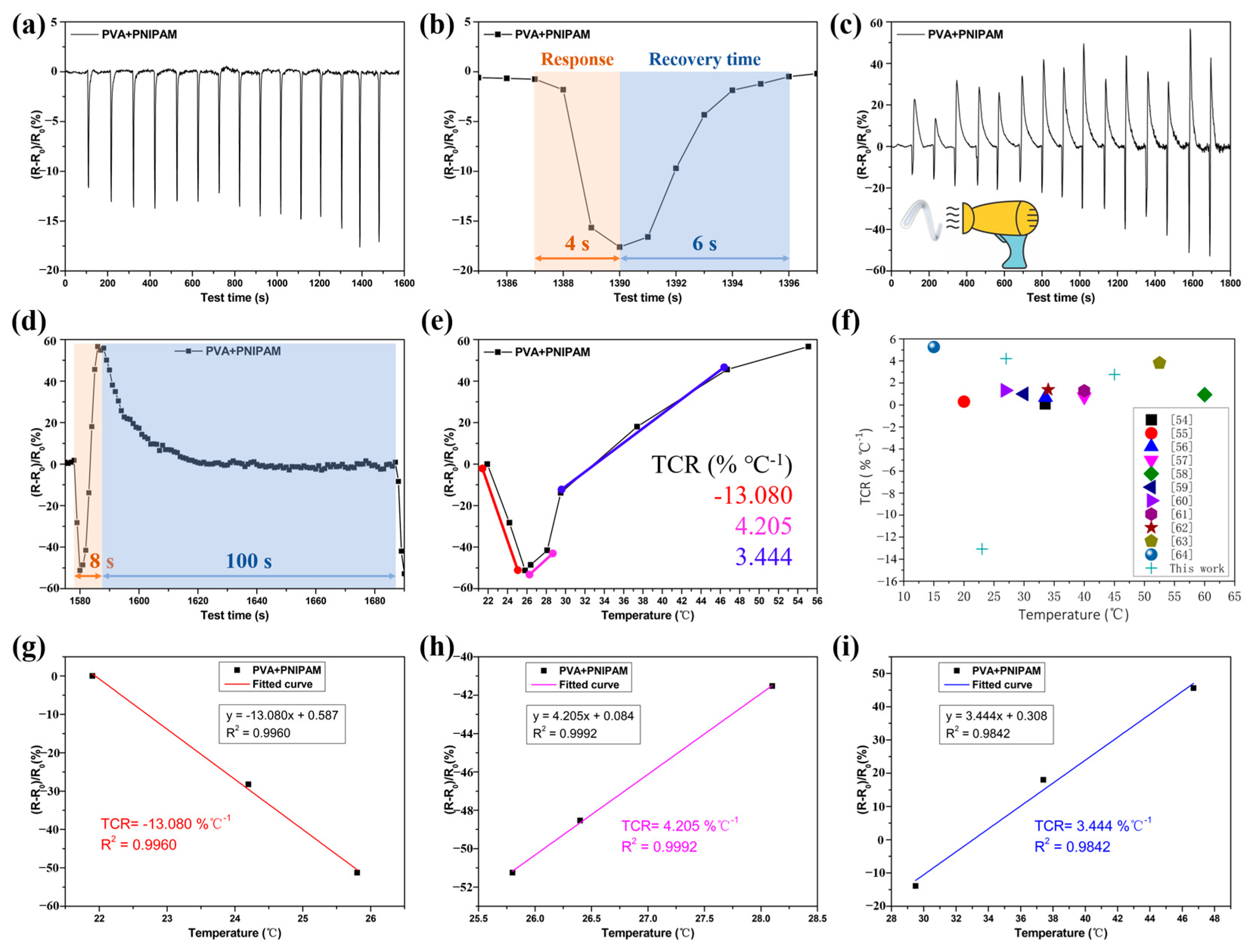
2.3.2. PVA/GL/PNIPAM Frost-Resistant Hydrogel Fibers as Temperature Sensors
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation Method of Multifunctional Biomimetic Hydrogel Fibers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagrat, G.; Samantha, J.P.; Daniel, C.C.; Daniel, W.S.; Chelsea, L.F.; Alexander, J.Z.; Paul, T.G.; Nicholas, J.C.; John, P.G.; Anderson, H.T.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar]
- Yang, H.; Ji, M.; Yang, M.; Shi, M.; Pan, Y.; Zhou, Y.; Qi, H.J.; Suo, Z.; Tang, J. Fabricating Hydrogels to Mimic Biological Tissues of Complex Shapes and High Fatigue Resistance. Matter 2021, 4, 1935–1946. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Rlectrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, P.; Ji, Z.; Chen, Z.; Wang, J.; Ling, W.; Liu, J.; Hu, M.; Zhi, C.; Huang, Y. Rechargeable Quasi-Solid-State Aqueous Hybrid Al3+/H+ Battery with 10,000 Ultralong Cycle Stability and Smart Switching Capability. Nano Res. 2021, 14, 4154–4162. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D.J.L.; Liu, T.; He, G.; Parkin, I.P. Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Adv. Mater. 2021, 33, 2007548. [Google Scholar] [CrossRef]
- Hsu, C.P.; Mandal, J.; Ramakrishna, S.N.; Spencer, N.D.; Isa, L. Exploring the Roles of Roughness, Friction and Adhesion in Discontinuous Shear Thickening by means of Thermo-Responsive Particles. Nat. Commun. 2021, 12, 1477. [Google Scholar] [CrossRef]
- Duan, J.; Yu, B.; Liu, K.; Li, J.; Yang, P.; Xie, W.; Xue, G.; Liu, R.; Wang, H.; Zhou, J.P.-N. Conversion in Thermogalvanic Cells Induced by Thermo-Sensitive Nanogels for Body Heat Harvesting. Nano Energy 2019, 57, 473–479. [Google Scholar] [CrossRef]
- Cao, M.; Shen, Y.; Yan, Z.; Wei, Q.; Jiao, T.; Shen, Y.; Han, Y.; Wang, Y.; Wang, S.; Xia, Y.; et al. Extraction-Like Removal of Organic Dyes from Polluted Water by the Graphene Oxide/PNIPAM Composite System. Chem. Eng. J. 2021, 405, 126647. [Google Scholar] [CrossRef]
- Cao, S.; Tong, X.; Dai, K.; Xu, Q. A Super-Stretchable and Tough Functionalized Boron Nitride/PEDOT:PSS/Poly(N-isopropylacrylamide) Hydrogel with Self-healing, Adhesion, Conductive and Photothermal Activity. J. Mater. Chem. A 2019, 7, 8204–8209. [Google Scholar] [CrossRef]
- Alsaid, Y.; Wu, S.; Wu, D.; Du, Y.; Shi, L.; Khodambashi, R.; Rico, R.; Hua, M.; Yan, Y.; Zhao, Y.; et al. Tunable Sponge-Like Hierarchically Porous Hydrogels with Simultaneously Enhanced Diffusivity and Mechanical Properties. Adv. Mater. 2021, 33, 2008235. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ozden, S.; Bizmark, N.; Arnold, C.B.; Datta, S.S.; Priestley, R.D. A Bioinspired Elastic Hydrogel for Solar-Driven Water Purification. Adv. Mater. 2021, 33, 2007833. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Gao, G.; Wei, S.; Jian, Y.; Le, X.; Lu, W.; Liu, Q.; Zhang, J.; Chen, T. Asymmetric Bilayer CNTs-Elastomer/Hydrogel Composite as Soft Actuators with Sensing Performance. Chem. Eng. J. 2021, 415, 128988. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Han, M.; Palmer, L.C.; Rogers, J.A.; Huang, Y.; Stupp, S.I. Synergistic Photoactuation of Bilayered Spiropyran Hydrogels for Predictable Origami-like Shape Change. Matter 2021, 4, 1377–1390. [Google Scholar] [CrossRef]
- Son, H.; Byun, E.; Yoon, Y.J.; Nam, J.; Song, S.H.; Yoon, C. Untethered Actuation of Hybrid Hydrogel Gripper via Ultrasound. ACS Macro Lett. 2020, 9, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yamamoto, S.; Sueyoshi, K.; Inadomi, T.; Kato, R.; Miyamoto, N. Perovskite Nanosheet Hydrogels with Mechanochromic Structural Color. Angew. Chem. Int. Ed. Engl. 2021, 60, 8466–8471. [Google Scholar] [CrossRef]
- Feng, H.; Fu, Q.; Du, W.; Zhu, R.; Ge, X.; Wang, C.; Li, Q.; Su, L.; Yang, H.; Song, J. Quantitative Assessment of Copper (II) in Wilson’s Disease Based on Photoacoustic Imaging and Ratiometric Surface-Enhanced Raman Scattering. ACS Nano 2021, 15, 3402–3414. [Google Scholar] [CrossRef]
- Li, X.; Zhao, D.; Shea, K.J.; Li, X.; Lu, X. In-Situ Formed Thermogelable Hydrogel Photonic Crystals Assembled by Thermosensitive IPN Nanogels. Mater. Horiz. 2021, 8, 932–938. [Google Scholar] [CrossRef]
- Deng, H.; Xu, X.; Zhang, C.; Su, J.W.; Huang, G.; Lin, J. Deterministic Self-Morphing of Soft-Stiff Hybridized Polymeric Films for Acoustic Metamaterials. ACS Appl. Mater. Interfaces 2020, 12, 13378–13385. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, Y.; Ali, Z.; Yin, H.; Sheng, F.; Lin, J.; Wang, B.; Hou, Y. Near-Infrared Light and Tumor Microenvironment Dual Responsive Size-Switchable Nanocapsules for Multimodal Tumor Theranostics. Nat. Commun. 2019, 10, 4418. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Chen, Z.; Gao, R.; Pan, L.; Deng, J.; Ye, X.; Zhang, J.; Zhang, S.; Mei, C.; et al. Microenvironment-Triggered Degradable Hydrogel for Imaging Diagnosis and Combined Treatment of Intraocular Choroidal Melanoma. ACS Nano 2020, 14, 15403–15416. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, Y.; Zhang, D.; Zhang, H.; Zhao, Y. Morphological Hydrogel Microfibers with MXene Encapsulation for Electronic Skin. Research 2021, 2021, 7065907. [Google Scholar] [CrossRef]
- Oh, J.H.; Hong, S.Y.; Park, H.; Jin, S.W.; Jeong, Y.R.; Oh, S.Y.; Yun, J.; Lee, H.; Kim, J.W.; Ha, J.S. Fabrication of High-Sensitivity Skin-Attachable Temperature Sensors with Bioinspired Microstructured Adhesive. ACS Appl. Mater. Interfaces 2018, 10, 7263–7270. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhang, X.; Li, W.; Tang, Y.; Bai, Y. Quickly Self-Healing Hydrogel at Room Temperature with High Conductivity Synthesized through Simple Free Radical Polymerization. J. Appl. Polym. Sci. 2019, 136, 47379. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.Z.; Zhang, W.; Yuan, W.; El-Demellawi, J.K.; Zhang, P.; Di Fabrizio, E.; Dong, X.; Alshareef, H.N. Ti3C2Tx MXene-Activated Fast Gelation of Stretchable and Self-Healing Hydrogels: A Molecular Approach. ACS Nano 2021, 15, 2698–2706. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, G.; Guo, J.; Liu, Y.; Ren, J.; Kong, T.; Zhao, Y. Vitamin Metal–Organic Framework-Laden Microfibers from Microfluidics for Wound Healing. Mater. Horiz. 2018, 5, 1137–1142. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Sun, L.; Zhang, X.; Zhao, Y. Microfluidic Generation of Microsprings with Ionic Liquid Encapsulation for Flexible Electronics. Research 2019, 2019, 6906275. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jin, Z.; Peng, L.; Zhao, F.; Xiao, D.; Jin, Y.; Yu, G. Stretchable All-Gel-State Fiber-Shaped Supercapacitors Enabled by Macromolecularly Interconnected 3D Graphene/Nanostructured Conductive Polymer Hydrogels. Adv. Mater. 2018, 30, 1800124. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Shi, R.; Xu, Y.; Zhang, L.; Fang, R.; Zhao, T.; Qi, S.; Jiang, L.; Liu, M. Super-Tough and Strong Nanocomposite Fibers by Flow-Induced Alignment of Carbon Nanotubes on Grooved Hydrogel Surfaces. Sci. China Mater. 2019, 62, 1332–1340. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kumar, H.; Guo, C.; Shin, J.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. Development of Antifreezing, Printable, Adhesive, Tough, Biocompatible, High-Water Content Hydrogel for Versatile Applications. ACS Appl. Mater. Interfaces 2023, 15, 16034–16045. [Google Scholar] [CrossRef]
- He, P.; Wu, J.; Pan, X.; Chen, L.; Liu, K.; Gao, H.; Wu, H.; Cao, S.; Huang, L.; Ni, Y. Anti-Freezing and Moisturizing Conductive Hydrogels for Strain Sensing and Moist-Electric Generation Applications. J. Mater. Chem. A 2020, 8, 3109–3118. [Google Scholar] [CrossRef]
- Jian, C.S.; Shuo, C.; Li, J.S.; Yi, F.G.; Lu, Z.Z.; Shu, L.W.; Hui, X.X.; Qing, B.G.; Zheng, W.Y. Mechanically and Electronically Robust Transparent Organohydrogel Fibers. Adv. Mater. 2020, 32, 1906994. [Google Scholar]
- Bai, Y.; Liu, R.; Liu, Y.; Wang, Y.; Wang, X.; Xiao, H.; Yuan, G. Concentrated Hydrogel Electrolyte for Integrated Supercapacitor with High Capacitance at Subzero Temperature. Sci. China Chem. 2021, 64, 852–860. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, Conductive Self-Healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Ed. Engl. 2017, 56, 14159–14163. [Google Scholar] [CrossRef]
- Peng, S.; Liu, S.; Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Facile Preparation and Characterization of Poly(vinyl alcohol)-NaCl-glycerol Supramolecular Hydrogel Electrolyte. Eur. Polym. J. 2018, 106, 206–213. [Google Scholar] [CrossRef]
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W.; et al. Polyacrylamide/Chitosan-Based Conductive Double Network Hydrogels with Outstanding Electrical and Mechanical Performance at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Poly(vinyl alcohol) Hydrogels with Integrated Toughness, Conductivity, and Freezing Tolerance Based on Ionic Liquid/Water Binary Solvent Systems. ACS Appl. Mater. Interface 2021, 13, 29008–29020. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Z.; Song, H.; Chang, Y.; Hou, W.; Li, Y.; Han, G. High-Sensitivity and Extreme Environment-Resistant Sensors Based on PEDOT:PSS@PVA Hydrogel Fibers for Physiological Monitoring. ACS Appl. Mater. Interface 2022, 14, 35114–35125. [Google Scholar] [CrossRef]
- Wei, F.; Tong, L.; Fan, W.; Shu, J.W.; Sheng, B.G.; Yun, H.L.; Jin, L.L.; Hao, R.Y.; Rui, X.L.; Chan, W.; et al. An Antisweat Interference and Highly Sensitive Temperature Sensor Based on Poly(3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) Fiber Coated with Polyurethane/ Graphene for Real-Time Monitoring of Body Temperature. ACS Nano 2023, 17, 21073–21082. [Google Scholar]
- Shu, Y.Z.; Zi, G.Z.; Zhe, X.X.; Yu, F.H.; Xu, Y.; Tian, Y.Z.; Huan, J.L.; Elias, M.K.; Li, W.; Lei, J.; et al. Complex Multiphase Organohydrogels with Programmable Mechanics Toward Adaptive Soft-Matter Machines. Sci. Adv. 2020, 6, 1464. [Google Scholar]
- Anand, K.M.; Thomas, J.W.; Wen, Y.P.; Patricia, X.; Kai, Y.W.; Emmanuel, P.G.; Barbara, M.; Robert, F.S. Autonomic Perspiration in 3D-Printed Hydrogel Actuators. Sci. Robot. 2020, 5, 3918. [Google Scholar]
- Liang, S.; Tu, Y.; Chen, Q.; Jia, W.; Wang, W.; Zhang, L. Microscopic Hollow Hydrogel Springs, Necklaces and Ladders: A Tubular Robot as a Potential Vascular Scavenger. Mater. Horiz. 2019, 6, 2135–2142. [Google Scholar] [CrossRef]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef]
- Maleki, M.; Habibi, M.; Golestanian, R.; Ribe, N.M.; Bonn, D. Liquid Rope Coiling on a Solid Surface. Phys. Rev. Lett. 2004, 93, 214502. [Google Scholar] [CrossRef]
- Su, J.S.; Ji, Y.P.; Jin, Y.L.; Ho, P.; Yong, D.P.; Kyu, B.L.; Chang, M.W.; Sang, H.L. “On the Fly” Continuous Generation of Alginate Fibers Using a Microfluidic Device. Langmuir 2007, 23, 9104–9108. [Google Scholar]
- Tottori, S.; Takeuchi, S. Formation of Liquid Rope Coils in a Coaxial Microfluidic Device. RSC Adv. 2015, 5, 33691–33695. [Google Scholar] [CrossRef]
- Grolman, J.M.; Zhang, D.; Smith, A.M.; Moore, J.S.; Kilian, K.A. Rapid 3D Extrusion of Synthetic Tumor Microenvironments. Adv. Mater. 2015, 27, 5512–5517. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, F.; Shang, L.; Cheng, Y.; Gu, Z.; Zhao, Y. Bioinspired Helical Microfibers from Microfluidics. Adv. Mater. 2017, 29, 1605765. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic Spinning of Micro- and Nano-Scale Fibers for Tissue Engineering. Lab. Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xie, R.; Liu, Y.; Luo, G.; Ding, M.; Liang, Q. Bioinspired Microfibers with Embedded Perfusable Helical Channels. Adv. Mater. 2017, 29, 1701664. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, J. Thermoresponsive PEDOT:PSS/PNIPAM Conductive Hydrogels as Wearable Resistive Sensors for Breathing Pattern Detection. Polym. J. 2022, 54, 793–801. [Google Scholar] [CrossRef]
- Wei, N.H.; Guang, Y.L.; Shang, Q.Z.; Yong, W.; Jin, W.; Qing, W.L.; Xue, T.Z. Polypyrrole/Silver Coaxial Nanowire Aero-Sponges for Temperature-Independent Stress Sensing and Stress-Triggered Joule Heating. ACS Nano 2015, 9, 4244–4251. [Google Scholar]
- Youn, D.Y.; Jung, U.; Naqi, M.; Choi, S.J.; Lee, M.G.; Lee, S.; Park, H.J.; Kim, I.D.; Kim, S. Wireless Real-Time Temperature Monitoring of Blood Packages: Silver Nanowire-Embedded Flexible Temperature Sensors. ACS Appl. Mater. Interfaces 2018, 10, 44678–44685. [Google Scholar] [CrossRef] [PubMed]
- Honda, W.; Harada, S.; Ishida, S.; Arie, T.; Akita, S.; Takei, K. High-Performance, Mechanically Flexible, and Vertically Integrated 3D Carbon Nanotube and InGaZnO Complementary Circuits with a Temperature Sensor. Adv. Mater. 2015, 27, 4674–4680. [Google Scholar] [CrossRef]
- Yang, H.; Qi, D.; Liu, Z.; Chandran, B.K.; Wang, T.; Yu, J.; Chen, X. Soft Thermal Sensor with Mechanical Adaptability. Adv. Mater. 2016, 28, 9175–9181. [Google Scholar] [CrossRef]
- Gu, J.; Huang, J.; Chen, G.; Hou, L.; Zhang, J.; Zhang, X.; Yang, X.; Guan, L.; Jiang, X.; Liu, H. Multifunctional Poly(vinyl alcohol) Nanocomposite Organohydrogel for Flexible Strain and Temperature Sensor. ACS Appl. Mater. Interfaces 2020, 12, 40815–40827. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Jiang, X.W.; Pooi, S.L. Stretchable Graphene Thermistor with Tunable Thermal Index. ACS Nano 2015, 9, 2130–2137. [Google Scholar]
- Yang, X.; Cao, L.; Wang, J.; Chen, L. Sandwich-like Polypyrrole/Reduced Graphene Oxide Nanosheets Integrated Gelatin Hydrogel as Mechanically and Thermally Sensitive Skin-Like Bioelectronics. ACS Sustain. Chem. Eng. 2020, 8, 10726–10739. [Google Scholar]
- Pang, Q.; Hu, H.; Zhang, H.; Qiao, B.; Ma, L. Temperature-Responsive Ionic Conductive Hydrogel for Strain and Temperature Sensors. ACS Appl. Mater. Interfaces 2022, 14, 26536–26547. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Liu, J.; Gu, J.; Zhu, J.; Huang, B.; Bai, J.; Guo, J.; Yang, X.; Guan, L. High Toughness Multifunctional Organic Hydrogels for Flexible Strain and Temperature Sensor. J. Mater. Chem. A 2021, 9, 23243–23255. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Liao, L.; Zhang, H.; Zhou, H.; Zhou, W.; Ren, T.; Sun, Z.; Lu, Y.; Nie, Z.; et al. Approaching Intrinsic Dynamics of MXenes Hybrid Hydrogel for 3D Printed Multimodal Intelligent Devices with Ultrahigh Superelasticity and Temperature Sensitivity. Nat. Commun. 2022, 13, 3420. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Guan, M.; Yao, R.; Qing, Y.; Hou, X.; Zhang, J. Facile Formation of Multifunctional Biomimetic Hydrogel Fibers for Sensing Applications. Gels 2024, 10, 590. https://doi.org/10.3390/gels10090590
Jia M, Guan M, Yao R, Qing Y, Hou X, Zhang J. Facile Formation of Multifunctional Biomimetic Hydrogel Fibers for Sensing Applications. Gels. 2024; 10(9):590. https://doi.org/10.3390/gels10090590
Chicago/Turabian StyleJia, Mengwei, Mingle Guan, Ryan Yao, Yuan Qing, Xiaoya Hou, and Jie Zhang. 2024. "Facile Formation of Multifunctional Biomimetic Hydrogel Fibers for Sensing Applications" Gels 10, no. 9: 590. https://doi.org/10.3390/gels10090590
APA StyleJia, M., Guan, M., Yao, R., Qing, Y., Hou, X., & Zhang, J. (2024). Facile Formation of Multifunctional Biomimetic Hydrogel Fibers for Sensing Applications. Gels, 10(9), 590. https://doi.org/10.3390/gels10090590






