Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Chemical Analysis
2.1.2. Characterization of the Ag Nanoparticles
Color Change and UV–Visible Spectroscopy Analysis
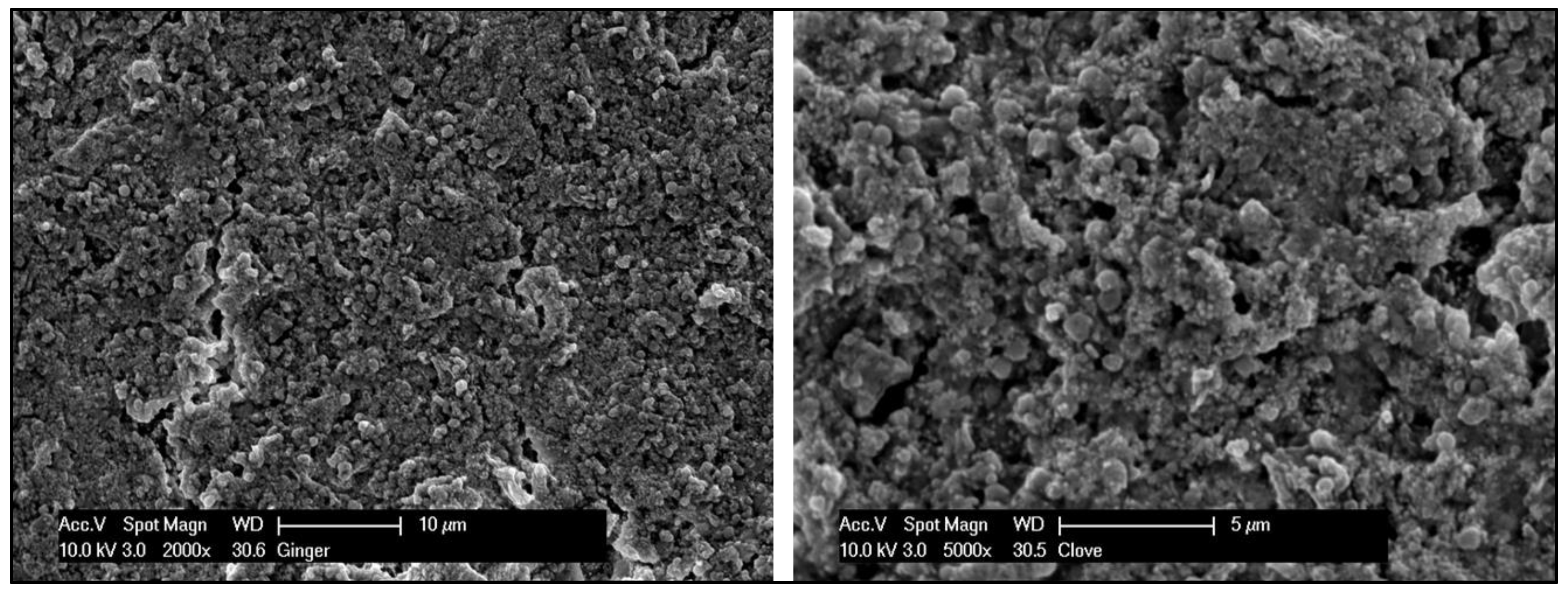
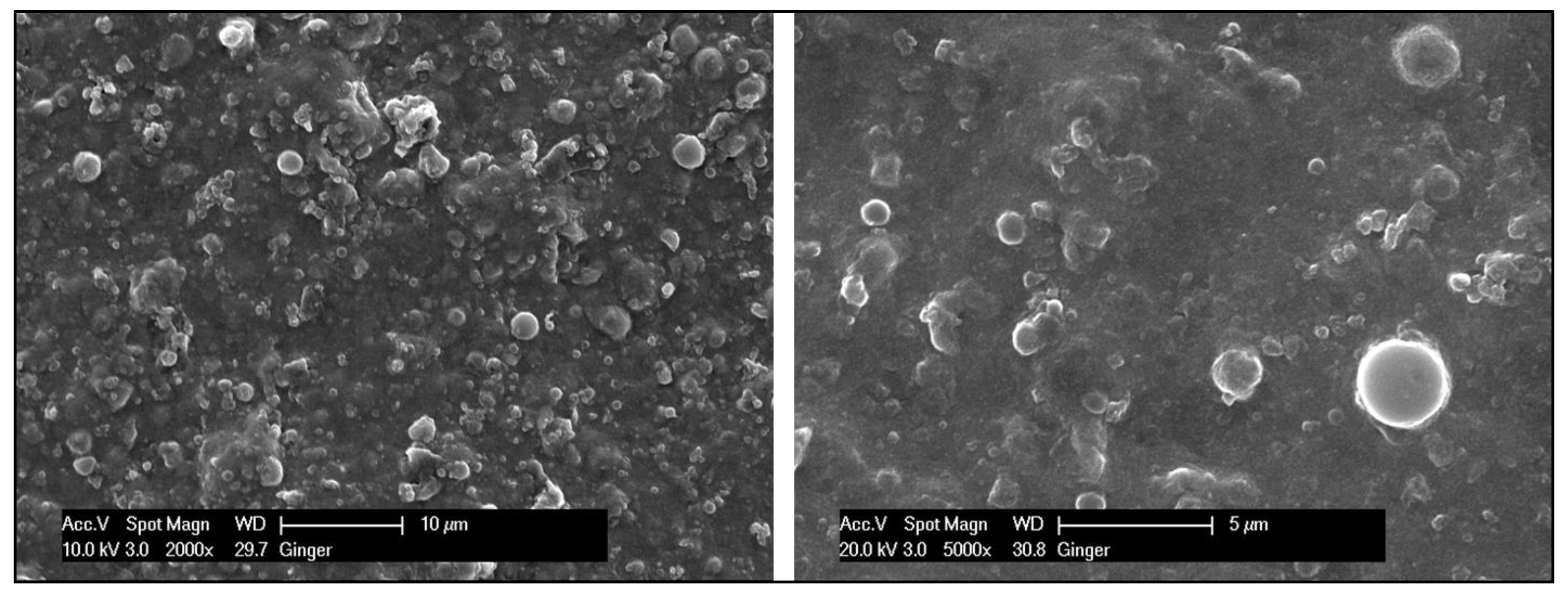
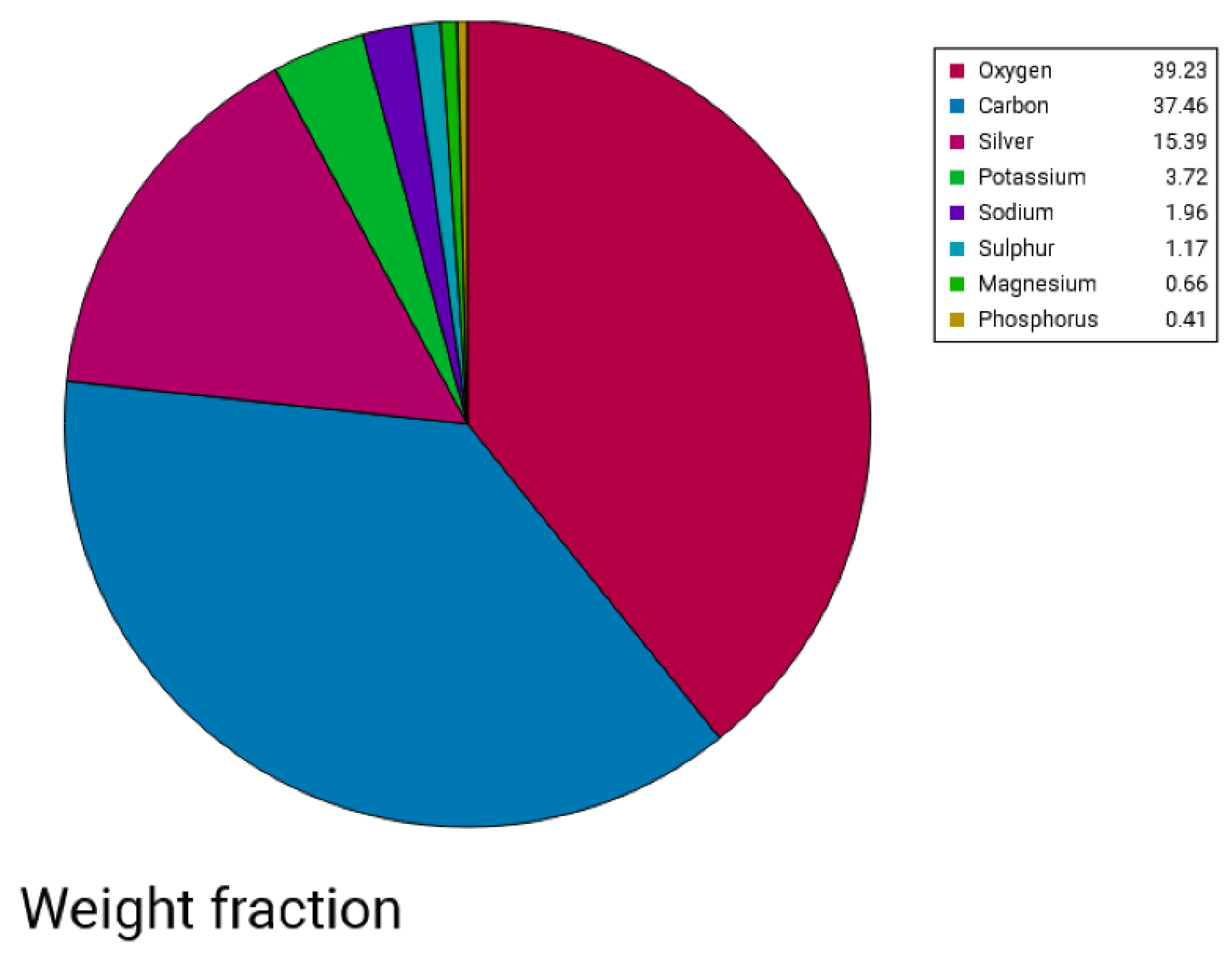
Scanning Electron Microscopy—EDX
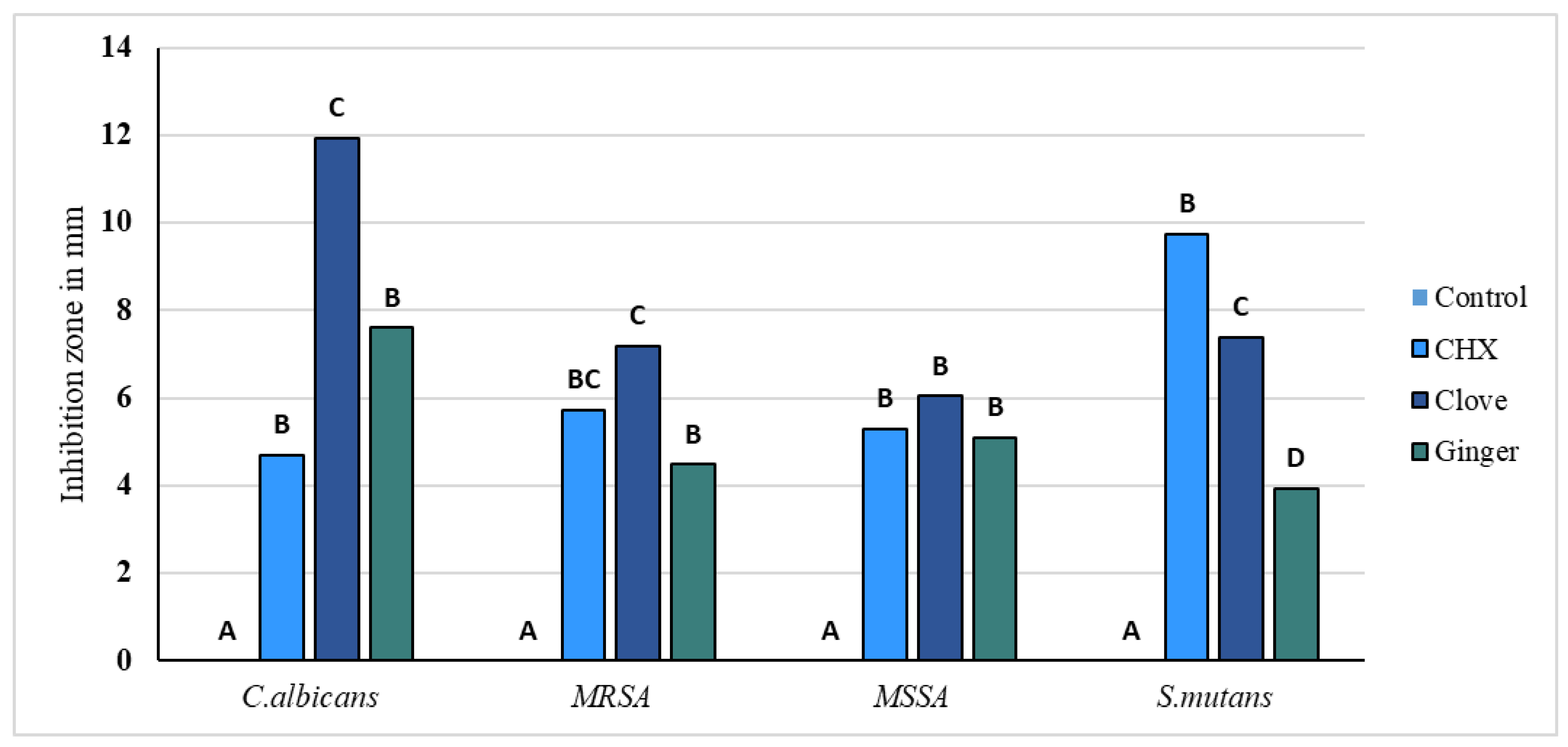
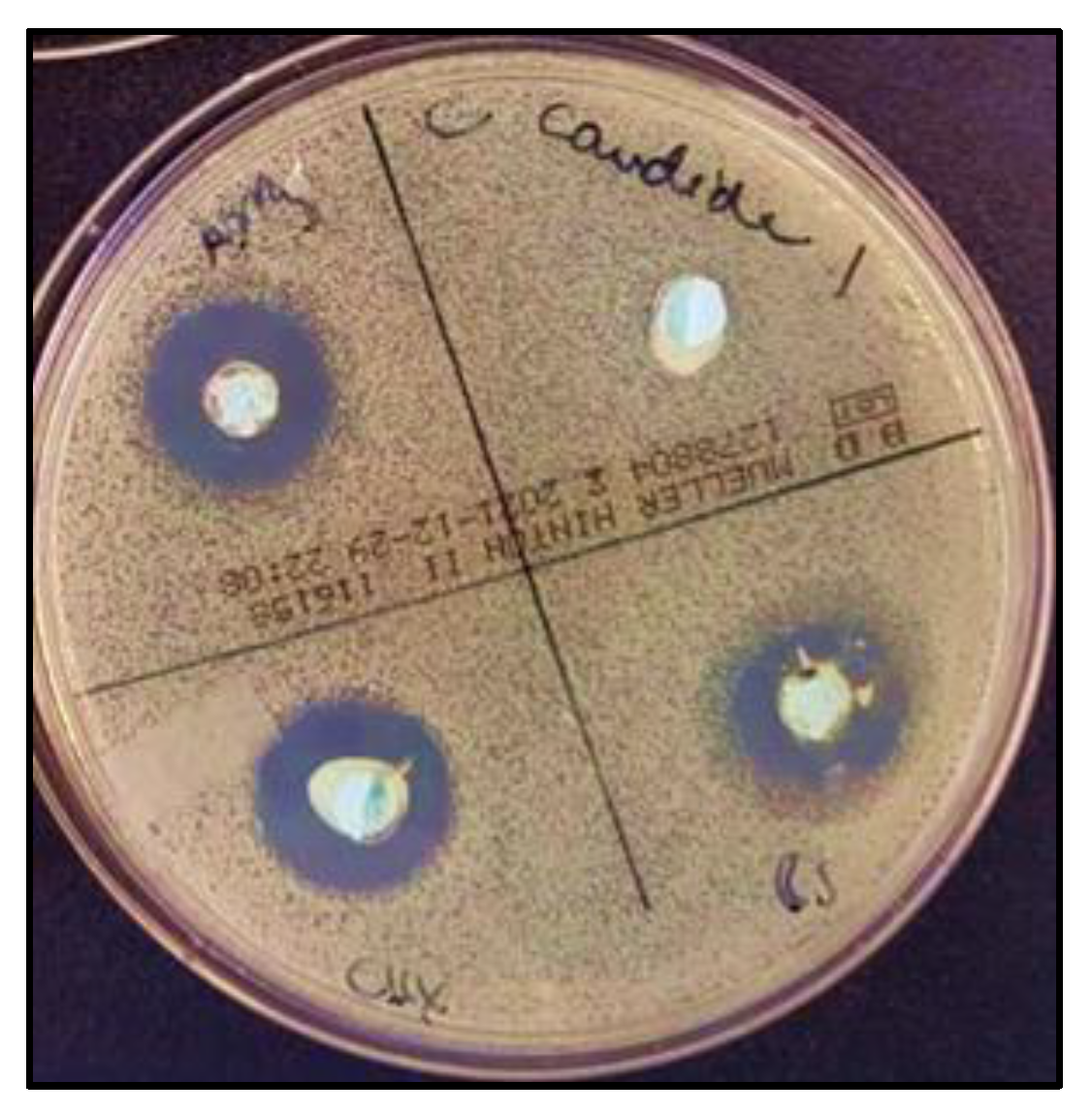
2.1.3. Antimicrobial Activity
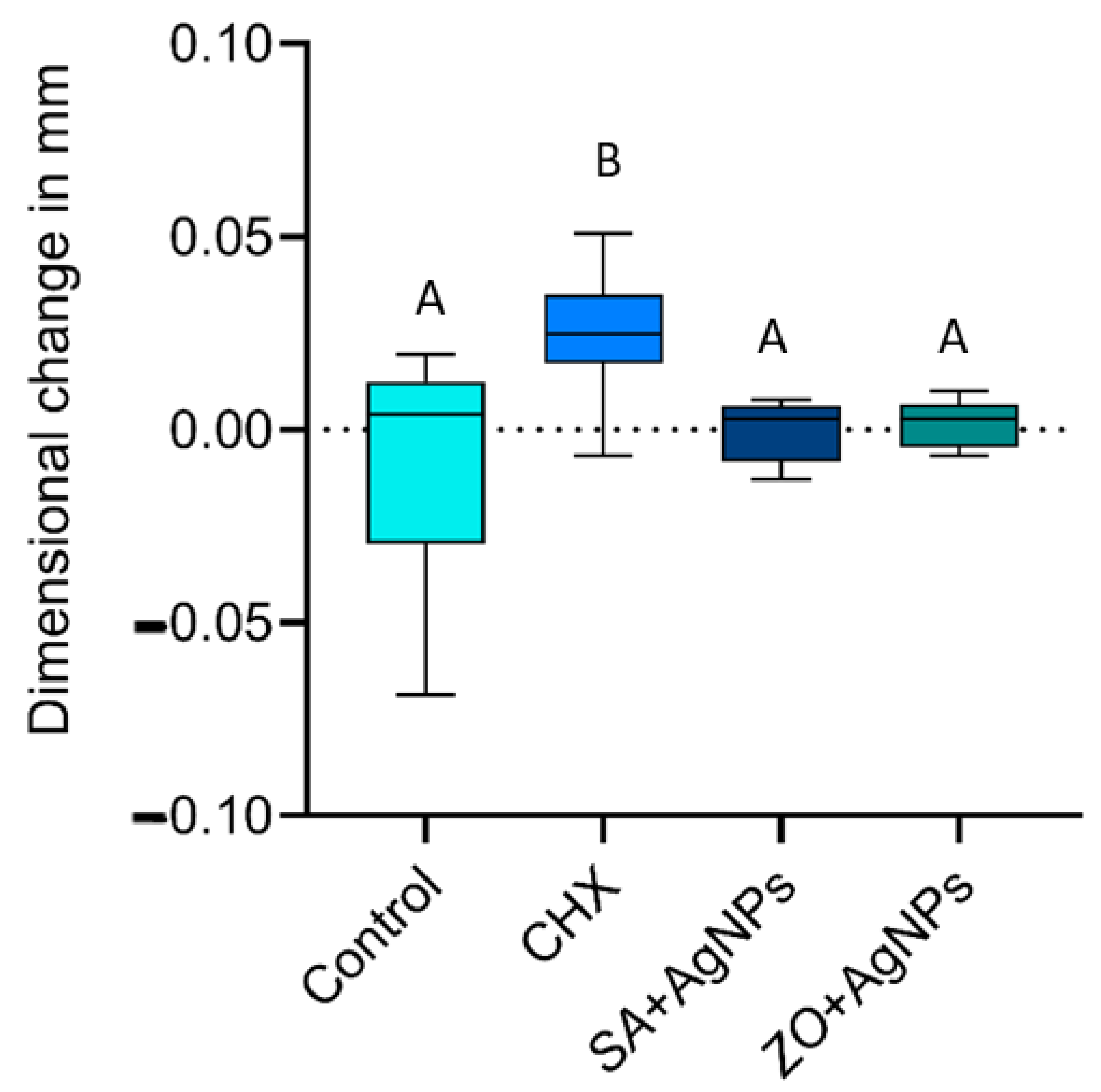
2.1.4. Dimensional Accuracy
2.2. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Antimicrobial Modification Solutions
4.3. Experimental Groups
- Negative Control: Alginate was mixed with distilled water.
- Positive Control: Alginate was mixed with a 0.2% chlorhexidine solution instead of water.
- Modified Groups:
- ○
- In one group, alginate was mixed with a solution of silver nitrate and the prepared clove extract (5:1).
- ○
- In the other group, alginate was mixed with a solution of silver nitrate and the prepared ginger extract (5:1).
4.4. Chemical Analysis of Plant Extracts
4.5. Characterization of Silver Nanoparticles
4.5.1. Scanning Electron Microscopy–Energy-Dispersive X-ray Spectroscopy
4.5.2. UV–Vis Spectroscopy
4.6. Analysis of Antimicrobial Activity
4.7. Dimensional Changes
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nandini, V.V.; Venkatesh, K.V.; Nair, K.C. Alginate impressions: A practical perspective. J. Conserv. Dent. 2008, 11, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.J. Some properties of alginate impression materials relevant to clinical practice. Br. Dent. J. 1966, 121, 463–467. [Google Scholar] [PubMed]
- Wang, J.; Wan, Q.; Chao, Y.; Chen, Y. A self-disinfecting irreversible hydrocolloid impression material mixed with chlorhexidine solution. Angle Orthod. 2007, 77, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Badrian, H.; Ghasemi, E.; Khalighinejad, N.; Hosseini, N. The effect of three different disinfection materials on alginate impression by spray method. ISRN Dent. 2012, 2012, 695151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Castro, D.T.; Kreve, S.; Oliveira, V.C.; Alves, O.L.; Dos Reis, A.C. Development of an Impression Material with Antimicrobial Properties for Dental Application. J. Prosthodont. 2019, 28, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.A.; Palenik, C.J.; Setcos, J.C.; Miller, C.H. Antimicrobial activities of dental impression materials. Dent. Mater. 1998, 14, 399–404. [Google Scholar] [CrossRef]
- Al-Jabrah, O.; Al-Shumailan, Y.; Al-Rashdan, M. Antimicrobial effect of 4 disinfectants on alginate, polyether, and polyvinyl siloxane impression materials. Int. J. Prosthodont. 2007, 20, 299–307. [Google Scholar]
- Dorner, A.R.; Ferraz da Silva, J.; Uemura, E.S.; Borges, A.L.; Fernandes Junior, V.; Yamamoto, E.T. Effect of disinfection of irreversible hydrocolloid impression materials with 1% sodium hypochlorite on surface roughness and dimensional accuracy of dental stone casts. J. Prosthodont. 2014, 23, 363–368. [Google Scholar] [CrossRef]
- Muzaffar, D.; Braden, M.; Parker, S.; Patel, M.P. The effect of disinfecting solutions on the dimensional stability of dental alginate impression materials. Dent. Mater. 2012, 28, 749–755. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials, 12th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2012. [Google Scholar]
- Ismail, H.A.; Asfour, H.; Shikho, S.A. A self-disinfecting irreversible hydrocolloid impression material mixed with povidone iodine powder. Eur. J. Dent. 2016, 10, 507–511. [Google Scholar] [CrossRef]
- Ramer, M.S.; Gerhardt, D.E.; McNally, K. Accuracy of irreversible hydrocolloid impression material mixed with disinfectant solutions. J. Prosthodont. 1993, 2, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, M.; Hasanzadeh, N.; Soleimani, F.; Shafaee, H. Antimicrobial and physical properties of alginate impression material incorporated with silver nanoparticles. Dent. Res. J. 2019, 16, 372–376. [Google Scholar]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular Basis of the Therapeutical Potential of Clove (Syzygium aromaticum L.) and Clues to Its Anti-COVID-19 Utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef] [PubMed]
- Jardón-Romero, E.A.; Lara-Carrillo, E.; González-Pedroza, M.G.; Sánchez-Mendieta, V.; Salmerón-Valdés, E.N.; Toral-Rizo, V.H.; Olea-Mejía, O.F.; López-González, S.; Morales-Luckie, R.A. Antimicrobial Activity of Biogenic Silver Nanoparticles from Syzygium aromaticum against the Five Most Common Microorganisms in the Oral Cavity. Antibiotics 2022, 11, 834. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2018, 7, 96–108. [Google Scholar] [CrossRef]
- Hu, D.; Gao, T.; Kong, X.; Ma, N.; Fu, J.; Meng, L.; Duan, X.; Hu, C.Y.; Chen, W.; Feng, Z.; et al. Ginger (Zingiber officinale) extract mediated green synthesis of silver nanoparticles and evaluation of their antioxidant activity and potential catalytic reduction activities with Direct Blue 15 or Direct Orange 26. PLoS ONE 2022, 17, e0271408. [Google Scholar] [CrossRef]
- Singer, L.; Karacic, S.; Szekat, C.; Bierbaum, G.; Bourauel, C. Biological properties of experimental dental alginate modified for self-disinfection using green nanotechnology. Clin. Oral Investig. 2023, 27, 6677–6688. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Mussin, J.; Robles-Botero, V.; Casañas-Pimentel, R.; Rojas, F.; Angiolella, L.; San Martín-Martínez, E.; Giusiano, G. Antimicrobial and cytotoxic activity of green synthesis silver nanoparticles targeting skin and soft tissue infectious agents. Sci. Rep. 2021, 11, 14566. [Google Scholar] [CrossRef] [PubMed]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials 2019, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), 336–343. [Google Scholar] [CrossRef]
- Ajlouni, A.W.; Hamdan, E.H.; Alshalawi, R.A.E.; Shaik, M.R.; Khan, M.; Kuniyil, M.; Alwarthan, A.; Ansari, M.A.; Khan, M.; Alkhathlan, H.Z.; et al. Green Synthesis of Silver Nanoparticles Using Aerial Part Extract of the Anthemis pseudocotula Boiss. Plant and Their Biological Activity. Molecules 2022, 28, 246. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose-Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, S.; Singh, R.P. Green synthesis of silver nanoparticles using ginger extract: Characterization and antibacterial activity. Mater. Sci. Eng. C 2017, 71, 1064–1072. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Heel, K.A. Use of multiparameter flow cytometry to determine the effects of monoterpenoids and phenylpropanoids on membrane polarity and permeability in staphylococci and enterococci. Int. J. Antimicrob. Agents 2012, 40, 239–245. [Google Scholar] [CrossRef]
- Al Mutairi, J.F.; Al-Otibi, F.; Alhajri, H.M.; Alharbi, R.I.; Alarifi, S.; Alterary, S.S. Antimicrobial Activity of Green Silver Nanoparticles Synthesized by Different Extracts from the Leaves of Saudi Palm Tree (Phoenix dactylifera L.). Molecules 2022, 27, 3113. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, e0256748. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhao, I.S.; Duffin, S.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. Revitalising silver nitrate for caries management. Int. J. Environ. Res. Public Health 2018, 15, 80. [Google Scholar] [CrossRef]
- Aoyama, N.; Hayakawa, I.; Akiba, N.; Minakuchi, S. Effect of high molecular weight sodium on the viscosity and characteristics of alginate impression materials. Prosthodont. Res. Pract. 2007, 6, 239–245. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Thombare, R.U. Dimensional Changes of Alginate Dental Impression Materials-An Invitro Study. J. Clin. Diagn. Res. 2015, 9, ZC98–ZC102. [Google Scholar] [CrossRef]
- ISO 21563:2021; Dentistry—Test Method for Alginate Impression Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Tan, H.K.; Hooper, P.M.; Buttar, I.A.; Wolfaardt, J.F. Effects of disinfecting irreversible hydrocolloid impressions on the resultant gypsum casts, part III: Dimensional changes. J. Prosthet. Dent. 1993, 70, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Vannort, R. Introduction to Dental Materials, 4th ed.; Mosby: St. Louis, MI, USA, 2013; pp. 189–191. [Google Scholar]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Keilig, L.; Fichte, M.; Bourauel, C. Evaluation of the properties of a new super quick-setting (2 min) polyether impression material. Clin. Oral Investig. 2023, 27, 3673–3682. [Google Scholar] [CrossRef]
- Santana, P.; Miranda, M.; Payrol, J.; Silva, M.; Hernández, V.; Peralta, E. Gas chromatography-mass spectrometry study from the leaves fractions obtained of vernonanthura patens (Kunth) H. Rob. Int. J. Org. Chem. 2013, 3, 105–109. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Ahsan Bajwa, A.; Parveen, A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation. Molecules 2020, 25, 3324. [Google Scholar] [CrossRef]
- Annamalai, J.; Nallamuthu, T. Green synthesis of silver nanoparticles: Characterization and determination of antibacterial potency. Appl. Nanosci. 2016, 6, 259–265. [Google Scholar] [CrossRef]
- Council on Dental Materials, Instruments, and Equipment. American National Standard/American Dental Association (ANSI/ADA), Specification No. 18, Alginate Impression Materials 1992; American Dental Association: Chicago, IL, USA, 1992. [Google Scholar]
- ISO Specification NO.4823:2000; Dentistry—Elastomeric Impression Materials. International Organization for Standardization: Geneva, Switzerland, 2000.




| NO. | Retention Time | Name | Area Sum % |
|---|---|---|---|
| 1 | 5.322 | α-Cedrol | 1.75 |
| 2 | 5.683 | Caryophyllene | 0.42 |
| 3 | 5.741 | Methyleugenol | 0.33 |
| 4 | 6.446 | Gallic acid | 1.89 |
| 5 | 6.725 | Patchoulol | 1.18 |
| 6 | 7.053 | 2-Heptanol | 1.16 |
| 7 | 7.701 | Longiborneol | 0,47 |
| 8 | 7.807 | Isobornyl acetate | 0.77 |
| 9 | 8.012 | Caffeic acid | 0.96 |
| 11 | 8.316 | 2-Hexadecanol | 1.28 |
| 12 | 8.632 | Salicylic acid | 0.34 |
| 10 | 8.8 | Phytol | 0.7 |
| 13 | 9.14 | Carveol | 2.2 |
| 14 | 9.513 | β-Curcumene | 0.89 |
| 15 | 9.792 | Chavico | 1.14 |
| 16 | 9.86 | Cinnamyl alcohol | 1.45 |
| 17 | 10.46 | Flavone,3′,4′,6,7-tetra methoxy | 0.71 |
| 18 | 10.57 | p-Cymen-7-ol | 1.03 |
| 19 | 10.74 | Cubebol | 0.77 |
| 20 | 10.85 | Ylangene | 0.47 |
| 21 | 11.12 | Eugenol | 29.46 |
| 22 | 11.25 | γ-Muurolene | 1.61 |
| 23 | 11.28 | α-copaen | 0.8 |
| 24 | 11.61 | Isoeugenol | 0.74 |
| 25 | 11.79 | β-Caryophyllen | 10.12 |
| 26 | 12.08 | α-Humulene | 2.26 |
| 27 | 12.13 | β-Elemen | 1.08 |
| 28 | 12.27 | γ-Cadinene | 0.58 |
| 29 | 12.51 | Alloaromadendren | 0.67 |
| 30 | 12.74 | δ-Cadinene | 3.72 |
| 31 | 12.86 | α-Cubebene | 0.42 |
| 32 | 12.90 | α-Cedrene | 0.39 |
| 33 | 12.95 | Retinol | 0.38 |
| 34 | 13.11 | Caryophyllene epoxide | 0.58 |
| 35 | 13.16 | α-Ionol | 0,92 |
| 36 | 13.37 | Widdrol | 0.77 |
| 37 | 13.38 | Elemol | 1 |
| 38 | 13.41 | Ledene | 0.77 |
| 39 | 13.53 | β-Patchoulene | 2.57 |
| 40 | 13.88 | α-Ylangene | 0.59 |
| 41 | 13.94 | Sclareol | 0.39 |
| 42 | 13.99 | β-Ionone | 0.43 |
| 43 | 14.12 | Epiglobulol | 0.3 |
| 44 | 14.17 | Thunbergol | 0.67 |
| 45 | 14,31 | Spathulenol | 1.11 |
| 46 | 14.43 | 4′-Hydroxy-5-methoxy flavone | 1.17 |
| 47 | 15.61 | Shyobunol | 0.87 |
| 48 | 15.86 | Syringic acid | 0.92 |
| 49 | 16.24 | β Carotene | 1.87 |
| 50 | 16.64 | Afromosin 7-O-glucoside | 0.86 |
| 51 | 16.89 | Flavone, 5-hydroxy-3,3′,4′,6,7-pentamethoxy- | 0.52 |
| 52 | 18.09 | 3′,4′,5′,5,6,7-Hexamethoxyflavone | 1.15 |
| 53 | 18.31 | Oleyl oleate | 2.05 |
| 54 | 18.63 | Ascaridole | 1.06 |
| 55 | 19.36 | Vitexin | 1.54 |
| 56 | 20.74 | Homovanillic Acid | 1.52 |
| 57 | 21.10 | Flavone, 3,5,7-tri methoxy- | 1.18 |
| 58 | 21.61 | γ-Selinene | 1.12 |
| 59 | 22.77 | Dehydrodieugenol | 0.74 |
| 60 | 23.08 | Geranyl isovalerate | 1.18 |
| NO. | Retention Time | Name | Area Sum % |
|---|---|---|---|
| 1 | 3.908 | 7-Hydroxy-4-methyl-3-phenyl coumarin | 2.23 |
| 2 | 4.486 | 2′,5′-Dimethoxyflavone | 1.18 |
| 3 | 4.662 | 2-Hydroxychalcone | 1.26 |
| 4 | 4.851 | 3′,4′,5′,5,6,7-Hexamethoxyflavone | 1.21 |
| 5 | 5.089 | Clovane | 1.32 |
| 6 | 5.458 | Luteolin 6-C-glucoside | 0.51 |
| 7 | 5.782 | Swertisin | 1.14 |
| 8 | 6.389 | 7,3′,4′,5′-Tetramethoxyflavanone | 1.54 |
| 9 | 6.651 | 3,2′,4′,5′-Tetramethoxyflavone | 0.72 |
| 10 | 6.987 | Isopulegol | 1.53 |
| 11 | 7.463 | Cubebol. | 0.75 |
| 12 | 7.643 | 7,2′,4′-Trimethoxyflavone | 0.64 |
| 13 | 8.041 | Gallic acid | 1.44 |
| 14 | 8.316 | Gentisic acid | 1.23 |
| 15 | 8.685 | α-Cedrol | 0.63 |
| 16 | 8.947 | Epicubebol | 2.25 |
| 17 | 9.157 | Caryophyllene epoxide | 1.17 |
| 18 | 9.509 | Sclareo | 1.46 |
| 19 | 9.636 | 3,6,3′,4′-Tetramethoxyflavone | 0.31 |
| 20 | 10.428 | Casticin | 1.01 |
| 21 | 11.174 | Patchoulane | 1.15 |
| 22 | 12.027 | Caryophyllene oxide | 5.21 |
| 23 | 12.294 | 3,7,8,2′-Tetramethoxyflavone | 2.16 |
| 24 | 13.254 | 2-Cyclohexen-1-one | 1.38 |
| 25 | 13.356 | 2,6-Dimethylphenol | 2.03 |
| 26 | 13.717 | 2′,4′-Dimethoxy-3-hydroxy-6-methyl flavone | 1.85 |
| 27 | 13.975 | Calderol | 1.49 |
| 28 | 14.5 | 3,6,2′,3′-Tetramethoxyflavone | 1.34 |
| 29 | 15.009 | Vanillic acid | 2.51 |
| 30 | 15.173 | Flavone, 3,5,7-trimethoxy- | 1.88 |
| 31 | 15.456 | Gardenin | 0.9 |
| 32 | 15.841 | Flavone, 4′,5,6,7-tetramethoxy | 3.2 |
| 33 | 16.522 | 5,7,2′-Trimethoxyflavone | 2.57 |
| 34 | 16.653 | Oleic Acid | 3.78 |
| 35 | 16.961 | Farnesol | 2.08 |
| 36 | 17.219 | Corymbolone | 1.84 |
| 37 | 17.724 | Digoxigenin | 4.04 |
| 38 | 18.282 | Isolongifolol | 1.93 |
| 39 | 18.54 | Decanoic acid | 2.19 |
| 40 | 19.274 | β Carotene | 0.75 |
| 41 | 21.571 | β-Ionone | 10.19 |
| 42 | 22.543 | β-Stigmasterol | 16.75 |
| 43 | 23.064 | Betulin | 5.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, L.; Beuter, L.; Karacic, S.; Bierbaum, G.; Karacic, J.; Bourauel, C. Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control. Gels 2024, 10, 600. https://doi.org/10.3390/gels10090600
Singer L, Beuter L, Karacic S, Bierbaum G, Karacic J, Bourauel C. Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control. Gels. 2024; 10(9):600. https://doi.org/10.3390/gels10090600
Chicago/Turabian StyleSinger, Lamia, Leonie Beuter, Sabina Karacic, Gabriele Bierbaum, Jesenko Karacic, and Christoph Bourauel. 2024. "Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control" Gels 10, no. 9: 600. https://doi.org/10.3390/gels10090600
APA StyleSinger, L., Beuter, L., Karacic, S., Bierbaum, G., Karacic, J., & Bourauel, C. (2024). Enhancing Dental Alginate with Syzygium aromaticum, Zingiber officinale and Green Silver Nanoparticles: A Nature-Enhanced Approach for Superior Infection Control. Gels, 10(9), 600. https://doi.org/10.3390/gels10090600







