Cellulose Fiber with Enhanced Mechanical Properties: The Role of Co-Solvents in Gel-like NMMO System
Abstract
1. Introduction
2. Results and Discussion
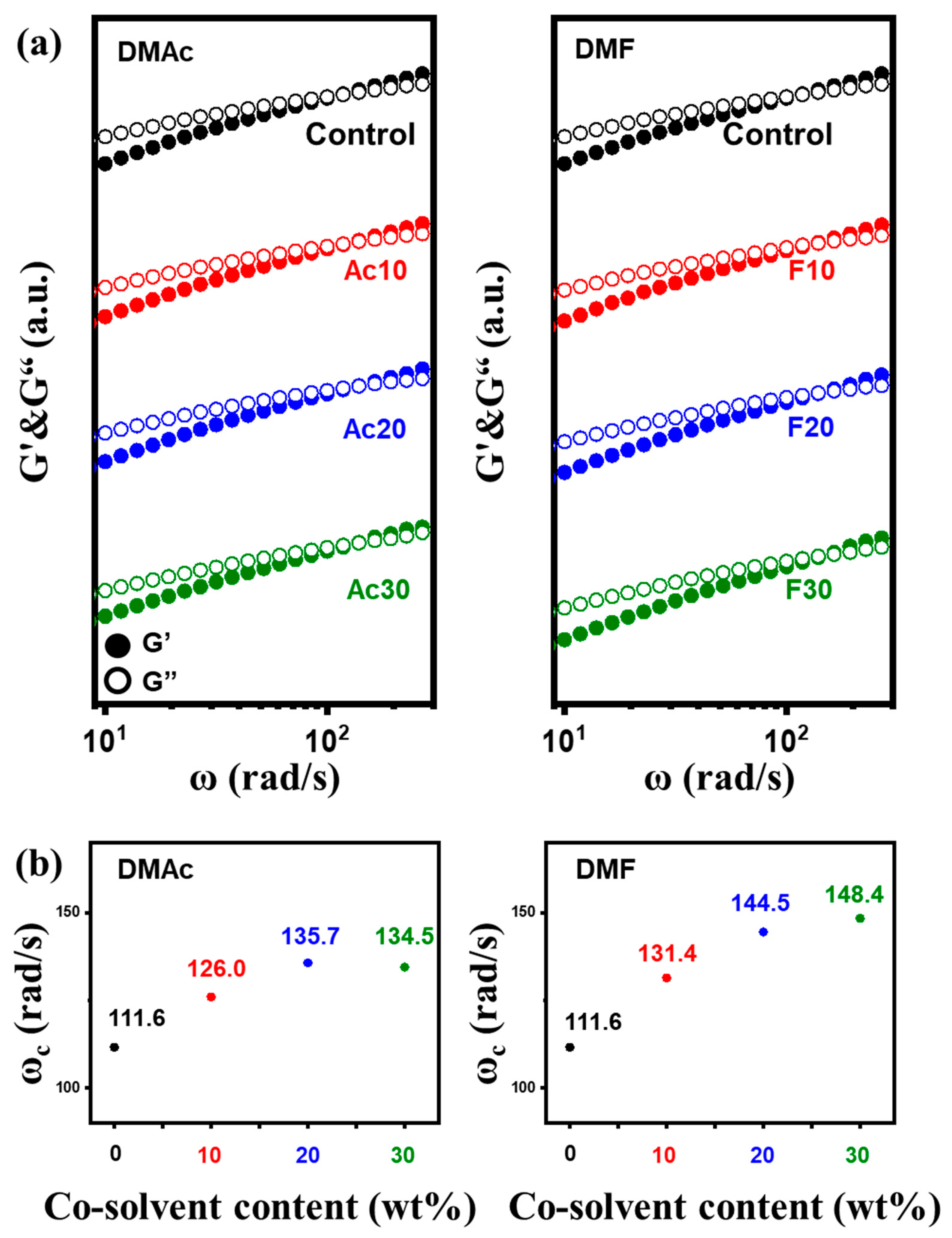
2.1. Rheological Behaviors
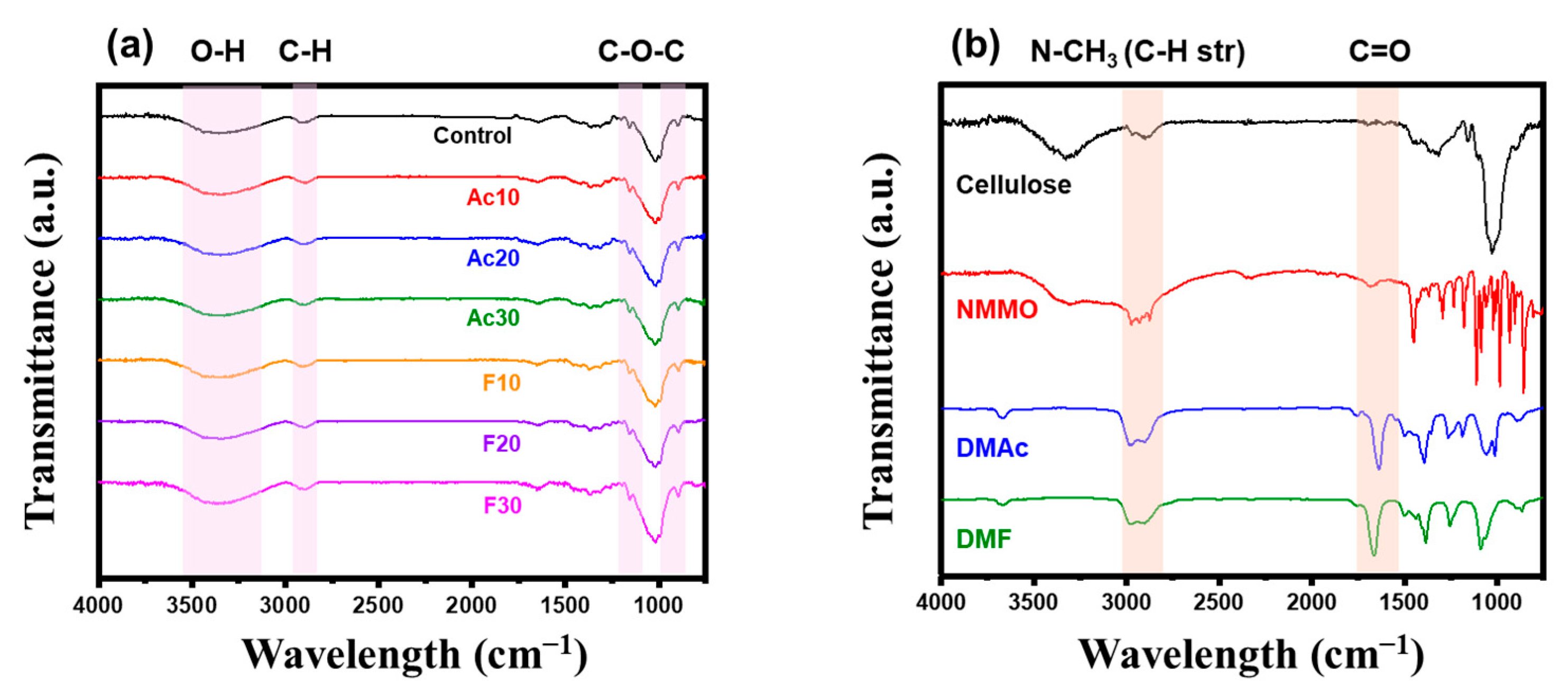
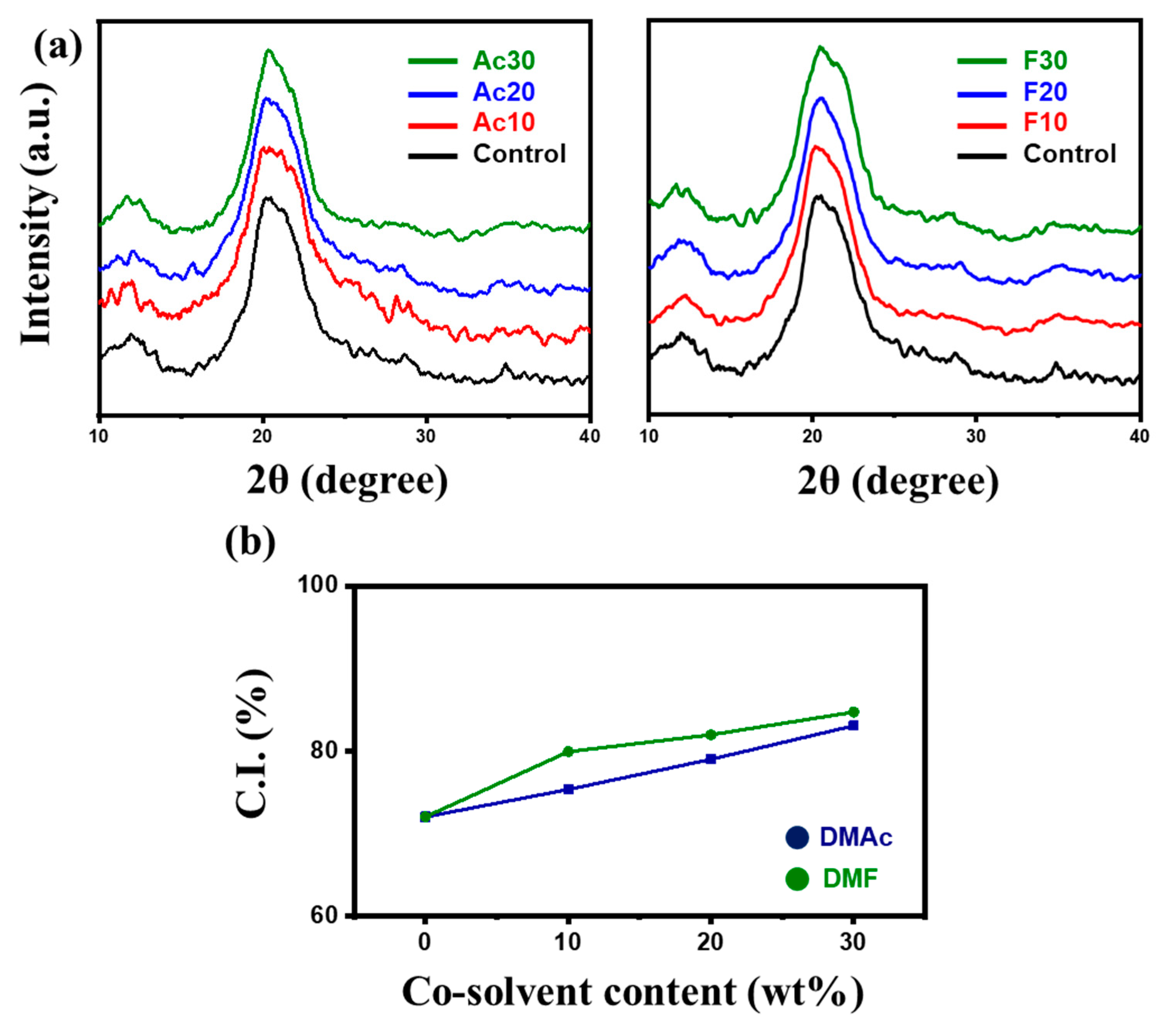
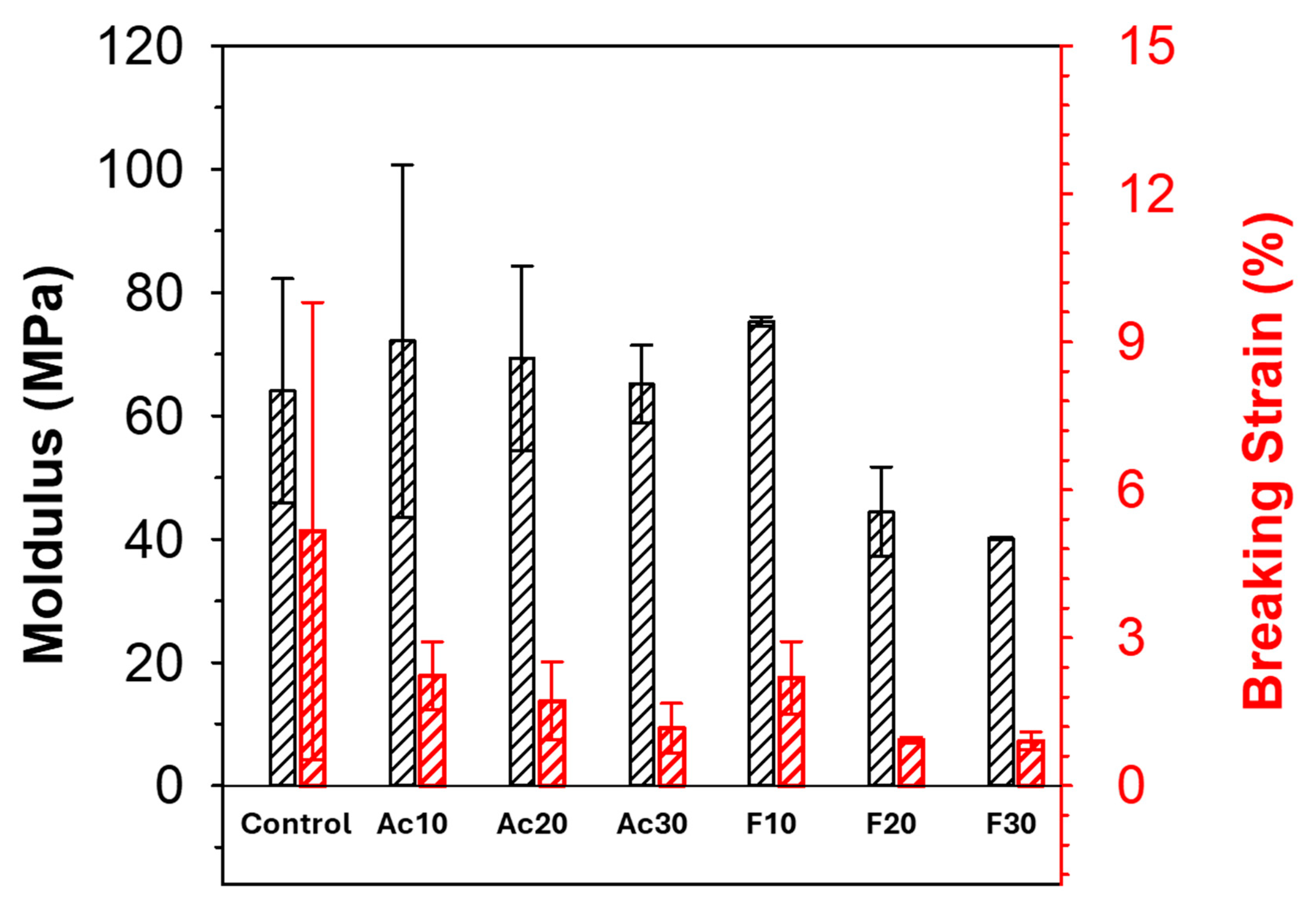
2.2. Characterization of Regenerated Fiber
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cellulose Solution and Regenerated Fiber Fabrication
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Periyasamy, A.P. Environmentally Friendly Approach to the Reduction of Microplastics during Domestic Washing: Prospects for Machine Vision in Microplastics Reduction. Toxics 2023, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.; Felix, F. Melting cellulose. Cellulose 2005, 12, 159–165. [Google Scholar] [CrossRef]
- Chen, J.; Guan, Y.; Wang, K.; Zhang, X.; Xu, F.; Sun, R. Combined effects of raw materials and solvent systems on the preparation and properties of regenerated cellulose fibers. Carbohydr. Polym. 2015, 128, 147–153. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Chung, K.-H.; Lee, W.-S. Structure of regenerated cellulose fibers from DMAc/LiCl solution. Text. Res. J. 1998, 68, 65–69. [Google Scholar] [CrossRef]
- Klar, V.; Orelma, H.; Rautkoski, H.; Kuosmanen, P.; Harlin, A. Spinning Approach for Cellulose Fiber Yarn Using a Deep Eutectic Solvent and an Inclined Channel. ACS Omega 2018, 3, 10918–10926. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Zhou, J.; Li, H.; Chen, H.; Jin, H. Novel fibers prepared from cellulose in NaOH/urea aqueous solution. Macromol. Rapid Commun. 2004, 25, 1558–1562. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.; Wang, Y.; Franz, J.; White, J.; Holladay, J. Interactions between cellulose and N-methylmorpholine-N-oxide. Carbohydr. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Braverman, L.; Romanov, V.; Lunina, O.; Belasheva, T.; Finger, G. Rheological properties of concentrated cellulose solutions in N-methylmorpholine-N-oxide. Fibre Chem. 1990, 22, 397–400. [Google Scholar] [CrossRef]
- Han, S.-Y.; Park, C.-W.; Febrianto, F.; Kim, N.-H.; Lee, S.-H. Pretreatment with [EMIM] Ac/DMAc co-solvent to improve enzymatic saccharification of pussy willow (Salix gracilistyla Miq.). BioResources 2020, 15, 187. [Google Scholar]
- Byrne, N.; Leblais, A.; Fox, B. Preparation of polyacrylonitrile–natural polymer composite precursors for carbon fiber using ionic liquid co solvent solutions. J. Mater. Chem. A 2014, 2, 3424–3429. [Google Scholar]
- Ahn, Y.; Hu, D.H.; Hong, J.H.; Lee, S.H.; Kim, H.J.; Kim, H. Effect of co-solvent on the spinnability and properties of electrospun cellulose nanofiber. Carbohydr. Polym. 2012, 89, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Magalhaes, S.; Pedrosa, J.F.S.; Ferreira, P.J.T.; Gamelas, J.A.F.; Rasteiro, M.G. Rheology of Suspensions of TEMPO-Oxidised and Cationic Cellulose Nanofibrils-The Effect of Chemical Pre-Treatment. Gels 2024, 10, 367. [Google Scholar] [CrossRef]
- Liao, J.; Pham, K.A.; Breedveld, V. Rheological characterization and modeling of cellulose nanocrystal and TEMPO-oxidized cellulose nanofibril suspensions. Cellulose 2020, 27, 3741–3757. [Google Scholar] [CrossRef]
- Kim, T.; Song, Y.; Ahn, J.; Kim, M.; Ko, E.; Kim, H. Rheological interpretation of intermediate physical state of gel and liquid crystalline phases in cellulose solution and their synergetic effects on the mechanical property. Cellulose 2021, 28, 10863–10874. [Google Scholar]
- Gilbert, R.-D.; Patton, P. Liquid crystal formation in cellulose and cellulose derivatives. Prog. Polym. Sci. 1983, 9, 115–131. [Google Scholar]
- Chaffey, C. Mechanisms and equations for shear thinning and thickening in dispersions. Colloid Polym. Sci. 1977, 255, 691–698. [Google Scholar] [CrossRef]
- Saiz, C.A.; Darvishmanesh, S.; Buekenhoudt, A.; Van der Bruggen, B. Shortcut applications of the Hansen solubility parameter for organic solvent nanofiltration. J. Membr. Sci. 2018, 546, 120–127. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Ramzan, M.; Mirza, M.A.; Khuroo, T. GastroPlus-and HSPiP-oriented predictive parameters as the basis of valproic acid-loaded mucoadhesive cationic nanoemulsion gel for improved nose-to-brain delivery to control convulsion in humans. Gels 2023, 9, 603. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F. The influence of polymer and ion solvation on the conformational properties of flexible polyelectrolytes. Gels 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liao, J.; Gulgunje, P.V.; Chang, H.; Arias-Monje, P.J.; Ramachandran, J.; Breedveld, V.; Kumar, S. Rheological behavior and fiber spinning of polyacrylonitrile (PAN)/Carbon nanotube (CNT) dispersions at high CNT loading. Polymer 2021, 215, 123369. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.; Zhang, Y.; Zhao, J. Study on the chain entanglement of polyvinyl alcohol fiber during the dry-jet wet spinning process. Fibers Polym. 2015, 16, 345–353. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Smith, B. Organic nitrogen compounds III: Secondary and tertiary amines. Spectroscopy 2019, 34, 22–26. [Google Scholar]
- Ren, Y.-K.; Liu, S.-D.; Duan, B.; Xu, Y.-F.; Li, Z.-Q.; Huang, Y.; Hu, L.-H.; Zhu, J.; Dai, S.-Y. Controllable intermediates by molecular self-assembly for optimizing the fabrication of large-grain perovskite films via one-step spin-coating. J. Alloys Compd. 2017, 705, 205–210. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Wang, S.; Lu, F.; Sun, R. Impact of regeneration process on the crystalline structure and enzymatic hydrolysis of cellulose obtained from ionic liquid. Carbohydr. Polym. 2014, 111, 400–403. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- He, T. Polymer strength and chain conformation. Die Makromol. Chem. Macromol. Chem. Phys. 1987, 188, 2489–2494. [Google Scholar] [CrossRef]



| δD | δP | δH | Ra | |
|---|---|---|---|---|
| Cellulose | 15.6 | 17.2 | 20.4 | - |
| NMMO | 8.6 | 8.6 | 15.4 | 18.9 |
| DMAc | 16.8 | 11.5 | 10.2 | 12.3 |
| DMF | 17.4 | 13.7 | 11.3 | 10.4 |
| Sample Codes | NMMO Concentration (wt%) | DMAc Concentration (wt%) | DMF Concentration (wt%) | Total Solvent (g) |
|---|---|---|---|---|
| Control | 100 | - | - | 20 |
| Ac10 | 90 | 10 | - | 20 |
| Ac20 | 80 | 20 | - | 20 |
| Ac30 | 70 | 30 | - | 20 |
| F10 | 90 | - | 10 | 20 |
| F20 | 80 | - | 20 | 20 |
| F30 | 70 | - | 30 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, D.; Kim, H. Cellulose Fiber with Enhanced Mechanical Properties: The Role of Co-Solvents in Gel-like NMMO System. Gels 2024, 10, 607. https://doi.org/10.3390/gels10090607
Kim S, Lee D, Kim H. Cellulose Fiber with Enhanced Mechanical Properties: The Role of Co-Solvents in Gel-like NMMO System. Gels. 2024; 10(9):607. https://doi.org/10.3390/gels10090607
Chicago/Turabian StyleKim, Suhnue, Darae Lee, and Hyungsup Kim. 2024. "Cellulose Fiber with Enhanced Mechanical Properties: The Role of Co-Solvents in Gel-like NMMO System" Gels 10, no. 9: 607. https://doi.org/10.3390/gels10090607
APA StyleKim, S., Lee, D., & Kim, H. (2024). Cellulose Fiber with Enhanced Mechanical Properties: The Role of Co-Solvents in Gel-like NMMO System. Gels, 10(9), 607. https://doi.org/10.3390/gels10090607





