Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application
Abstract
1. Introduction
2. Results and Discussion
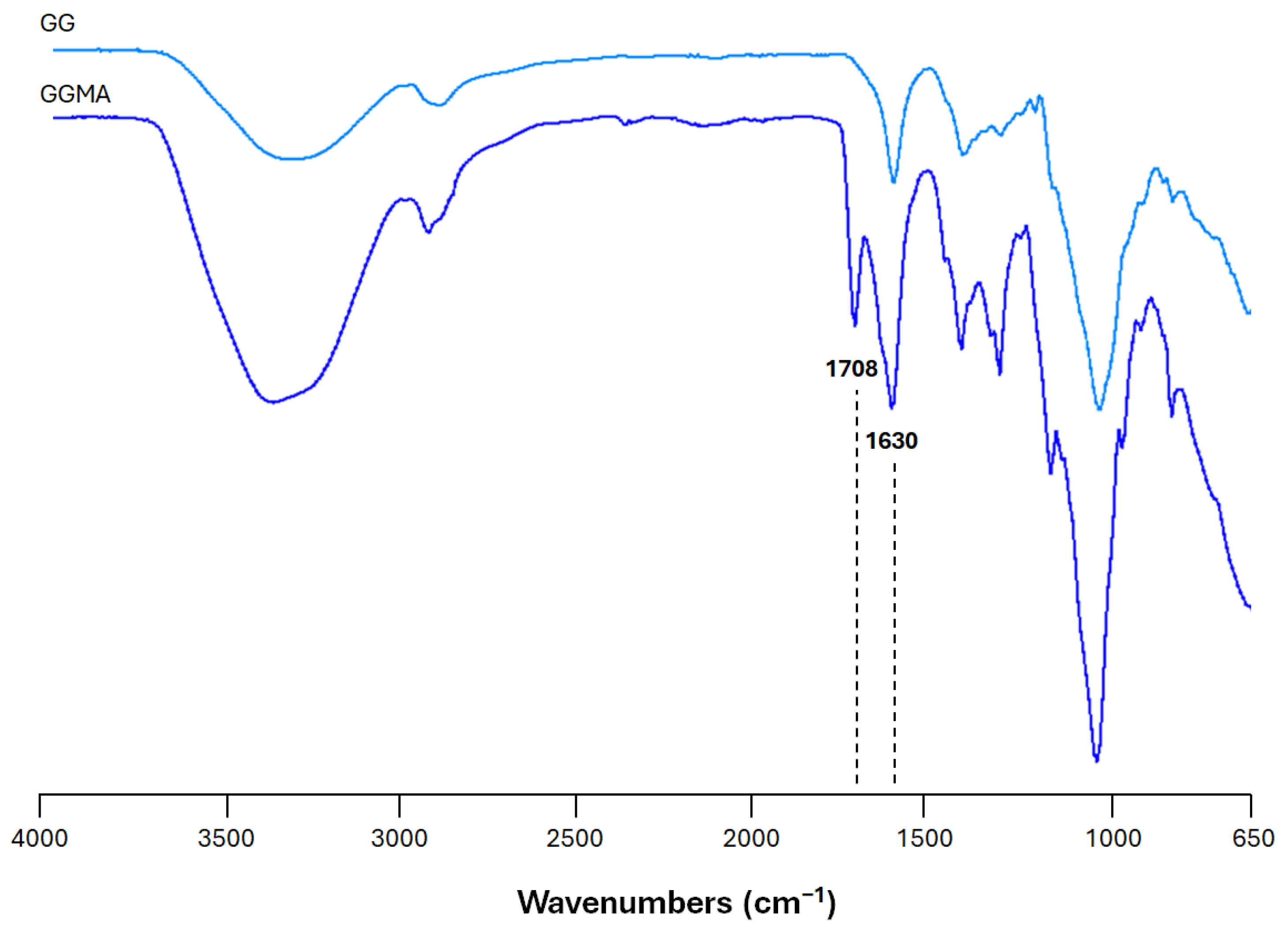
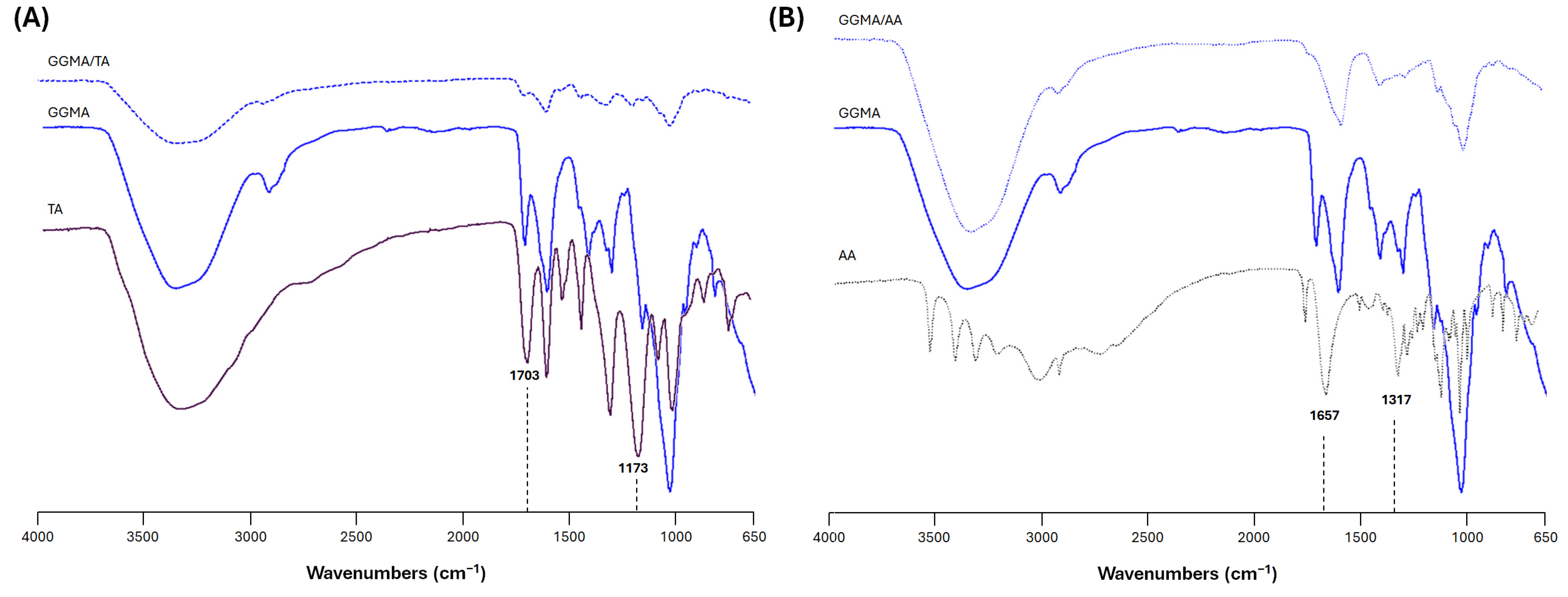
2.1. Physicochemical Characterization of the Bioink
2.2. Property of Hydrogel Patches
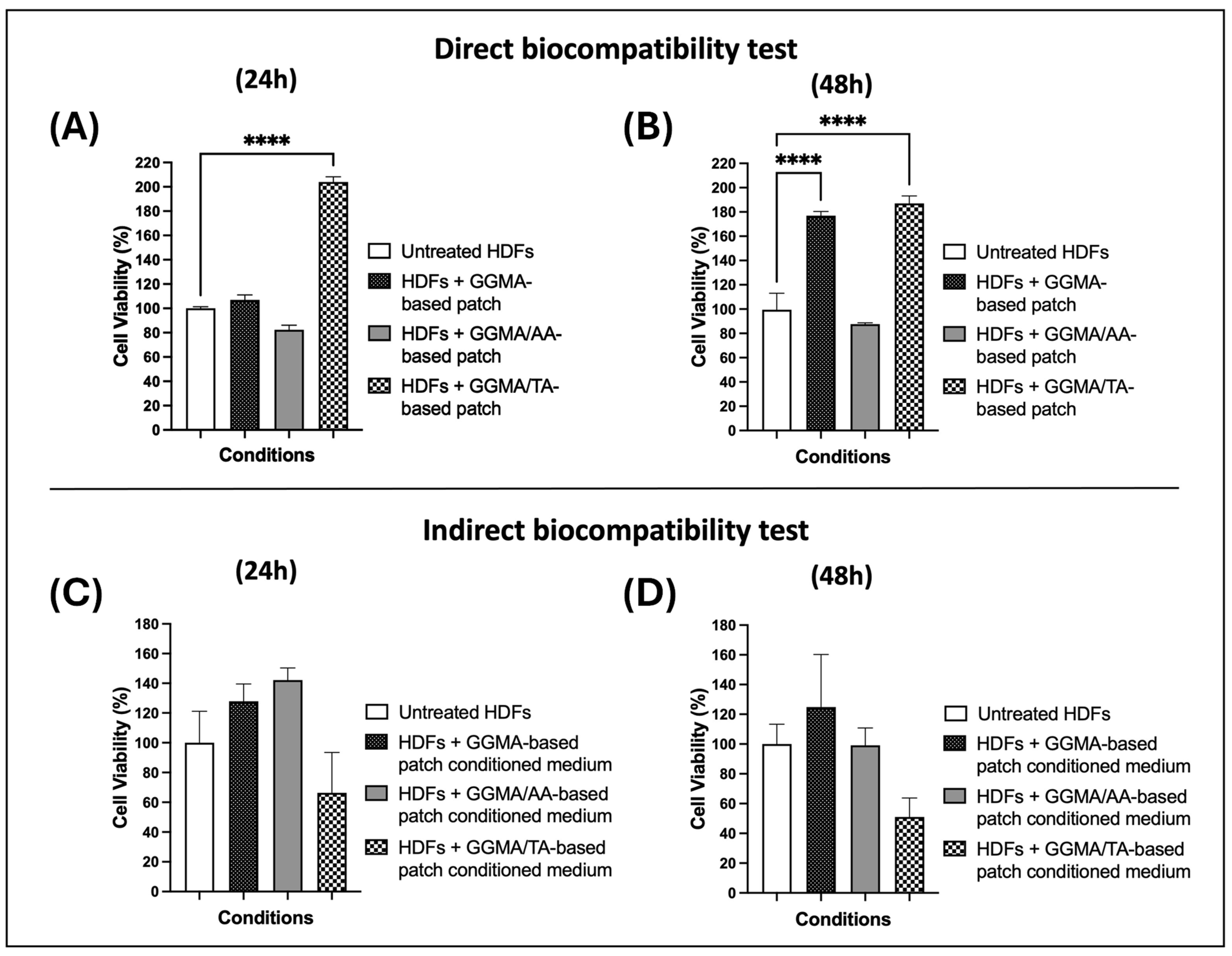
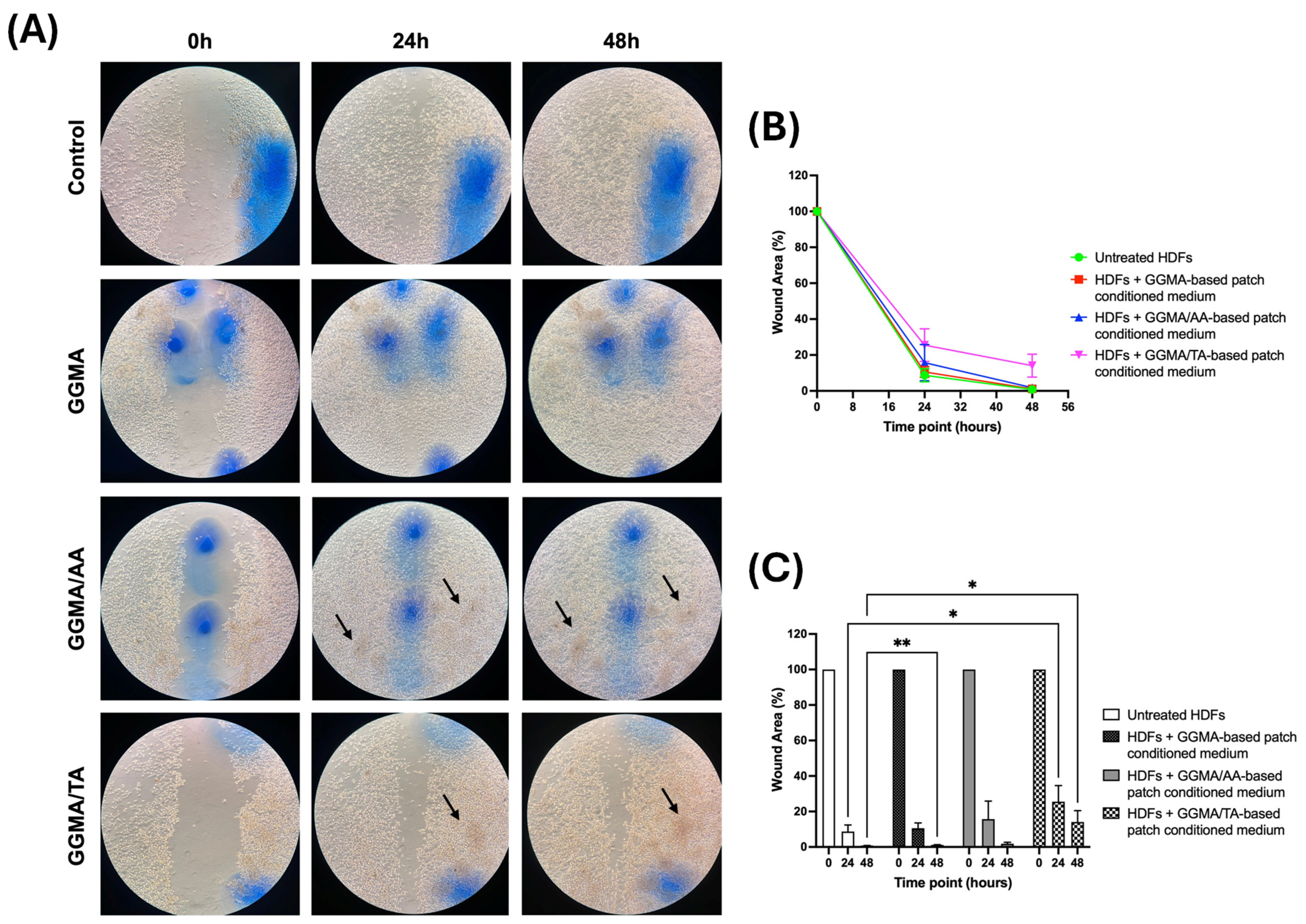
2.3. In Vitro Assays
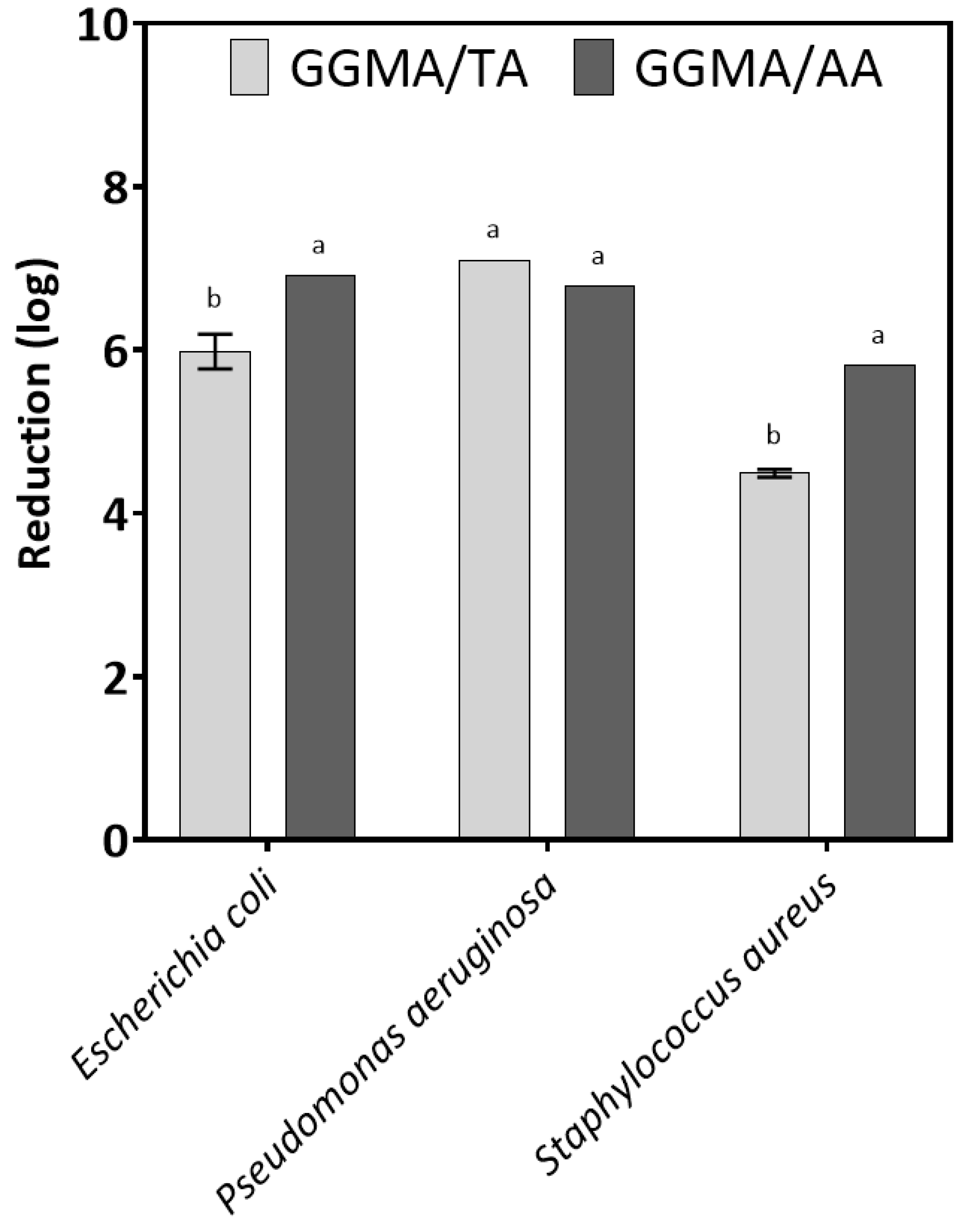
2.4. Antimicrobial Properties
3. Conclusions
4. Materials and Methods
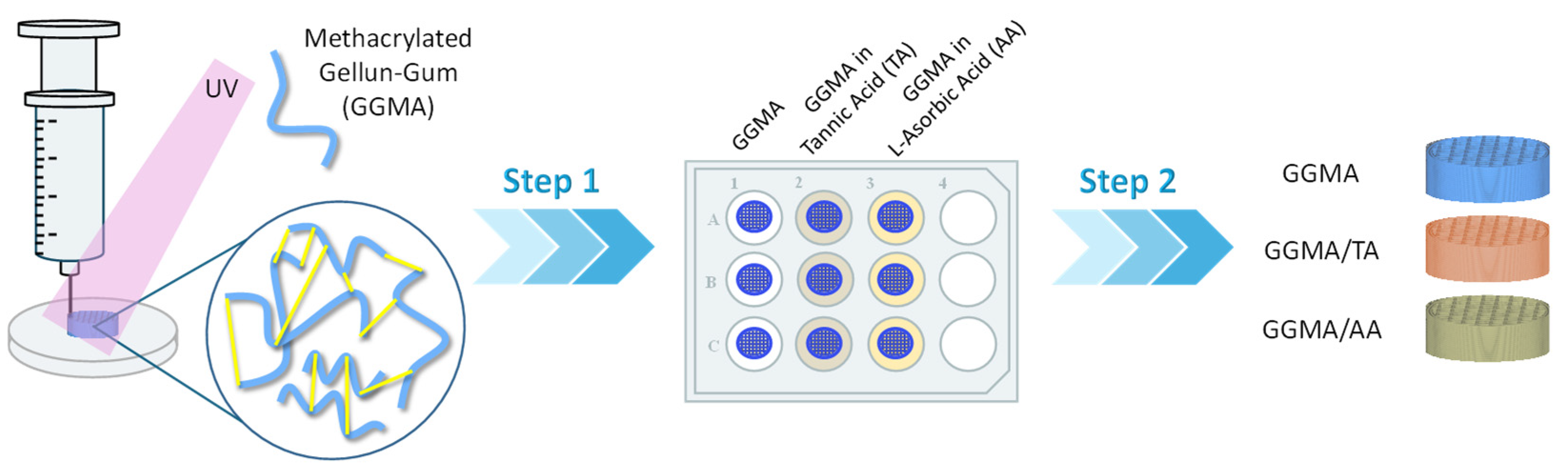
4.1. Synthesis and Characterization of Photocrosslinkable GGMA
4.2. Preparation of GGMA Patches
4.3. Characterization of GGMA Patches
4.3.1. Physicochemical Characterization
4.3.2. Morphological Analysis
4.3.3. Mechanical Analysis
4.3.4. Swelling Studies
4.3.5. Release Studies
4.3.6. Antioxidant Test
4.4. Biological Characterization
4.4.1. Cell Culture
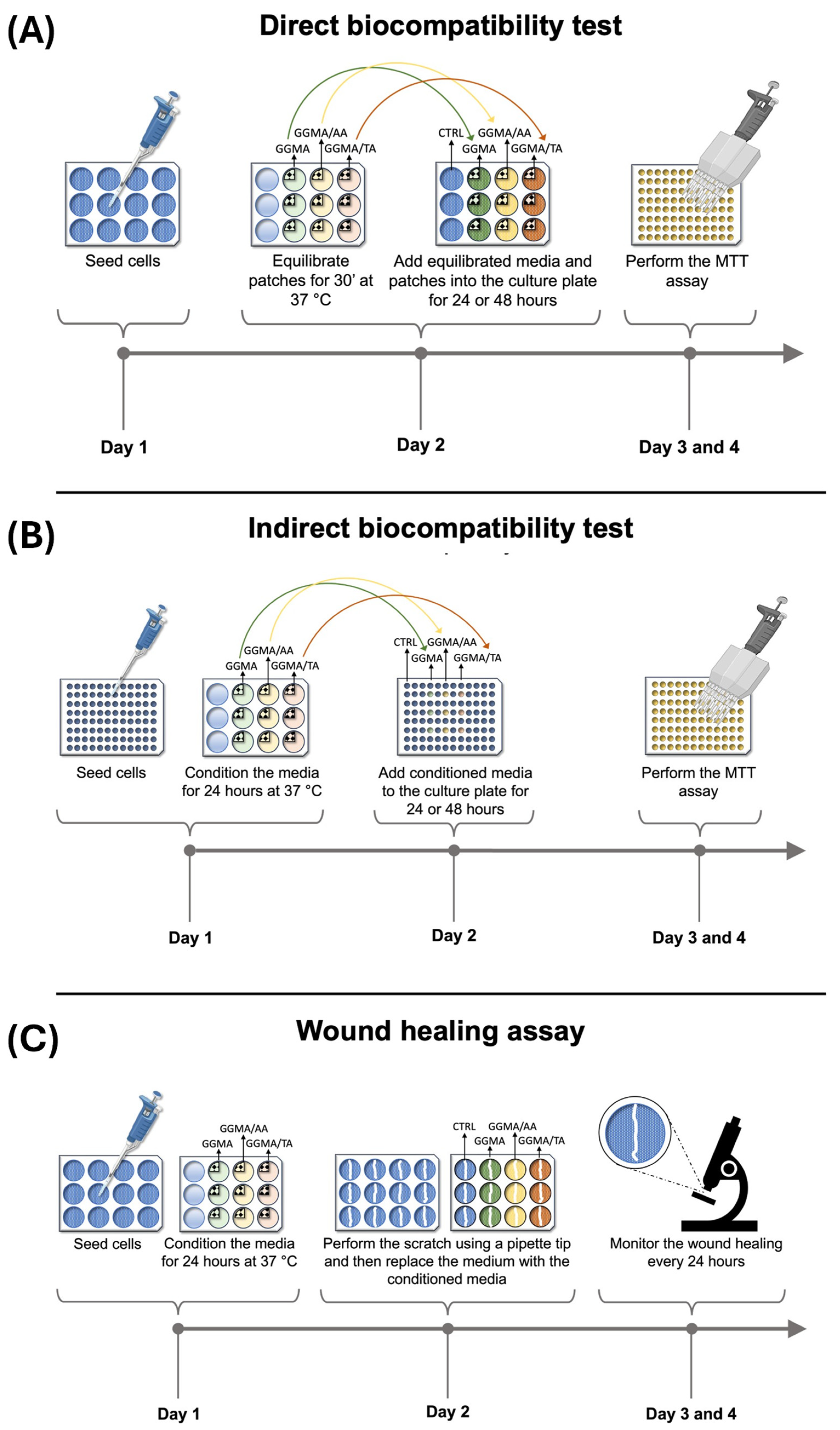
4.4.2. Biocompatibility Direct Test According to ISO 10993-5
4.4.3. Biocompatibility Indirect Test According to ISO 10993-5
4.4.4. Wound Healing Assay
4.5. Antimicrobial Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of Mesenchymal Stem Cells Delivered from Methacrylated Hyaluronic Acid Patch Improve the Regenerative Properties of Endothelial and Dermal Cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Álvarez-Echazú, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. CMC 2019, 25, 5782–5797. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, U.; Ronca, A.; Scialla, S.; Soriente, A.; Manini, P.; Phua, J.W.; Ottenheim, C.; Pezzella, A.; Calabrese, G.; Raucci, M.G.; et al. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials 2023, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.B.; Saudagar, P.S.; Singhal, R.S.; Pandey, A. Statistical Approach to Optimization of Fermentative Production of Gellan Gum from Sphingomonas Paucimobilis ATCC 31461. J. Biosci. Bioeng. 2006, 102, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, I.; Khalid, S.H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In Vitro and In Vivo Evaluation of Gellan Gum Hydrogel Films: Assessing the Co Impact of Therapeutic Oils and Ofloxacin on Wound Healing. Int. J. Biol. Macromol. 2021, 166, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Gellan Gum in Wound Dressing Scaffolds. Polymers 2022, 14, 4098. [Google Scholar] [CrossRef]
- Mohd, S.S.; Abdullah, M.A.A.; Mat Amin, K.A. Gellan Gum/Clay Hydrogels for Tissue Engineering Application: Mechanical, Thermal Behavior, Cell Viability, and Antibacterial Properties. J. Bioact. Compat. Polym. 2016, 31, 648–666. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-Loaded Hydrogels Facilitates Wound Healing Using Photodynamic Therapy by In Vitro and In Vivo Evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin Loaded Gellan Gum–Chitosan Hydrogels (GGCH-HGs) for Effective Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Reczyńska-Kolman, K.; Hartman, K.; Kwiecień, K.; Brzychczy-Włoch, M.; Pamuła, E. Composites Based on Gellan Gum, Alginate and Nisin-Enriched Lipid Nanoparticles for the Treatment of Infected Wounds. Int. J. Mol. Sci. 2021, 23, 321. [Google Scholar] [CrossRef]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine Gellan Gum–Collagen Interpenetrating Network Hydrogels as Mechanically Enhanced Anti-Inflammatory Biologic Wound Dressings for Burn Wound Therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef]
- Li, W.; Jian, X.; Zou, Y.; Wu, L.; Huang, H.; Li, H.; Hu, D.; Yu, B. Corrigendum: The Fabrication of a Gellan Gum-Based Hydrogel Loaded with Magnesium Ions for the Synergistic Promotion of Skin Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1335918. [Google Scholar] [CrossRef]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and Characterization of Scaffolds Containing Different Amounts of Allantoin for Skin Tissue Engineering. Sci. Rep. 2021, 11, 16164. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Thevi, T.; Abas, A.L.; Rajan, M. The Effects of Vitamin C on Wound Healing—Systematic Review. Indian J. Surg. 2024, 86, 23–29. [Google Scholar] [CrossRef]
- Moores, J. Vitamin C: A Wound Healing Perspective. Br. J. Community Nurs. 2013, 18, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A.; Yager, D.R.; Natarajan, R. Vitamin C Promotes Wound Healing through Novel Pleiotropic Mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Umachigi, S.P.; Jayaveera, K.N.; Ashok Kumar, C.K.; Kumar, G.S.; Vrushabendra Swamy, B.M.; Kishore Kumar, D.V. Studies on Wound Healing Properties of Quercus infectoria. Trop. J. Pharm. Res. 2008, 7, 913–919. [Google Scholar] [CrossRef]
- Su, X.; Liu, X.; Wang, S.; Li, B.; Pan, T.; Liu, D.; Wang, F.; Diao, Y.; Li, K. Wound-Healing Promoting Effect of Total Tannins from Entada phaseoloides (L.) Merr. in Rats. Burns 2017, 43, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and Properties of Tannic Acid Cross-linked Collagen Scaffold and Its Application in Wound Healing. J. Biomed. Mater. Res. 2013, 101B, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Wekwejt, M.; Małek, M.; Ronowska, A.; Michno, A.; Pałubicka, A.; Zasada, L.; Klimek, A.; Kaczmarek-Szczepańska, B. Hyaluronic Acid/Tannic Acid Films for Wound Healing Application. Int. J. Biol. Macromol. 2024, 254, 128101. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; D’Amora, U.; Gardin, C.; Leo, S.; Dalla Paola, L.; Tremoli, E.; Giuliani, A.; Calzà, L.; Ronca, A.; Ambrosio, L.; et al. Stem Cell-Derived Small Extracellular Vesicles Embedded into Methacrylated Hyaluronic Acid Wound Dressings Accelerate Wound Repair in a Pressure Model of Diabetic Ulcer. J. Nanobiotechnol. 2023, 21, 469. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, W.; Cui, Z.; Huang, J.; Cao, H.; Li, Y. 3D Printed Alginate/Gelatin-Based Porous Hydrogel Scaffolds to Improve Diabetic Wound Healing. Giant 2023, 16, 100185. [Google Scholar] [CrossRef]
- Wahyono, T.; Astuti, D.A.; Gede Wiryawan, I.K.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in The Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Sønderskov, S.M.; Xia, D.; Wu, X.; Liang, C.; Dong, M. Tannic Acid-Functionalized 3D Porous Nanofiber Sponge for Antibiotic-Free Wound Healing with Enhanced Hemostasis, Antibacterial, and Antioxidant Properties. J. Nanobiotechnol. 2023, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Zasada, L.; D’Amora, U.; Pałubicka, A.; Michno, A.; Ronowska, A.; Wekwejt, M. Bioactivation of Konjac Glucomannan Films by Tannic Acid and Gluconolactone Addition. ACS Appl. Mater. Interfaces 2024, 16, 46102–46112. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A Green Method for the Synthesis of Copper Nanoparticles Using L-Ascorbic Acid. Matéria (Rio J.) 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Wagner, B.A.; Buettner, G.R. Stability of Aqueous Solutions of Ascorbate for Basic Research and for Intravenous Administration. Adv. Redox Res. 2023, 9, 100077. [Google Scholar] [CrossRef] [PubMed]
- Brites, A.; Ferreira, M.; Bom, S.; Grenho, L.; Claudio, R.; Gomes, P.S.; Fernandes, M.H.; Marto, J.; Santos, C. Fabrication of Antibacterial and Biocompatible 3D Printed Manuka-Gelatin Based Patch for Wound Healing Applications. Int. J. Pharm. 2023, 632, 122541. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef]
- Kalra, A.; Lowe, A. An Overview of Factors Affecting the Skins Youngs Modulus. J. Aging Sci. 2016, 4, 1000156. [Google Scholar] [CrossRef]
- Guo, R.; Merkel, A.R.; Sterling, J.A.; Davidson, J.M.; Guelcher, S.A. Substrate Modulus of 3D-Printed Scaffolds Regulates the Regenerative Response in Subcutaneous Implants through the Macrophage Phenotype and Wnt Signaling. Biomaterials 2015, 73, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Dacrory, S.; D’Amora, U.; Longo, A.; Hasanin, M.S.; Soriente, A.; Fasolino, I.; Kamel, S.; Al-Shemy, M.T.; Ambrosio, L.; Scialla, S. Chitosan/Cellulose Nanocrystals/Graphene Oxide Scaffolds as a Potential pH-Responsive Wound Dressing: Tuning Physico-Chemical, pro-Regenerative and Antimicrobial Properties. Int. J. Biol. Macromol. 2024, 278, 134643. [Google Scholar] [CrossRef]
- Aliabadi, M.; Chee, B.S.; Matos, M.; Cortese, Y.J.; Nugent, M.J.D.; De Lima, T.A.M.; Magalhães, W.L.E.; De Lima, G.G.; Firouzabadi, M.D. Microfibrillated Cellulose Films Containing Chitosan and Tannic Acid for Wound Healing Applications. J. Mater. Sci. Mater. Med. 2021, 32, 67. [Google Scholar] [CrossRef] [PubMed]
- Mangır, N.; Bullock, A.J.; Roman, S.; Osman, N.; Chapple, C.; MacNeil, S. Production of Ascorbic Acid Releasing Biomaterials for Pelvic Floor Repair. Acta Biomater. 2016, 29, 188–197. [Google Scholar] [CrossRef]
- León, P.G.; Rojas, A.M. Gellan Gum Films as Carriers of L-(+)-Ascorbic Acid. Food Res. Int. 2007, 40, 565–575. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Przekora, A. Cellular Response to Vitamin C-Enriched Chitosan/Agarose Film with Potential Application as Artificial Skin Substitute for Chronic Wound Treatment. Cells 2020, 9, 1185. [Google Scholar] [CrossRef]
- Ravi, D.; Rajalekshmy, G.P.; Rekha, M.R.; Joseph, R. Ascorbic Acid-Loaded Gellan-g-Poly(Ethylene Glycol) Methacrylate Matrix as a Wound-Healing Material. Int. J. Biol. Macromol. 2023, 251, 126243. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yang, T.; Ke, R.; Wang, C. Preparation and Characterization of pH-Responsive Sodium Alginate/Humic Acid/Konjac Hydrogel for L-Ascorbic Acid Controlled Release. Mat. Express 2019, 9, 563–569. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Xiao, Y.-B.; Hsu, S.; Chang, S.-W.; Chou, C.-C. Molecular Interactions of Tannic Acid and Matrix Metalloproteinases 2 and 9. Comput. Struct. Biotechnol. J. 2023, 21, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Singh, A.K.; Saneja, A. Preparation, Characterization, and Antioxidant Activity of L-Ascorbic Acid/HP-β-Cyclodextrin Inclusion Complex-Incorporated Electrospun Nanofibers. Foods 2023, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.D.C.; Porredon, C.; Gibert, C. Insight into the Antioxidant Activity of Ascorbic Acid-Containing Gelatin Nanoparticles in Simulated Chronic Wound Conditions. Antioxidants 2024, 13, 299. [Google Scholar] [CrossRef]
- ANSI/AAMI/ISO 10993-5:2009/(R)2014; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. AAMI: Arlington, VA, USA, 2009; ISBN 978-1-57020-355-8.
- Kaczmarek, B.; Miłek, O.; Nadolna, K.; Owczarek, A.; Kleszczyński, K.; Osyczka, A.M. Normal and Cancer Cells Response on the Thin Films Based on Chitosan and Tannic Acid. Toxicol. Vitr. 2020, 62, 104688. [Google Scholar] [CrossRef]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An Initial Evaluation of Gellan Gum as a Material for Tissue Engineering Applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Chaitrakoonthong, T.; Ampornaramveth, R.; Kamolratanakul, P. Rinsing with L-Ascorbic Acid Exhibits Concentration-Dependent Effects on Human Gingival Fibroblast In Vitro Wound Healing Behavior. Int. J. Dent. 2020, 2020, 4706418. [Google Scholar] [CrossRef] [PubMed]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Muraro, R.; Di Giovanni, P. Microbial Species Isolated from Infected Wounds and Antimicrobial Resistance Analysis: Data Emerging from a Three-Years Retrospective Study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Omrani, Z.; Abbasi, R.; Mirshafiei, M.; Yazdian, F. Poly (Tannic Acid) Based Nanocomposite as a Promising Potential in Biomedical Applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105568. [Google Scholar] [CrossRef]
- Przekwas, J.; Wiktorczyk, N.; Budzyńska, A.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation. Microorganisms 2020, 8, 553. [Google Scholar] [CrossRef]
- Di Muzio, L.; Simonetti, P.; Carriero, V.C.; Brandelli, C.; Trilli, J.; Sergi, C.; Tirillò, J.; Cairone, F.; Cesa, S.; Radocchia, G.; et al. Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings. Molecules 2022, 27, 2959. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Di Muzio, L.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S.; et al. Design of a Tunable Nanocomposite Double Network Hydrogel Based on Gellan Gum for Drug Delivery Applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Hua, L.; Qian, H.; Lei, T.; Zhang, Y.; Lei, P.; Hu, Y. 3D-Printed Porous Tantalum Coated with Antitubercular Drugs Achieving Antibacterial Properties and Good Biocompatibility. Macromol. Biosci. 2022, 22, 2100338. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Staroń, A.; Staroń, P.; Chmielowiec-Korzeniowska, A.; Drabik, A.; Tymczyna, L.; Banach, M. Preparation and of PVA-Based Compositions with Embedded Silver, Copper and Zinc Oxide Nanoparticles and Assessment of Their Antibacterial Properties. J. Nanobiotechnol. 2020, 18, 148. [Google Scholar] [CrossRef]
- Mndlovu, H.; Du Toit, L.C.; Kumar, P.; Choonara, Y.E. Tannic Acid-Loaded Chitosan-RGD-Alginate Scaffolds for Wound Healing and Skin Regeneration. Biomed. Mater. 2023, 18, 045009. [Google Scholar] [CrossRef] [PubMed]
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Standards Organization: Geneva, Switzerland, 2011.
- JIS. Antibacterial Products–Test for Antibacterial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan, 2010. [Google Scholar]
- Souli, M.; Galani, I.; Plachouras, D.; Panagea, T.; Armaganidis, A.; Petrikkos, G.; Giamarellou, H. Antimicrobial Activity of Copper Surfaces against Carbapenemase-Producing Contemporary Gram-Negative Clinical Isolates. J. Antimicrob. Chemother. 2013, 68, 852–857. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, F.; Vitale, A.M.; Picone, D.; De Cesare, N.; Swiontek Brzezinska, M.; Kaczmarek-Szczepanska, B.; Ronca, A.; Zavan, B.; Bucchieri, F.; Szychlinska, M.A.; et al. Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application. Gels 2025, 11, 40. https://doi.org/10.3390/gels11010040
Scalia F, Vitale AM, Picone D, De Cesare N, Swiontek Brzezinska M, Kaczmarek-Szczepanska B, Ronca A, Zavan B, Bucchieri F, Szychlinska MA, et al. Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application. Gels. 2025; 11(1):40. https://doi.org/10.3390/gels11010040
Chicago/Turabian StyleScalia, Federica, Alessandra Maria Vitale, Domiziana Picone, Noemi De Cesare, Maria Swiontek Brzezinska, Beata Kaczmarek-Szczepanska, Alfredo Ronca, Barbara Zavan, Fabio Bucchieri, Marta Anna Szychlinska, and et al. 2025. "Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application" Gels 11, no. 1: 40. https://doi.org/10.3390/gels11010040
APA StyleScalia, F., Vitale, A. M., Picone, D., De Cesare, N., Swiontek Brzezinska, M., Kaczmarek-Szczepanska, B., Ronca, A., Zavan, B., Bucchieri, F., Szychlinska, M. A., & D’Amora, U. (2025). Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application. Gels, 11(1), 40. https://doi.org/10.3390/gels11010040














