Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications
Abstract
1. Introduction
2. PDA with Natural Polymer-Based Network Structure
2.1. Agarose
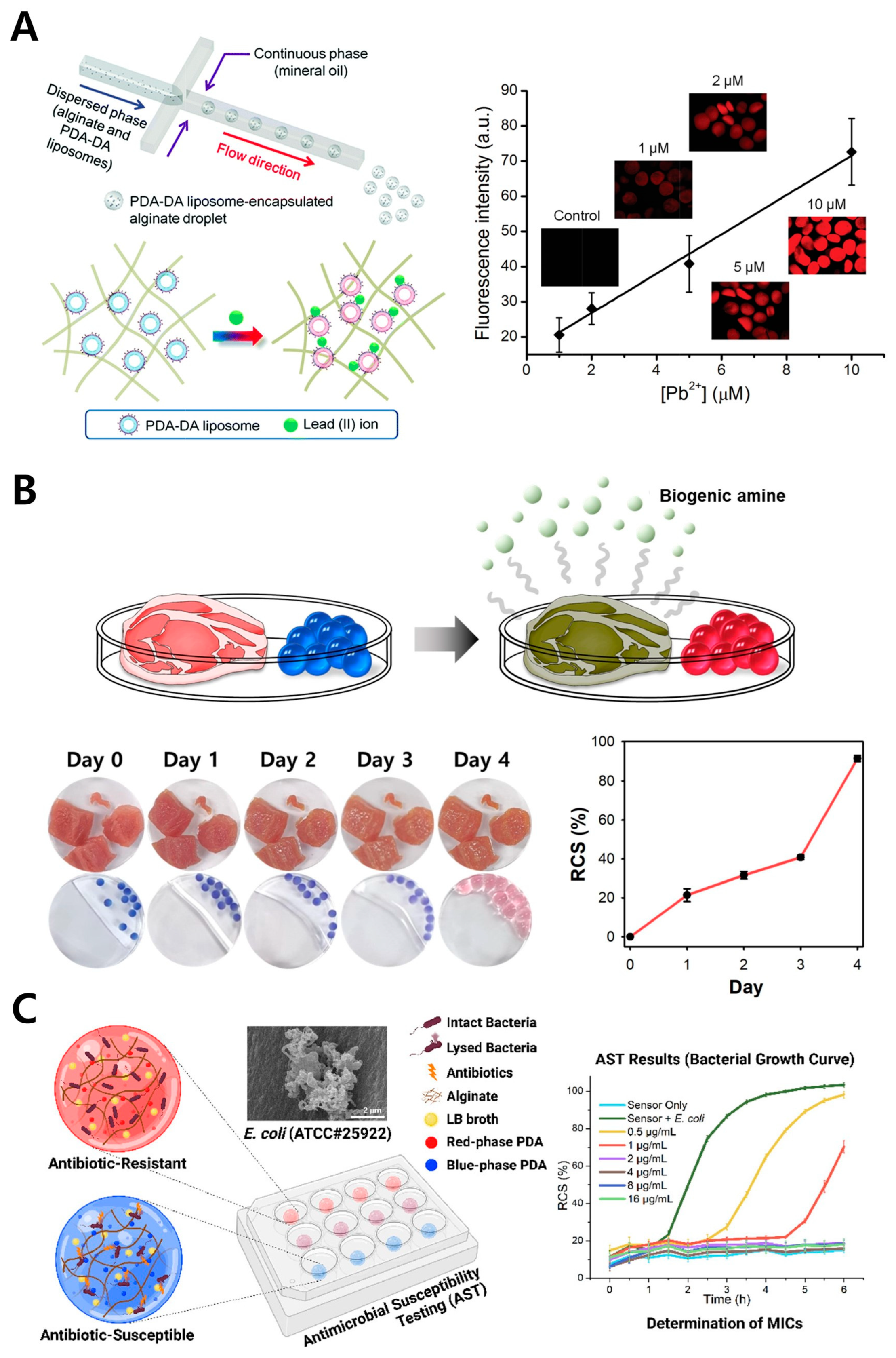
2.2. Alginate
2.3. Chitosan
3. PDA with Synthetic Polymer-Based Network Structure
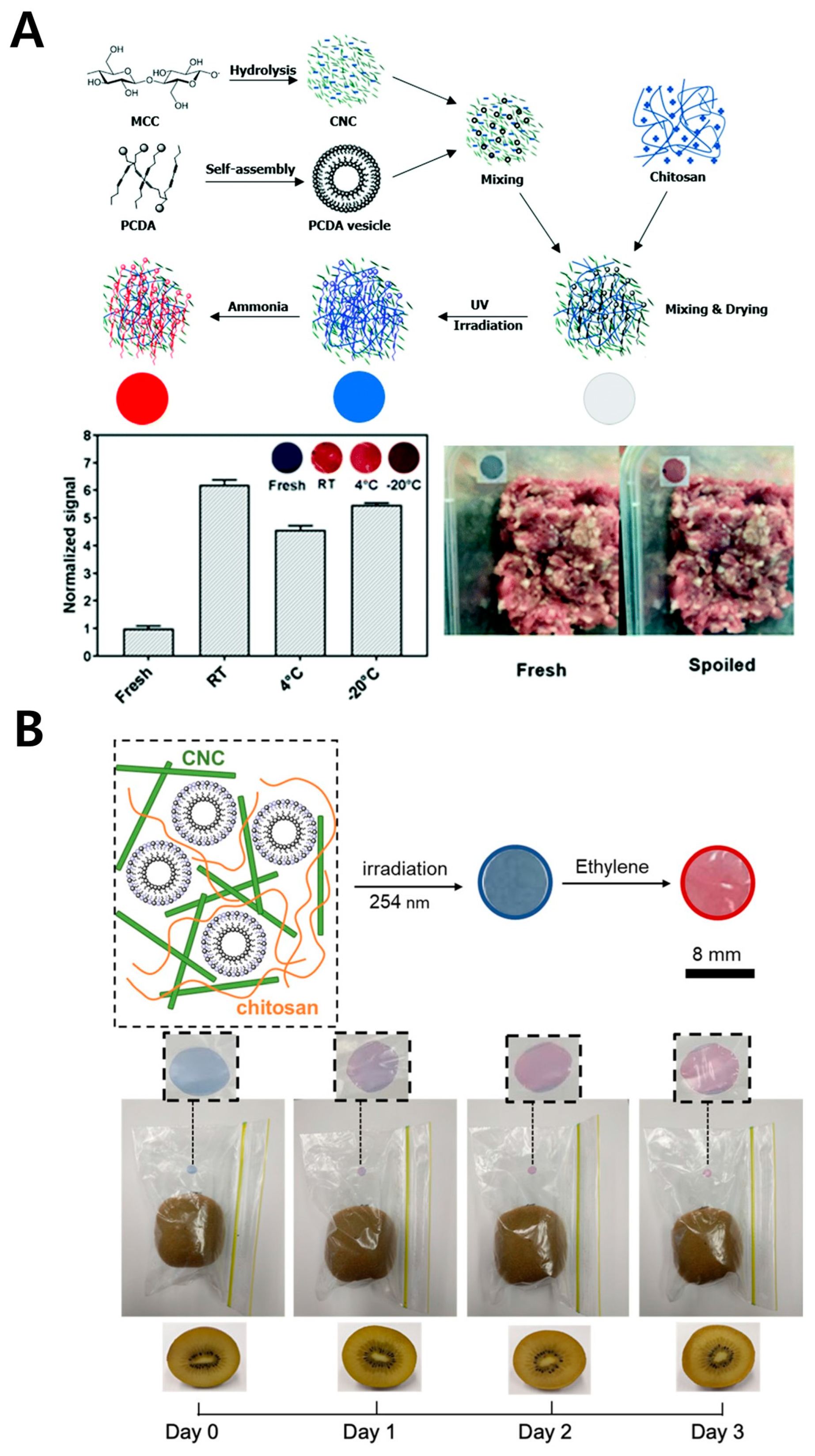
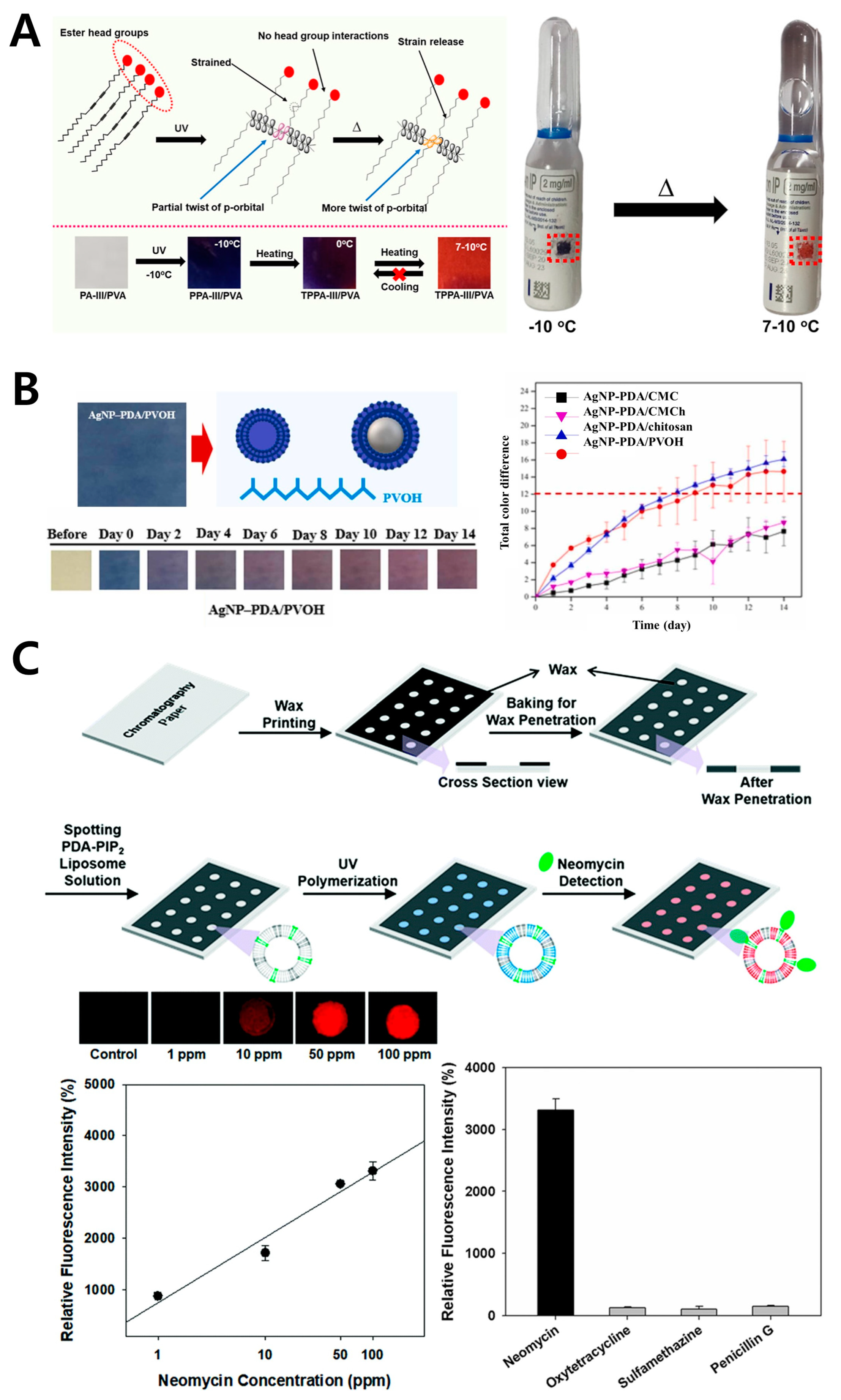
3.1. Poly(Vinyl Alcohol) (PVA)
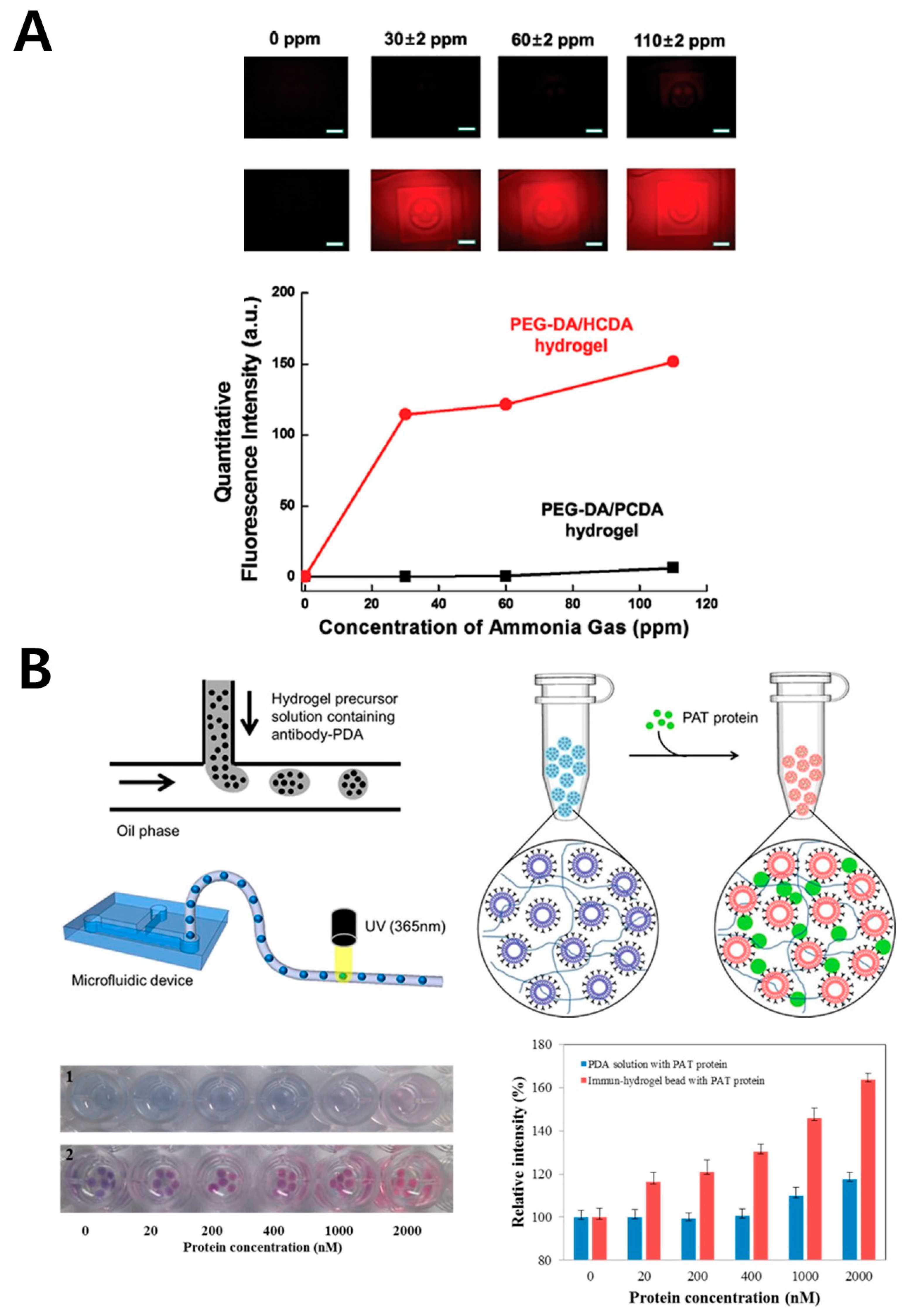
3.2. Poly(Ethylene Glycol) Diacrylate (PEG-DA)
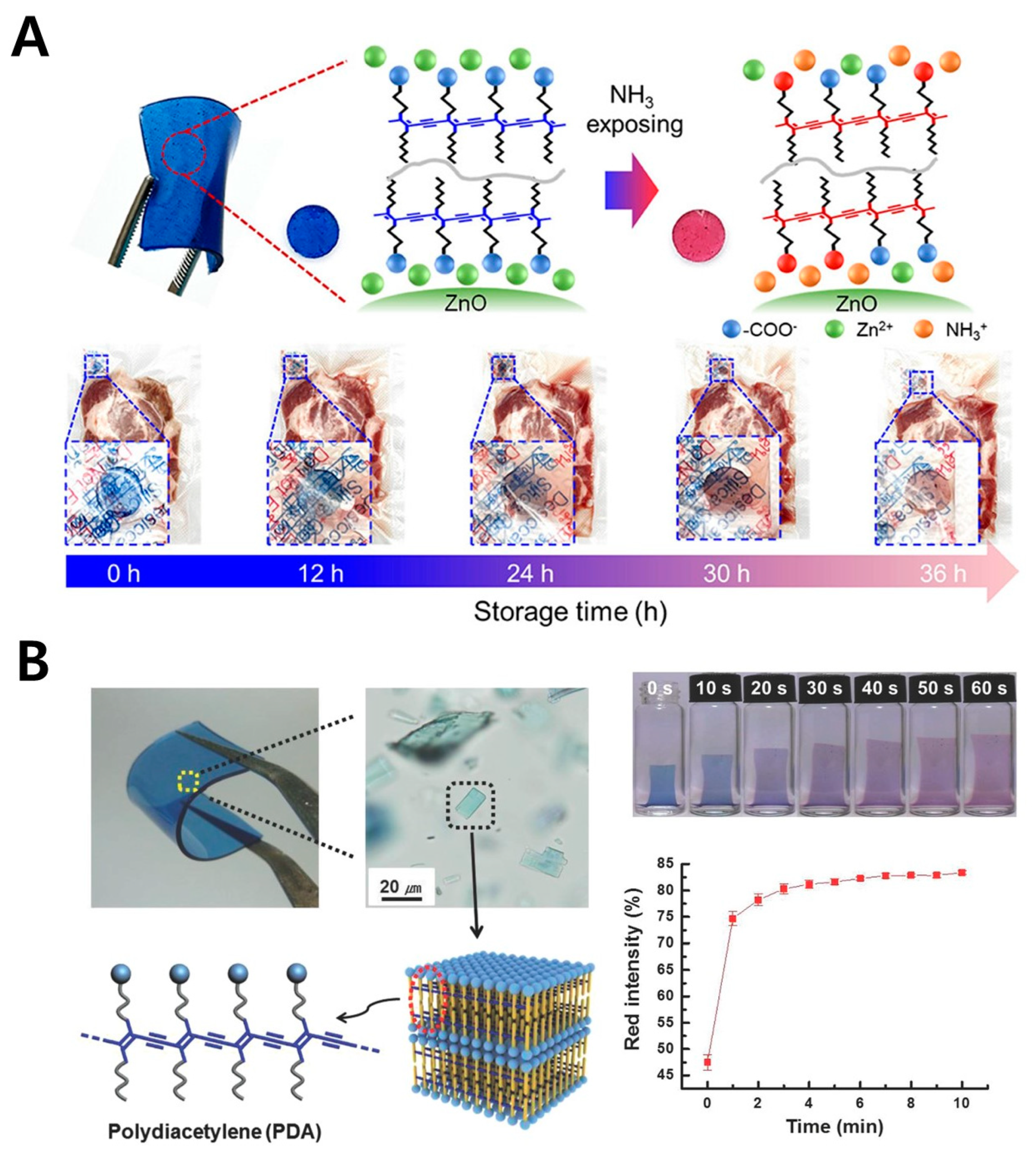
3.3. PDMS (Polydimethylsiloxane)
4. Other Polymeric Structure: Diacetylene (DA) Polymerized Gel
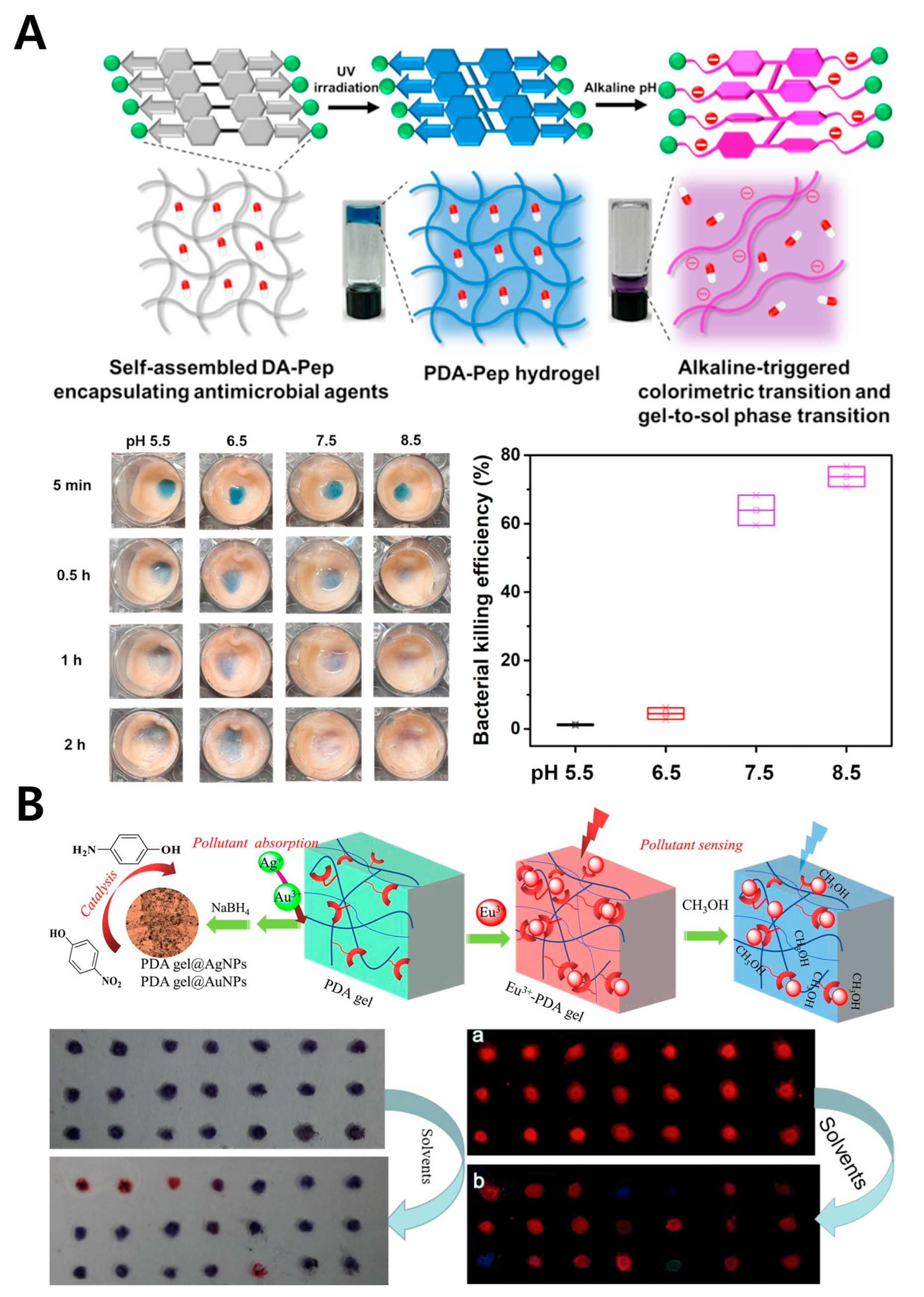
4.1. PDA-Incorporated Peptide Hydrogel
4.2. PDA-Gel
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent Progress and Growth in Biosensors Technology: A Critical Review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent Advances in Cortisol Sensing Technologies for Point-of-Care Application. Biosens Bioelectron 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-Free Optical Biosensors for Food and Biological Sensor Applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Weston, M.; Pham, A.H.; Tubman, J.; Gao, Y.; Tjandra, A.D.; Chandrawati, R. Polydiacetylene-Based Sensors for Food Applications. Mater. Adv. 2022, 3, 4088–4102. [Google Scholar] [CrossRef]
- Kim, Y.R.; Jung, S.; Ryu, H.; Yoo, Y.E.; Kim, S.M.; Jeon, T.J. Synthetic Biomimetic Membranes and Their Sensor Applications. Sensors 2012, 12, 9530–9550. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Peng, S.; Spevak, W.; Charych, D. Color and Chromism of Polydiacetylene Vesicles. Acc. Chem. Res. 1998, 31, 229–239. [Google Scholar] [CrossRef]
- Reppy, M.A.; Pindzola, B.A. Biosensing with Polydiacetylene Materials: Structures, Optical Properties and Applications. Chem. Commun. 2007, 42, 4317–4338. [Google Scholar] [CrossRef]
- Cho, E.; Jung, S. Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing. Molecules 2018, 23, 107. [Google Scholar] [CrossRef]
- Kim, C.; Hong, C.; Lee, K. Structures and Strategies for Enhanced Sensitivity of Polydiacetylene(PDA) Based Biosensor Platforms. Biosens. Bioelectron. 2021, 181, 113120. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Deb, R.; Suklabaidya, S.; Bhattacharjee, D.; Arshad Hussain, S. Polydiacetylene a Unique Material to Design Biosensors. Mater. Today Proc. 2022, 65, 2765–2772. [Google Scholar] [CrossRef]
- Qian, X.; Städler, B. Recent Developments in Polydiacetylene-Based Sensors. Chem. Mater. 2019, 31, 1196–1222. [Google Scholar] [CrossRef]
- Jung, Y.K.; Son, M.H. Polydiacetylene-Based Aptasensors for Rapid and Specific Colorimetric Detection of Malignant Exosomes. Talanta 2024, 268, 125342. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, F.; Wang, Y.; Zhang, W.; Chen, X. Polydiacetylene-Based Sensor for Highly Sensitive and Selective Pb2+ Detection. Dye. Pigment. 2015, 120, 307–313. [Google Scholar] [CrossRef]
- Suklabaidya, S.; Chakraborty, S.; Sarkar, S.; Paul, R.; Banik, H.; Chakraborty, A.; Bhattacharjee, D.; Majumdar, S.; Hussain, S.A. Polydiacetylene-N-1-Hexadecyl Imidazole Mixed Film and Its Application toward the Sensing of Volatile Organic Compounds, Gasoline, and Pollution Level in Car Exhaust. J. Phys. Chem. C 2021, 125, 15976–15986. [Google Scholar] [CrossRef]
- Jang, H.; Lee, P.; Kim, S.; Kim, S.M.; Jeon, T.J. An Antibacterial Microfluidic System with Fish Gill Structure for the Detection of Staphylococcus via Enzymatic Reaction on a Chromatic Polydiacetylene Material Caused by Lysostaphin. Microchim. Acta 2017, 184, 4563–4569. [Google Scholar] [CrossRef]
- Zhou, C.; You, T.; Jang, H.; Ryu, H.; Lee, E.S.; Oh, M.H.; Huh, Y.S.; Kim, S.M.; Jeon, T.J. Aptamer-Conjugated Polydiacetylene Colorimetric Paper Chip for the Detection of Bacillus Thuringiensis Spores. Sensors 2020, 20, 3124. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.V.; Musa, O.M.; Steed, J.W. Properties and Applications of Stimuli-Responsive Diacetylenes. Cryst. Growth Des. 2021, 21, 3614–3638. [Google Scholar] [CrossRef]
- Wen, J.T.; Roper, J.M.; Tsutsui, H. Polydiacetylene Supramolecules: Synthesis, Characterization, and Emerging Applications. Ind. Eng. Chem. Res. 2018, 57, 9037–9053. [Google Scholar] [CrossRef]
- Weston, M.; Tjandra, A.D.; Chandrawati, R. Tuning Chromatic Response, Sensitivity, and Specificity of Polydiacetylene-Based Sensors. Polym. Chem. 2020, 11, 166–183. [Google Scholar] [CrossRef]
- Huo, J.; Deng, Q.; Fan, T.; He, G.; Hu, X.; Hong, X.; Chen, H.; Luo, S.; Wang, Z.; Chen, D. Advances in Polydiacetylene Development for the Design of Side Chain Groups in Smart Material Applications-a Mini Review. Polym. Chem. 2017, 8, 7438–7445. [Google Scholar] [CrossRef]
- Pires, A.C.D.S.; Soares, N.d.F.F.; da Silva, L.H.M.; de Andrade, N.J.; Silveira, M.F.A.; de Carvalho, A.F. Polydiacetylene as a Biosensor: Fundamentals and Applications in the Food Industry. Food Bioproc. Tech. 2010, 3, 172–181. [Google Scholar] [CrossRef]
- Fang, F.; Meng, F.; Luo, L. Recent Advances on Polydiacetylene-Based Smart Materials for Biomedical Applications. Mater. Chem. Front. 2020, 4, 1089–1104. [Google Scholar] [CrossRef]
- Tjandra, A.D.; Pham, A.H.; Chandrawati, R. Polydiacetylene-Based Sensors To Detect Volatile Organic Compounds. Chem. Mater. 2022, 34, 2853–2876. [Google Scholar] [CrossRef]
- Lebègue, E.; Farre, C.; Jose, C.; Saulnier, J.; Lagarde, F.; Chevalier, Y.; Chaix, C.; Jaffrezic-Renault, N. Responsive Polydiacetylene Vesicles for Biosensing Microorganisms. Sensors 2018, 18, 599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xue, Y.; Lin, X.; Duan, M.; Hong, W.; Geng, L.; Zhou, J.; Fan, Y. Smartphone-Based Polydiacetylene Colorimetric Sensor for Point-of-Care Diagnosis of Bacterial Infections. Smart. Mater. Med. 2024, 5, 140–152. [Google Scholar] [CrossRef]
- Das, B.; Jo, S.; Zheng, J.; Chen, J.; Sugihara, K. Recent Progress in Polydiacetylene Mechanochromism. Nanoscale 2022, 14, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.L.; Vásquez, L.; Athanassiou, A.; Fragouli, D. Polymeric Hydrogels—A Promising Platform in Enhancing Water Security for a Sustainable Future. Adv. Mater. Interfaces 2021, 8, 2100580. [Google Scholar] [CrossRef]
- Ghassemi, Z.; Leach, J.B. Impact of Confinement within a Hydrogel Mesh on Protein Thermodynamic Stability and Aggregation Kinetics. Mol. Pharm. 2024, 21, 1137–1148. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent Advances in Polymers and Polymer Composites for Food Packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Thakuri, A.; Acharya, R.; Banerjee, M.; Chatterjee, A. Alendronate-Modified Polydiacetylene (PDA) Dual-Mode Sensor for the Selective Detection of Lead (II) Ions up to the NM Level in Solutions and Agarose Films. ACS Appl. Polym. Mater. 2024, 6, 1023–1032. [Google Scholar] [CrossRef]
- Lee, J.; Seo, S.; Kim, J. Colorimetric Detection of Warfare Gases by Polydiacetylenes toward Equipment-Free Detection. Adv. Funct. Mater. 2012, 22, 1632–1638. [Google Scholar] [CrossRef]
- Weston, M.; Kuchel, R.P.; Chandrawati, R. Digital Analysis of Polydiacetylene Quality Tags for Contactless Monitoring of Milk. Anal. Chim. Acta 2021, 1148, 238190. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Kuchel, R.P.; Chandrawati, R. A Polydiacetylene-Based Colorimetric Sensor as an Active Use-By Date for Plant-Based Milk Alternatives. Macromol. Rapid Commun. 2020, 41, 2000172. [Google Scholar] [CrossRef] [PubMed]
- Meir, D.; Silbert, L.; Volinsky, R.; Kolusheva, S.; Weiser, I.; Jelinek, R. Colorimetric/Fluorescent Bacterial Sensing by Agarose-Embedded Lipid / Polydiacetylene Films. J. Appl. Microbiol. 2008, 104, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Thakuri, A.; Acharya, R.; Banerjee, M.; Chatterjee, A. A Polydiacetylene (PDA) Based Dual-Mode Optical Sensor for the ppb Level Selective Detection of Biogenic Polyamines. Sens. Actuators B Chem. 2024, 409, 135573. [Google Scholar] [CrossRef]
- Ahmadi, N.; Kim, D.Y.; Shin, S.S.; Daradmare, S.; Kim, J.M.; Park, B.J. Gas-Shearing Microfluidic Fabrication of Polydiacetylene–Alginate Colorimetric Sensor Beads. Small Struct 2024, 6, 202400340. [Google Scholar] [CrossRef]
- Kang, D.H.; Jung, H.S.; Ahn, N.; Yang, S.M.; Seo, S.; Suh, K.Y.; Chang, P.S.; Jeon, N.L.; Kim, J.; Kim, K. Janus-Compartmental Alginate Microbeads Having Polydiacetylene Liposomes and Magnetic Nanoparticles for Visual Lead (II) Detection. ACS Appl. Mater. Interfaces 2014, 6, 10631–10637. [Google Scholar] [CrossRef]
- Wang, D.E.; Wang, Y.; Tian, C.; Zhang, L.; Han, X.; Tu, Q.; Yuan, M.; Chen, S.; Wang, J. Polydiacetylene Liposome-Encapsulated Alginate Hydrogel Beads for Pb2+ Detection with Enhanced Sensitivity. J. Mater. Chem. A Mater. 2015, 3, 21690–21695. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kim, J.; Kim, H.; Lim, J.; Seo, S.B.; Kang, B.; Kang, T.; Jung, J.; Seo, S.; et al. Polydiacetylene-Based Hydrogel Beads as Colorimetric Sensors for the Detection of Biogenic Amines in Spoiled Meat. Food Chem. 2023, 403, 134317. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jang, H.; Lee, D.; Jeong, W.; Bae, E.H.; Kim, H.; Choi, Y.S.; Shin, M.; Kim, S.M.; Jeon, T.J. Portable Colorimetric Hydrogel Beads for Point-of-Care Antimicrobial Susceptibility Testing. ACS Sens. 2023, 8, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jang, H.; Jeong, W.; Shim, J.; Kim, S.M.; Jeon, T.J. Thermohypersensitive Polydiacetylene Vesicles Embedded in Calcium-Alginate Hydrogel Beads. Korean J. Chem. Eng. 2023, 40, 398–404. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Schwarte, J.V.; Kareru, P.G.; Gachanja, A.N.; Fromm, K.M. Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets. Polymers 2023, 15, 1062. [Google Scholar] [CrossRef]
- Champaiboon, T.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. A Polydiacetylene Multilayer Film for Naked Eye Detection of Aromatic Compounds. Sens. Actuators B Chem. 2009, 139, 532–537. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; McConchie, R.; Dehghani, F.; Chandrawati, R. Polydiacetylene-Based Sensors to Detect Food Spoilage at Low Temperatures. J. Mater. Chem. C Mater. 2019, 7, 1919–1926. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Oveissi, F.; Chandrawati, R.; Dehghani, F.; Naficy, S. Naked-Eye Detection of Ethylene Using Thiol-Functionalized Polydiacetylene-Based Flexible Sensors. ACS Sens. 2020, 5, 1921–1928. [Google Scholar] [CrossRef]
- Park, S.; Lee, G.S.; Cui, C.; Ahn, D.J. Simple Detection of Food Spoilage Using Polydiacetylene/Poly (Vinyl Alcohol) Hybrid Films. Macromol. Res. 2016, 24, 380–384. [Google Scholar] [CrossRef]
- Goyal, S.; Sharma, D.; Deep, A.; Kumar, K.; Sharma, A.L. Polydiacetylene/Poly (Vinyl Alcohol) Composite Films as a Low-Temperature Irreversible Indicator for Drug Storage Applications. ACS Appl. Polym. Mater. 2024, 6, 4186–4194. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, K.; Son, Y.; Chang, P.S.; Kim, J.; Jung, H.S. Design of a Simple Paper-Based Colorimetric Biosensor Using Polydiacetylene Liposomes for Neomycin Detection. Analyst 2018, 143, 4623–4629. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, D.J. Fabrication of Sensory Structure Based on Poly (Ethylene Glycol)-Diacrylate Hydrogel Embedding Polydiacetylene. Korean J. Chem. Eng. 2017, 34, 2092–2095. [Google Scholar] [CrossRef]
- Jung, S.H.; Jang, H.; Lim, M.C.; Kim, J.H.; Shin, K.S.; Kim, S.M.; Kim, H.Y.; Kim, Y.R.; Jeon, T.J. Chromatic Biosensor for Detection of Phosphinothricin Acetyltransferase by Use of Polydiacetylene Vesicles Encapsulated within Automatically Generated Immunohydrogel Beads. Anal. Chem. 2015, 87, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Siribunbandal, P.; Osotchan, T.; Kim, Y.H.; Jaisutti, R. Highly Sensitive Colorimetric Ammonia Sensors Based on Polydiacetylene/Zinc Oxide Nanopellet-Embedded PDMS Films for Meat Spoilage Detection. ACS Appl. Polym. Mater. 2023, 5, 7786–7794. [Google Scholar] [CrossRef]
- Park, D.H.; Hong, J.; Park, I.S.; Lee, C.W.; Kim, J.M. A Colorimetric Hydrocarbon Sensor Employing a Swelling-Induced Mechanochromic Polydiacetylene. Adv. Funct. Mater. 2014, 24, 5186–5193. [Google Scholar] [CrossRef]
- Chen, W.; Hazoor, S.; Madigan, R.; Adones, A.A.; Chintapula, U.K.; Nguyen, K.T.; Tang, L.; Foss, F.W.; Dong, H. Alkaline-Responsive Polydiacetylene-Peptide Hydrogel for pH-Sensing and on-Demand Antimicrobial Release. Mater. Today Adv. 2022, 16, 100288. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Geng, L.; Feng, G.; Ren, J.; Yu, X. Polydiacetylene-Based Gels for Solvent Discrimination and Formation of Au/Ag Nanoparticles with Embedded Photocatalytic Performance. Mater. Des. 2021, 205, 109744. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xu, X.W.; Chen, F.Q.; Weng, H.F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar. Drugs 2023, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Roux, D.C.D.; Jeacomine, I.; Maîtrejean, G.; Caton, F.; Rinaudo, M. Characterization of Agarose Gels in Solvent and Non-Solvent Media. Polymers 2023, 15, 2162. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of Alginate Extraction Processes: Impact on Alginate Molecular Structure and Techno-Functional Properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Iacovino, S.; Cofelice, M.; Sorrentino, E.; Cuomo, F.; Messia, M.C.; Lopez, F. Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit. Gels 2024, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Ristić, I.; Nikolić, L.; Cakić, S.; Nikolić, V.; Tanasić, J.; Zvezdanović, J.; Krstić, M. Eco-Friendly Microwave Synthesis of Sodium Alginate-Chitosan Hydrogels for Effective Curcumin Delivery and Controlled Release. Gels 2024, 10, 637. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.; Cristiano, M.C.; Fresta, M.; Paolino, D.; Rafaniello, C. Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits. Gels 2022, 8, 431. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Oliveira, D.A.; McLamore, E.S.; Gomes, C.L. Rapid and Label-Free Listeria Monocytogenes Detection Based on Stimuli-Responsive Alginate-Platinum Thiomer Nanobrushes. Sci. Rep. 2022, 12, 21413. [Google Scholar] [CrossRef] [PubMed]
- Fatoni, A.; Wijonarko, A.; Anggraeni, M.D.; Hermawan, D.; Diastuti, H.; Zusfahair. Alginate NiFe2O4 Nanoparticles Cryogel for Electrochemical Glucose Biosensor Development. Gels 2021, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Potisatityuenyong, A.; Tumcharern, G.; Dubas, S.T.; Sukwattanasinitt, M. Layer-by-Layer Assembly of Intact Polydiacetylene Vesicles with Retained Chromic Properties. J. Colloid. Interface Sci. 2006, 304, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction Methods and Biomedical Applications of PVA-Based Hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Feng, W. PVA Hydrogel Properties for Biomedical Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Malka, E.; Margel, S. Engineering of PVA/PVP Hydrogels for Agricultural Applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liao, H.; Ye, J.; Wan, P.; Zhang, L. Polyvinyl Alcohol-Stabilized Liquid Metal Hydrogel for Wearable Transient Epidermal Sensors. ACS Appl. Mater. Interfaces 2019, 11, 47358–47364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Chae, S.K.; Lee, Y.B.; Lee, J.S.; Lee, G.S.; Kim, T.Y.; Ahn, D.J. Polydiacetylene Supramolecules Embedded in PVA Film for Strip-Type Chemosensors. Chem. Lett. 2006, 35, 560–561. [Google Scholar] [CrossRef]
- Saenjaiban, A.; Thanakkasaranee, S.; Jantanasakulwong, K.; Punyodom, W.; Lee, Y.S.; Singjai, P.; Reungsang, A.; Tanadchangsaeng, N.; Worajittiphon, P.; Panyathip, R.; et al. Influence of Biodegradable Polymer Films on Conformation of Polydiacetylene–Silver Nanocomposites as Colorimetric Time–Temperature Indicators. Sens. Actuators A Phys. 2024, 376, 115658. [Google Scholar] [CrossRef]
- Yuan, Y.; Tyson, C.; Szyniec, A.; Agro, S.; Tavakol, T.N.; Harmon, A.; Lampkins, D.R.; Pearson, L.; Dumas, J.E.; Taite, L.J. Bioactive Polyurethane–Poly (Ethylene Glycol) Diacrylate Hydrogels for Applications in Tissue Engineering. Gels 2024, 10, 108. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Gonella, S.; Domingues, M.F.; Miguel, F.; Moura, C.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Silva, J.C. Fabrication and Characterization of Porous PEGDA Hydrogels for Articular Cartilage Regeneration. Gels 2024, 10, 422. [Google Scholar] [CrossRef]
- De Masi, A.; Scognamiglio, P.L.; Battista, E.; Netti, P.A.; Causa, F. PEG-Based Cleavable Hydrogel Microparticles with Controlled Porosity for Permiselective Trafficking of Biomolecular Complexes in Biosensing Applications. J. Mater. Chem. B 2022, 10, 1980–1990. [Google Scholar] [CrossRef]
- Lee, N.Y.; Jung, Y.K.; Park, H.G. On-Chip Colorimetric Biosensor Based on Polydiacetylene (PDA) Embedded in Photopolymerized Poly (Ethylene Glycol) Diacrylate (PEG-DA) Hydrogel. Biochem. Eng. J. 2006, 29, 103–108. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Dou, M.; Dominguez, D.C.; Li, X.; Sanchez, J.; Scott, G. A Versatile PDMS/Paper Hybrid Microfluidic Platform for Sensitive Infectious Disease Diagnosis. Anal. Chem. 2014, 86, 7978–7986. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Qiao, Y.; Feng, H.; Li, J.; Fu, J.; Liang, F.; Lu, Z. PDMS-Based Microfluidic Devices Using Commoditized PCBs as Masters with No Specialized Equipment Required. RSC Adv. 2017, 7, 31603–31609. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Wang, Q.; Geng, H.; Cui, T.; Hu, Y.; Luo, Q.; Li, A.; Li, W.; Lin, Y.; et al. Inchworm-Inspired Soft Robot with Magnetic Driving Based on PDMS, EGaIn and NdFeB (PEN) Combination. Chem. Eng. J. 2023, 466, 142994. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. PDMS Lab-on-a-Chip Fabrication Using 3D Printed Templates. Lab. Chip. 2014, 14, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable Polydimethylsiloxane (PDMS) for Rapid Lab-on-a-Chip Prototyping. Lab. Chip. 2007, 7, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Park, D.H.; Kim, J.M. Development of Polydiacetylene-Polydimethylsiloxane Based Chloroform Sensor. Polymers 2016, 40, 769–773. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Di Domenico, E.G.; Sivori, F.; Truglio, M.; Del Giudice, A.; Fratoddi, I.; Chronopoulou, L.; Palocci, C. Biosynthesis of Peptide Hydrogel–Titania Nanoparticle Composites with Antibacterial Properties. Gels 2023, 9, 940. [Google Scholar] [CrossRef]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Fratoddi, I.; Chronopoulou, L.; Palocci, C. Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery. Gels 2023, 9, 953. [Google Scholar] [CrossRef]

| Polymer Type | Specific Polymer | Target Type | Specific Target | Limit of Detection | Practical Application | Application Area | Ref. |
|---|---|---|---|---|---|---|---|
| Natural polymer | Agarose | Heavy metal | Pb2+ | 3.2 ng/mL | Environmental water source | Environment | [31] |
| Warfare gas | DFP | 160 mg/m3 | N/A | Safety | [32] | ||
| Spoilage marker | FFA | N/A | Milk | Food | [33,34] | ||
| Contamination marker | Pathogenic bacteria | 102 cells | Blood, urine | Healthcare | [35] | ||
| Alginate | Disease marker | BPAs | 1.4 ng/mL | Serum, urine | Healthcare | [36] | |
| Organic solvent | IPA | 32 mg/mL | N/A | Environment | [37] | ||
| Heavy metal | Pb2+ | 200 ng/mL | N/A | Environment | [38,39] | ||
| Spoilage marker | BAs | 100 ng/mL | Pork meat | Food | [40] | ||
| Infection marker | Pathogenic bacteria | 10 CFU/mL | Blood | Healthcare | [41] | ||
| Freshness indicator | Temperature | N/A | N/A | Food | [42] | ||
| Chitosan | Infection marker | pH | N/A | N/A | Healthcare | [43] | |
| Waste marker | Aromatic compounds | 20 mM | N/A | Environment | [44] | ||
| Spoilage marker | Ammonia gas | 300 μg/mL | Beef product | Food | [45] | ||
| Food quality indicator | Ethylene | 600 μg/mL | Kiwi | Food | [46] | ||
| Synthetic polymer | PVA | Spoilage marker | Ammonia gas | 1000 μg/mL | Beef hairtail | Food | [47] |
| Storage stability indicator | Temperature | N/A | Vaccine vial | Healthcare | [48] | ||
| Antibiotics | Neomycin | 1 μg/mL | N/A | Healthcare | [49] | ||
| PEG-DA | Spoilage marker | Ammonia gas | N/A | N/A | Environment | [50] | |
| GMO marker | PAT protein | 20 nM | N/A | Food | [51] | ||
| PDMS | Spoilage marker | Ammonia gas | 160 μg/mL | Pork, chicken | Food | [52] | |
| Hydrocarbon | Kerosene | N/A | Diesel oil | Industrial | [53] | ||
| Others | Peptide gel | Infection marker | pH | 10 μM | Pig skin | Healthcare | [54] |
| PDA gel | Waste marker | Organic solvent | 12.5 mM | N/A | Environment | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Jeon, J.; Shin, M.; Kang, G.; Ryu, H.; Kim, S.M.; Jeon, T.-J. Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications. Gels 2025, 11, 66. https://doi.org/10.3390/gels11010066
Jang H, Jeon J, Shin M, Kang G, Ryu H, Kim SM, Jeon T-J. Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications. Gels. 2025; 11(1):66. https://doi.org/10.3390/gels11010066
Chicago/Turabian StyleJang, Huisoo, Junhyeon Jeon, Mingyeong Shin, Geonha Kang, Hyunil Ryu, Sun Min Kim, and Tae-Joon Jeon. 2025. "Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications" Gels 11, no. 1: 66. https://doi.org/10.3390/gels11010066
APA StyleJang, H., Jeon, J., Shin, M., Kang, G., Ryu, H., Kim, S. M., & Jeon, T.-J. (2025). Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications. Gels, 11(1), 66. https://doi.org/10.3390/gels11010066










