Structural Characterization of Ordered Mesoporous Silica Prepared by a Sol–Gel Process Using Urea-Based Cationic Gemini Surfactants
Abstract
1. Introduction
2. Results and Discussion
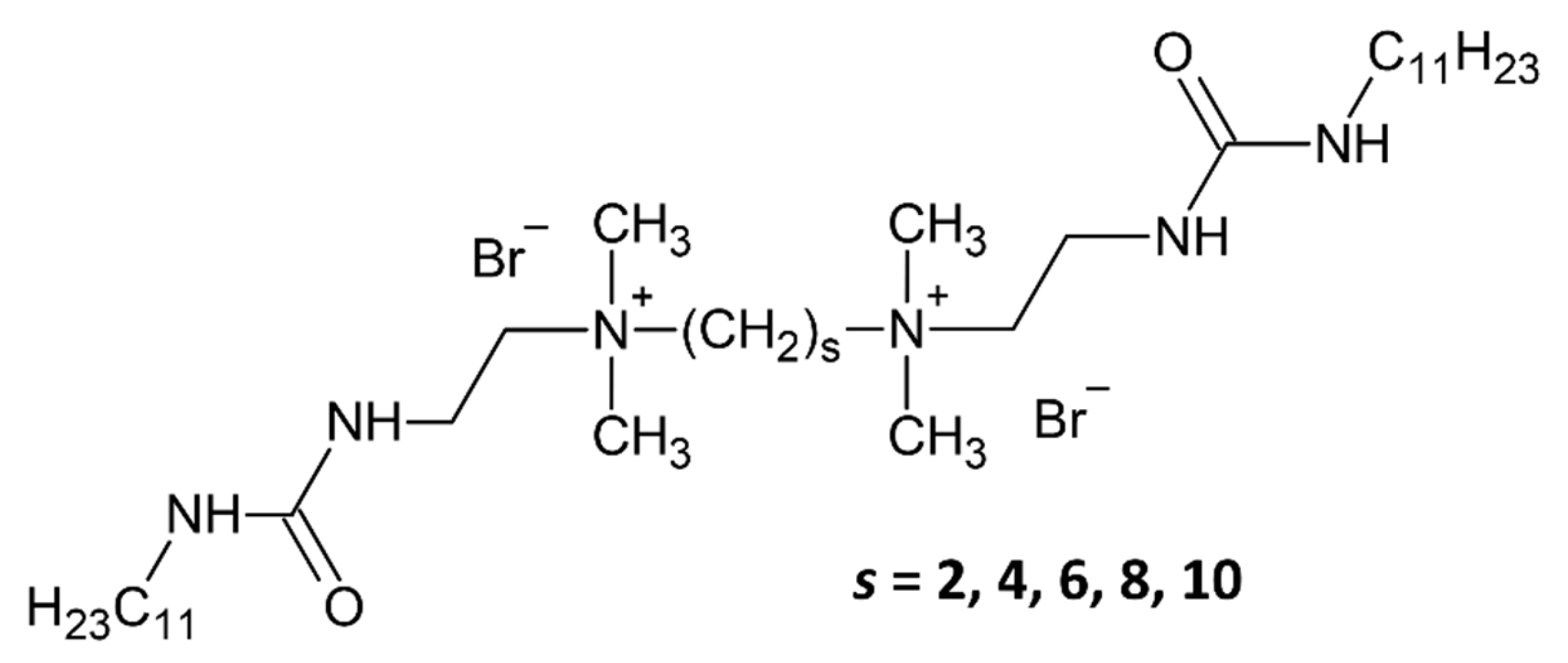
2.1. Samples
2.2. Infrared Spectroscopy
2.3. Microscale Structure
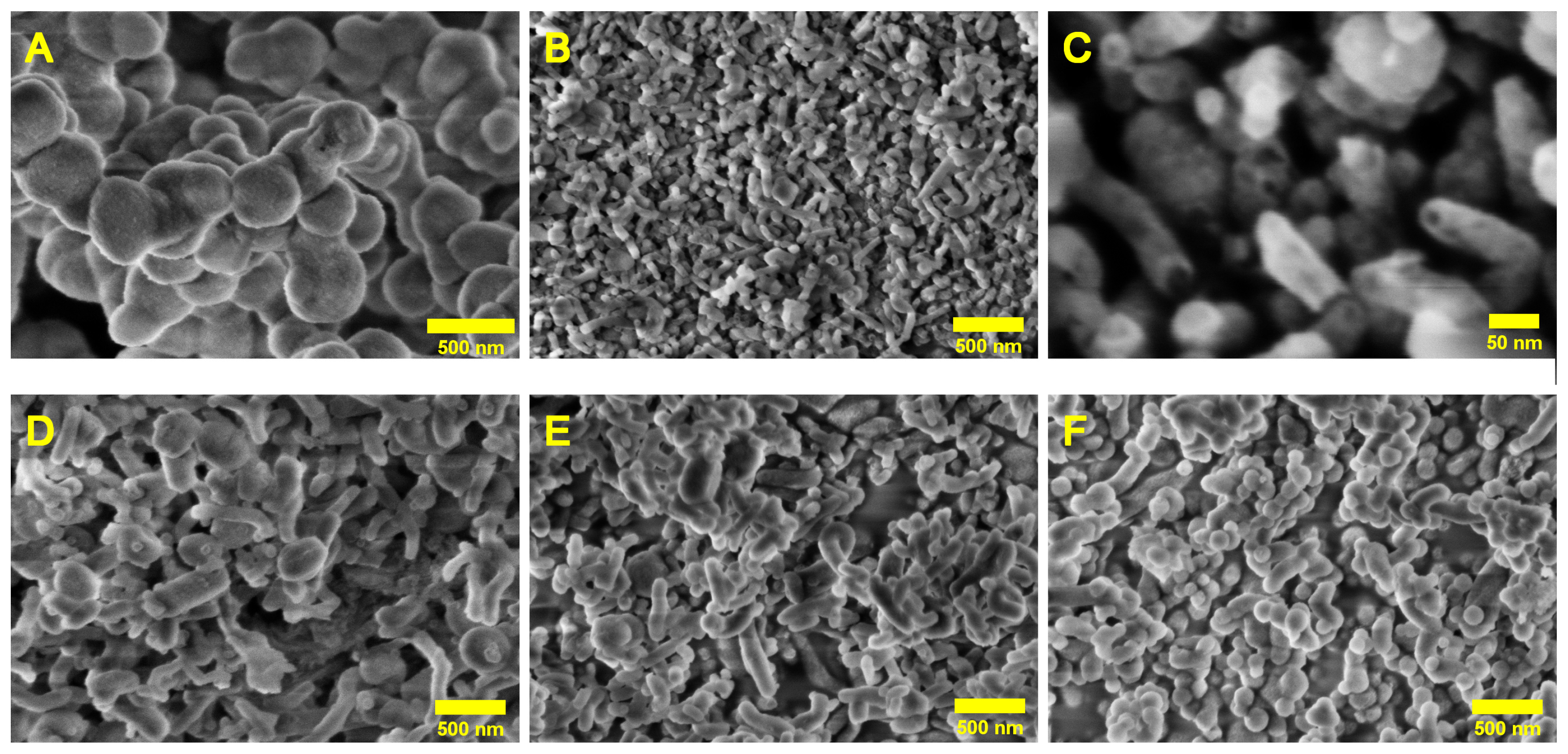
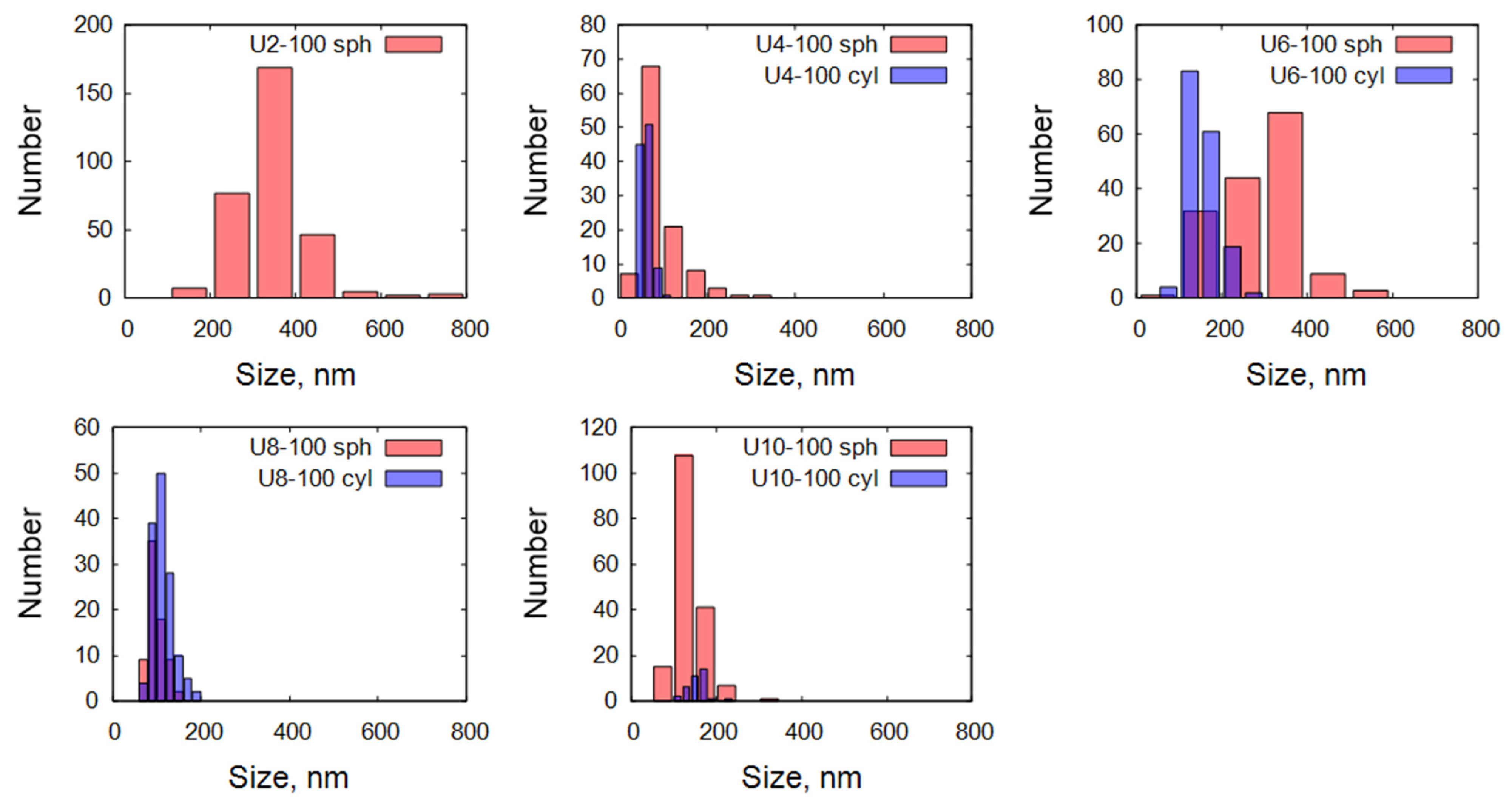
2.3.1. Scanning Electron Microscopy
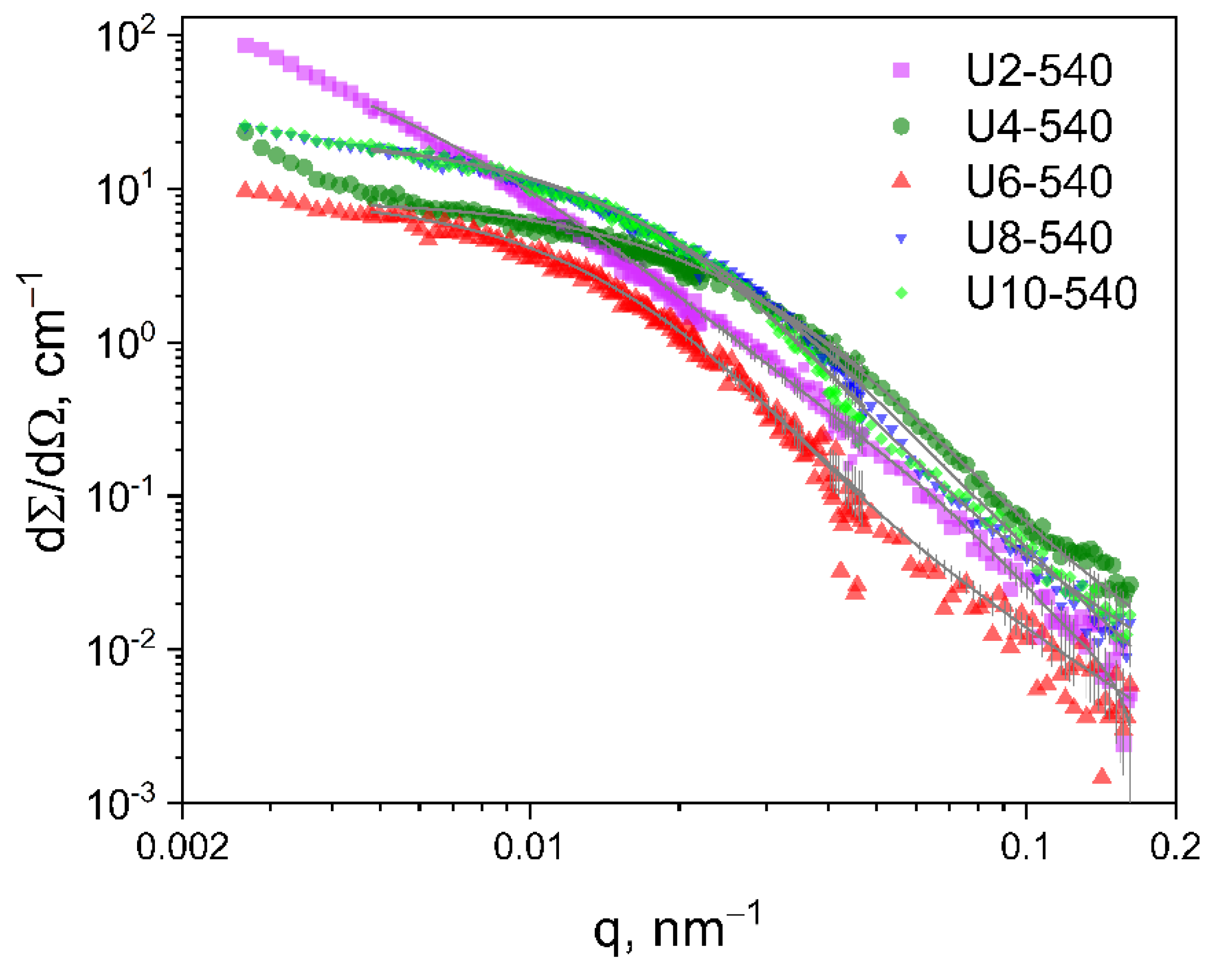
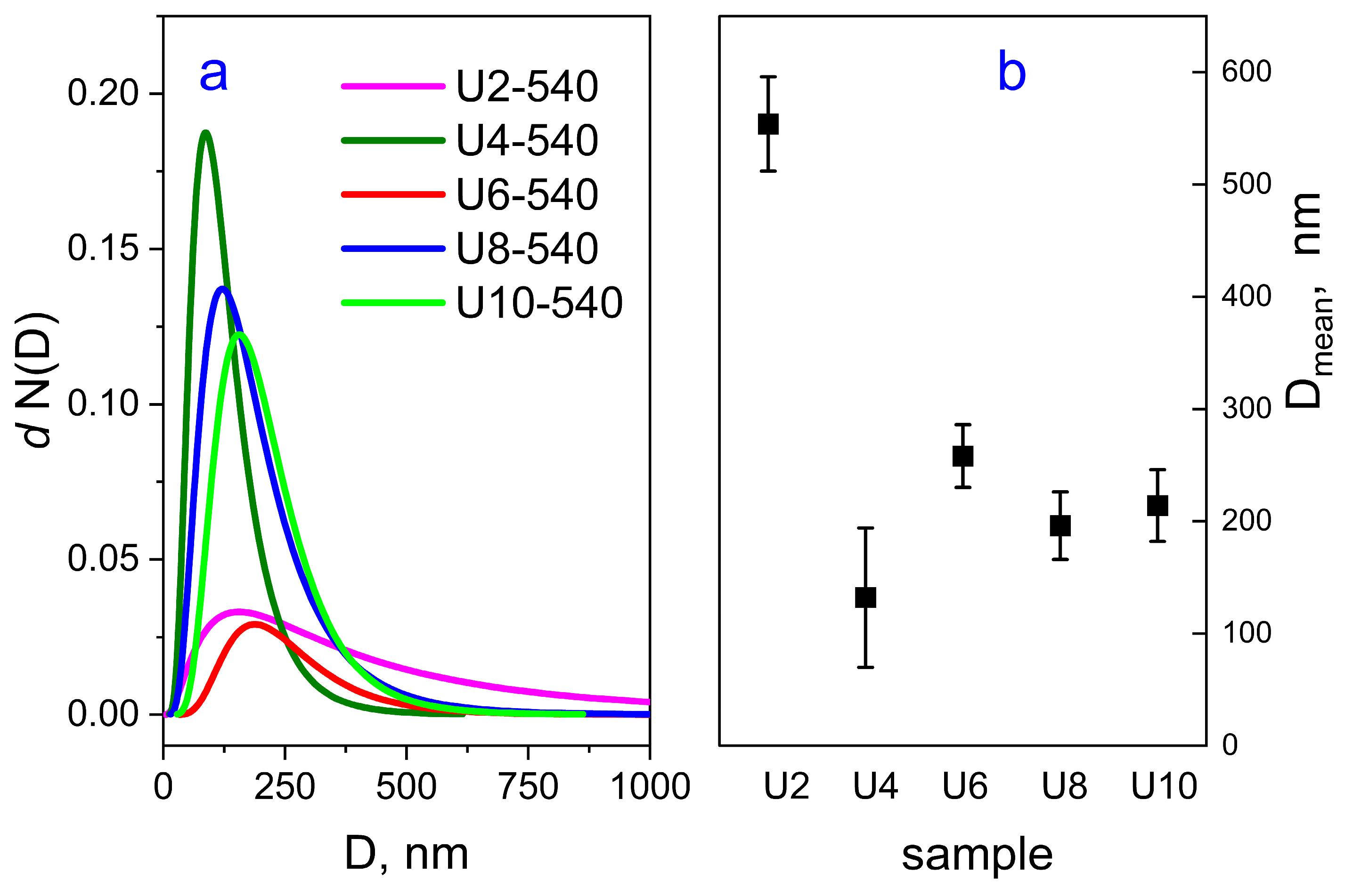
2.3.2. Ultra-Small-Angle Neutron Scattering
2.4. Nanoscale Structure
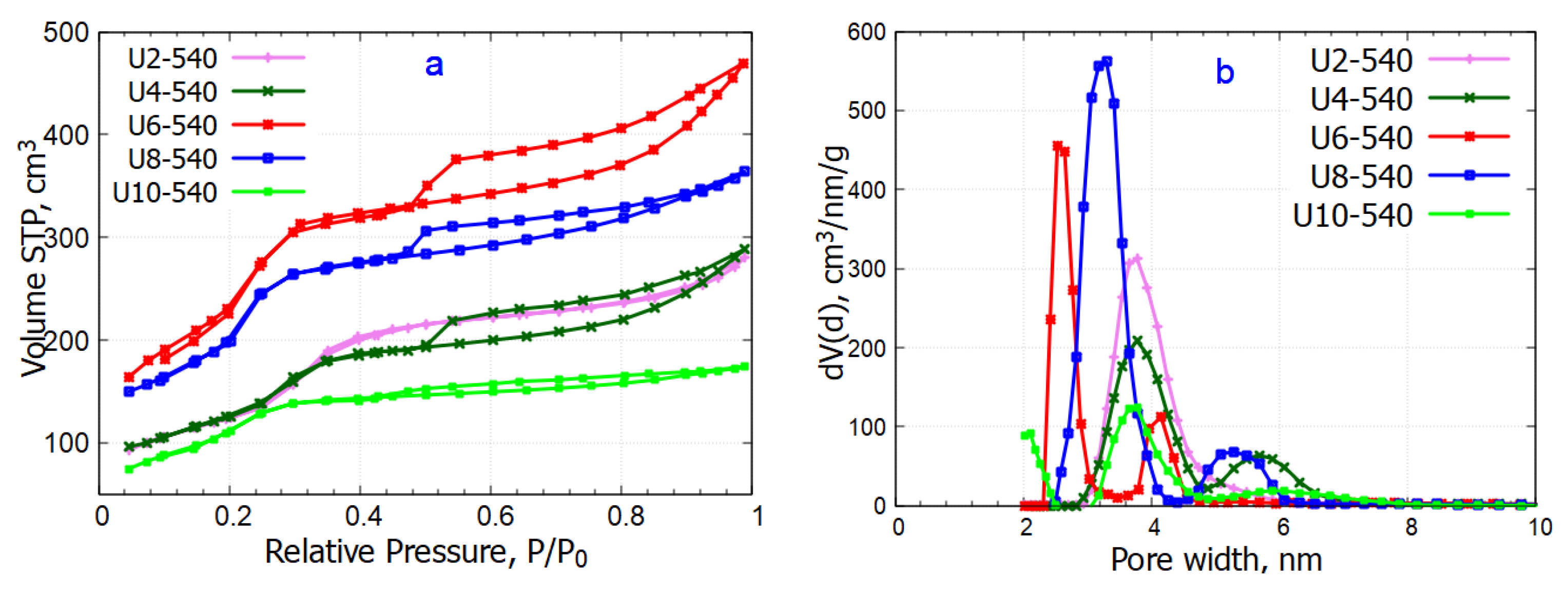
2.4.1. Nitrogen Sorption
2.4.2. Transmission Electron Microscopy
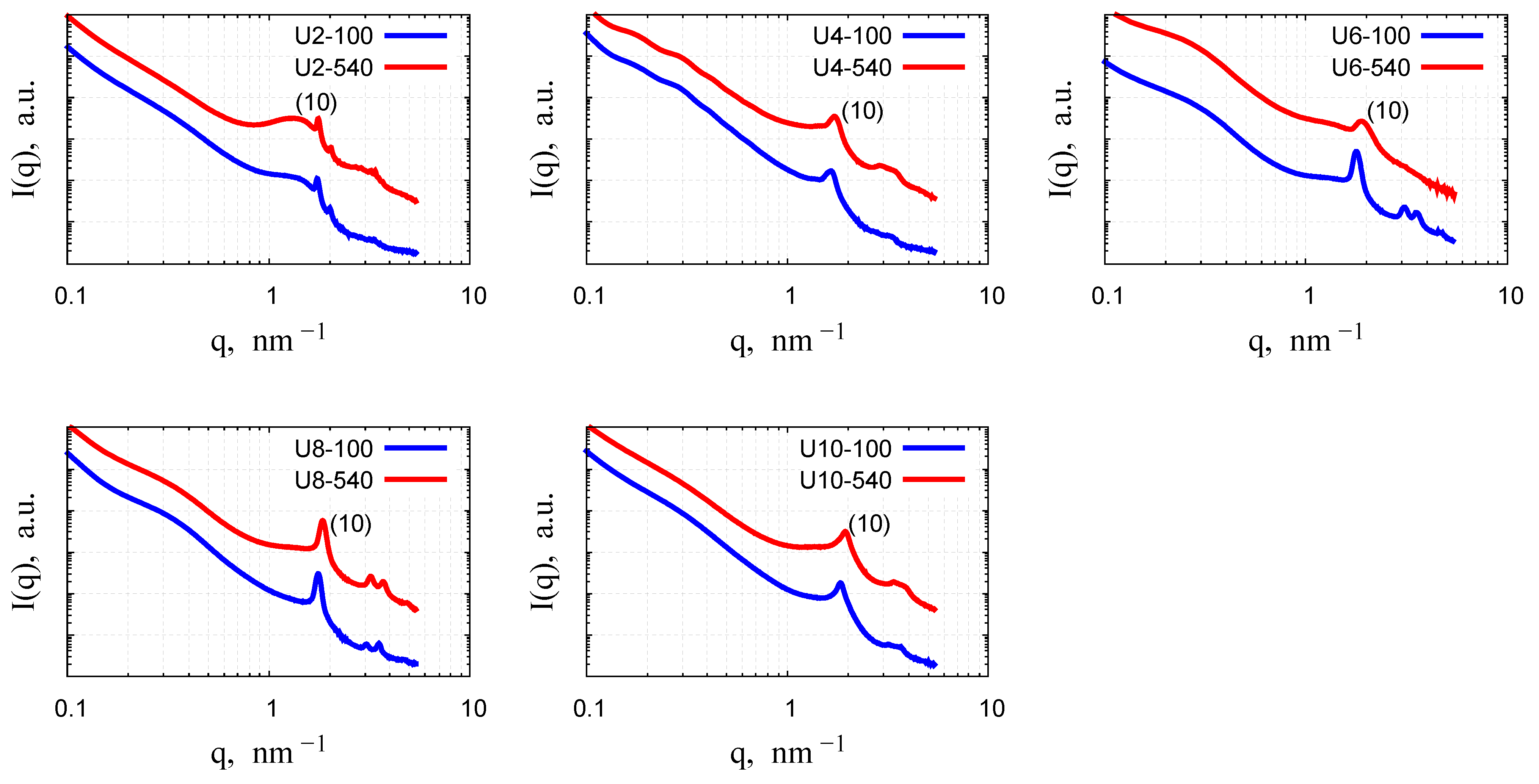
2.4.3. Small-Angle X-Ray Scattering
2.5. Influence of the Surfactant Spacer Length
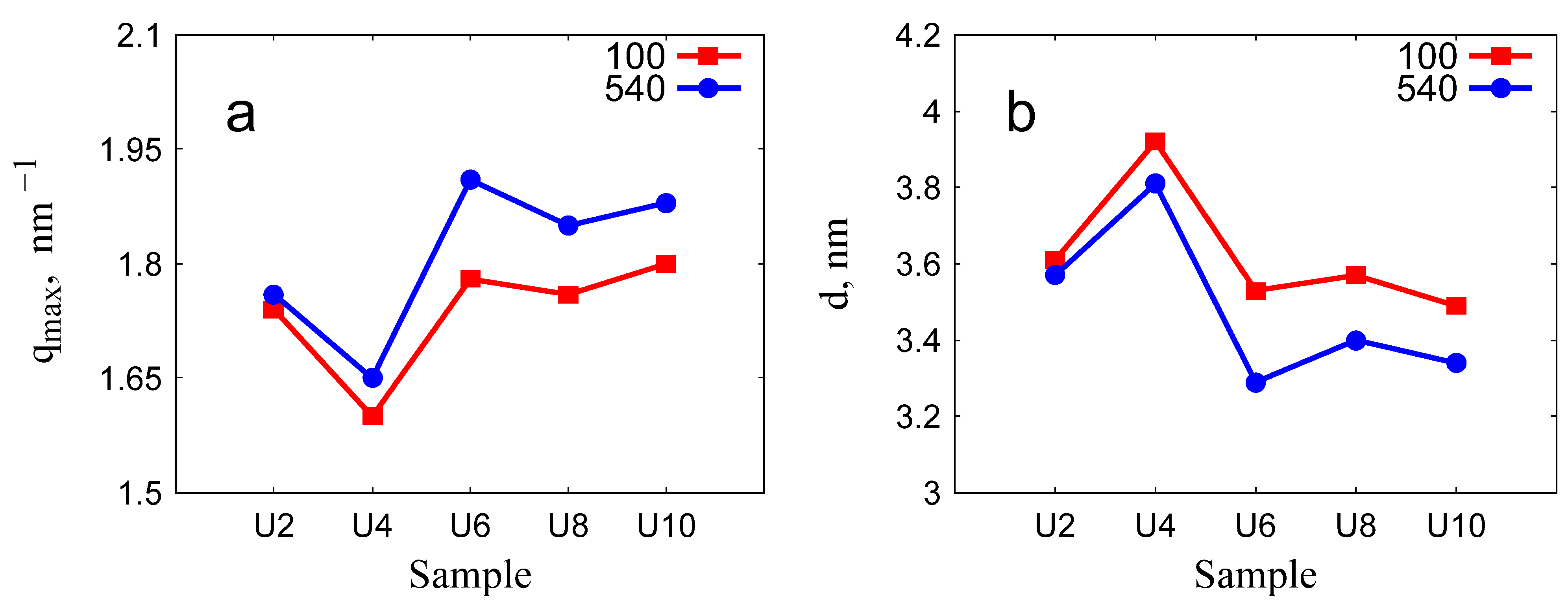
2.6. Lattice Spacing and Domain Size
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Urea Gemini Surfactants
4.2. Synthesis of Mesoporous Silica Materials
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Schuth, F.; Sing, K.S.W.; Weitkamp, J. Handbook of Porous Solids; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; 3141p. [Google Scholar]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified Zeolites: Preparation, Characterization, and Applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Mizukami, F.; Hanaoka, T. General and Simple Approach for Control Cage and Cylindrical Mesopores, and Thermal/Hydrothermal Stable Frameworks. J. Phys. Chem. B 2005, 109, 9255–9264. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Guerrero-Hernández, L.; Meléndez-Ortiz, H.I.; Cortez-Mazatan, G.Y.; Vaillant-Sánchez, S.; Peralta-Rodríguez, R.D. Gemini and Bicephalous Surfactants: A Review on Their Synthesis, Micelle Formation, and Uses. Int. J. Mol. Sci. 2022, 23, 1798. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Brycka, J.; Kowalczyk, I. Properties and Applications of Quaternary Ammonium Gemini Surfactant 12-6-12: An Overview. Molecules 2023, 28, 6336. [Google Scholar] [CrossRef]
- Voort, P.; Mathieu, M.; Mees, F.; Vansant, E.F.J. Synthesis of high-quality MCM-48 and MCM-41 by means of the GEMINI surfactant method. J. Phys. Chem. B 1998, 102, 8847–8851. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Y.; Li, Q.; Wang, J. Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template. Materials 2022, 15, 2780. [Google Scholar] [CrossRef]
- Wang, S.; Han, S.; Cui, X.; Qiu, X. Effects of the spacer length of gemini surfactants on the ordered pore of silica. J. Porous Mater. 2012, 19, 243–249. [Google Scholar] [CrossRef]
- Gianotti, E.; Berlier, G.; Costabello, K.; Coluccia, S.; Meneau, F. In situ synchrotron small-angle X-ray scattering study of MCM-41 crystallisation using Gemini surfactants. Catal. Today 2007, 126, 203–210. [Google Scholar] [CrossRef]
- Kurbonov, S.; Pisárčik, M.; Lukáč, M.; Czigány, Z.; Kovács, Z.; Tolnai, I.; Kriechbaum, M.; Ryukhtin, V.; Petrenko, V.; Avdeev, M.V.; et al. Ordered Mesoporous Silica Prepared with Biodegradable Gemini Surfactants as Templates for Environmental Applications. Materials 2025, 18, 773. [Google Scholar] [CrossRef] [PubMed]
- Kaczerewska, O.; Sousa, I.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Gemini Surfactant as a Template Agent for the Synthesis of More Eco-Friendly Silica Nanocapsules. Appl. Sci. 2020, 10, 8085. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mat. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Pisárčik, M.; Pupák, M.; Devínsky, F.; Almásy, L.; Tian, Q.; Bukovský, M. Urea-based gemini surfactants: Synthesis, aggregation behaviour and biological activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 385–396. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Matusoiu, F.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Svera, P.; Ianasi, C. Molybdate Recovery by Adsorption onto Silica Matrix and Iron Oxide Based Composites. Gels 2022, 8, 125. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Nicola, R.; Costişor, O.; Muntean, S.-G.; Nistor, M.-A.; Putz, A.-M.; Ianăşi, C.; Lazău, R.; Almásy, L.; Săcărescu, L. Mesoporous magnetic nanocomposites: A promising adsorbent for the removal of dyes from aqueous solutions. J. Porous Mater. 2020, 27, 413–428. [Google Scholar] [CrossRef]
- Lenza, R.F.S.; Vasconcelos, W.L. Preparation of Silica by Sol-Gel Method Using Formamide. Mat. Res. 2001, 4, 175–179. [Google Scholar] [CrossRef]
- Latypova, A.R.; Lebedev, M.D.; Tarasyuk, I.A.; Sidorov, A.I.; Rumyantsev, E.V.; Vashurin, A.S.; Marfin, Y.S. Sol-Gel Synthesis of Organically Modified Silica Particles as Efficient Palladium Catalyst Supports to Perform Hydrogenation Process. Catalysts 2021, 11, 1175. [Google Scholar] [CrossRef]
- Courtney, T.D.; Chang, C.-C.; Gorte, R.J.; Lobo, R.F.; Fan, W.; Nikolakis, V. Effect of water treatment on Sn-BEA zeolite: Origin of 960 cm−1 FTIR peak. Micropor. Mesopor. Mater. 2015, 210, 69–76. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Conolly, J.; Singh, M.; Buckley, C.E. Determination of size and ordering of pores in mesoporous silica using small angle neutron scattering. Physica B 2004, 350, 224–226. [Google Scholar] [CrossRef]
- Szegedi, A.; Popova, M.; Goshev, I.; Klébert, S.; Mihály, J. Controlled drug release on amine functionalized spherical MCM-41. J. Solid State Chem. 2012, 194, 257–263. [Google Scholar] [CrossRef]
- Goworek, J.; Kierys, A.; Gac, W.; Borówka, A.; Kusak, R. Thermal Degradation of CTAB in As-Synthesized MCM-41. J. Therm. Anal. Calorim. 2009, 96, 375–382. [Google Scholar] [CrossRef]
- Putz, A.-M.; Cecilia, S.; Ianăşi, C.; Dudás, Z.; Székely, K.N.; Plocek, J.; Sfârloaga, P.; Sacarescu, L.; Almásy, L. Pore ordering in mesoporous matrices induced by different directing agents. J. Porous Mater. 2015, 22, 321–331. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzalka, M.; Pikus, S.; Skibinska, M.; Blachnio, M.; Derylo-Marczewska, A. The structure of ordered mesoporous materials synthesized from aluminum phyllosilicate clay (Bentonite). Molecules 2023, 28, 2561. [Google Scholar] [CrossRef]
- Borówka, A.; Skrzypiec, K. Effects of temperature on the structure of mesoporous silica materials templated with cationic surfactants in a nonhydrothermal short-term synthesis route. J. Solid State Chem. 2021, 299, 122183. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.I.; Milanovic, M.; Stijepovic, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cui, K.; Jin, S. Temperature-driven structural evolution during preparation of MCM-41 mesoporous silica. Materials 2024, 17, 1711. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Danino, D.; Talmon, Y.; Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) Surfactants (Dimeric Surfactants). 5. Aggregation and Microstructure in Aqueous Solutions. Langmuir 1995, 11, 1448–1456. [Google Scholar] [CrossRef]
- Harrison, I.R.; Kozmiski, S.J.; Varnell, W.D.; Wang, J.-I. SAXS instrumental broadening and the order dependence of peak width. J. Polym. Sci. 1981, 19, 487–497. [Google Scholar] [CrossRef]
- Bergenholtz, J.; Ulama, J.; Zackrisson Oskolkova, M. Analysis of small-angle X-ray scattering data in the presence of significant instrumental smearing. J. Appl. Crystallogr. 2016, 49, 47–54. [Google Scholar] [CrossRef]
- Wagner, J.; Härtl, W.; Hempelmann, R. Characterization of Monodisperse Colloidal Particles: Comparison between SAXS and DLS. Langmuir 2000, 16, 4080–4085. [Google Scholar] [CrossRef]
- Strunz, P.; Saroun, J.; Mikula, P.; Lukas, P.; Eichhorn, F. Double-Bent-Crystal Small-Angle Neutron Scattering Setting and its Applications. J. Appl. Cryst. 1997, 30, 844–848. [Google Scholar] [CrossRef]
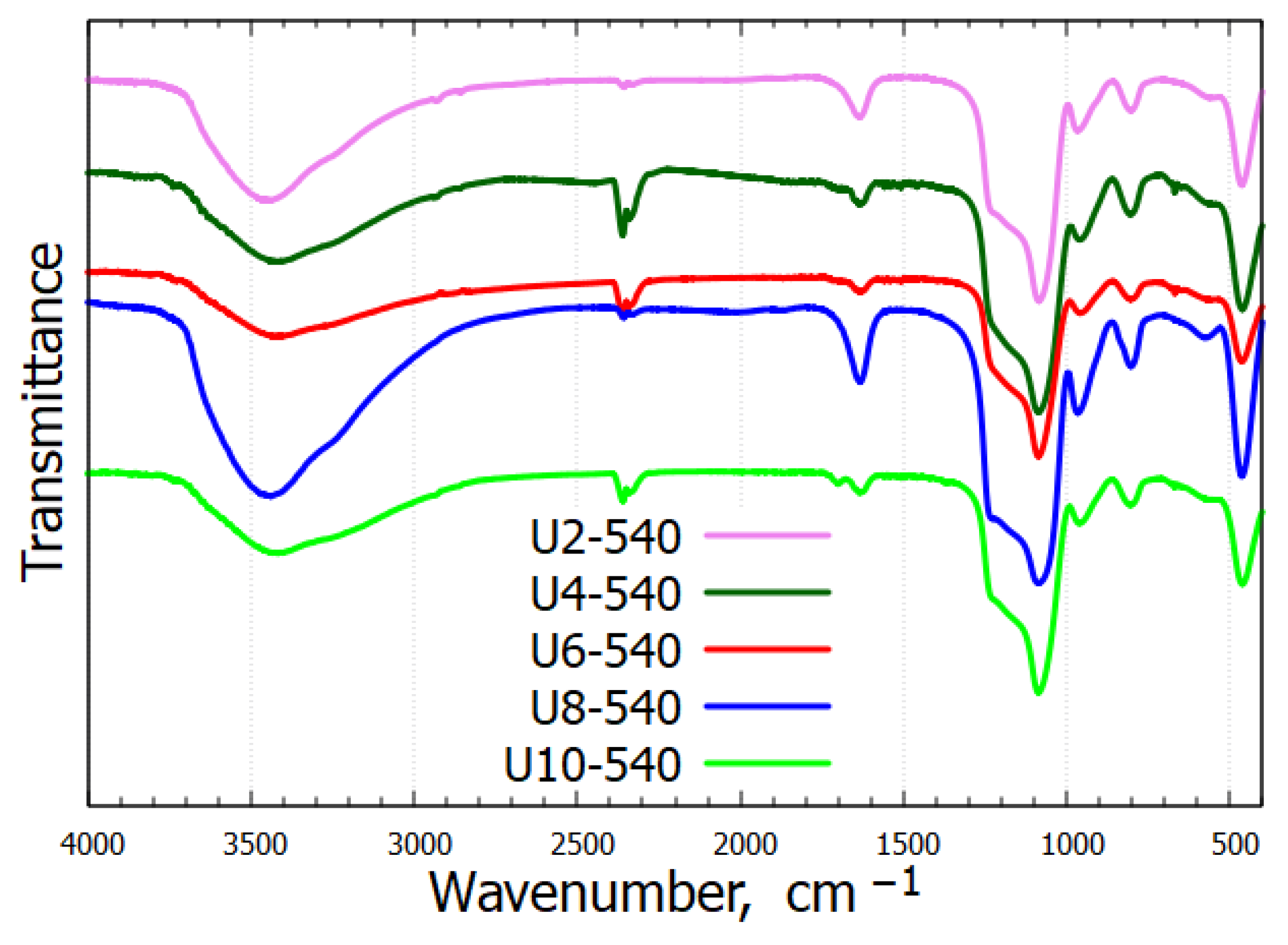


| Band Assignments | υ [cm−1] |
|---|---|
| Free silanol groups and molecular water [21] | 3455 |
| Molecular water and the SiO2 network [22] | 1636 |
| Asymmetric Si–O–Si stretching [23] | 1088 |
| Si–OH stretching [24,25,26] | 961 |
| Symmetric Si–O–Si stretching [23,24] | 806 |
| Si–O–Si bending [21,23] | 463 |
| Sample | Morphology | Diameter Range [nm] |
|---|---|---|
| U2-100 | 100% Spherical | Spherical: 100–800 |
| U4-100 | 50% Spherical, 50% Cylindrical | Spherical: 25–325, Cylindrical: 45–105 |
| U6-100 | 50% Spherical, 50% Cylindrical | Spherical: 50–550, Cylindrical: 75–275 |
| U8-100 | 35% Spherical, 65% Cylindrical | Spherical: 65–145, Cylindrical: 65–195 |
| U10-100 | 85% Spherical, 15% Cylindrical | Spherical: 75–325, Cylindrical: 110–230 |
| Sample | Specific Surface Area [m2/g] | Specific Micropore Area [m2/g] | Pore Diameter [nm] | Total Pore Volume [cm3/g] | Micropore Volume [cm3/g] | ||
|---|---|---|---|---|---|---|---|
| BJH(ads) | BJH(des) | DFT | |||||
| U2-540 | 481 | - | 3.1 | 3.3 | 3.8 | 0.43 | - |
| U4-540 | 464 | - | 3.5 | 4.3 | 3.8 | 0.45 | - |
| U6-540 | 910 | - | 3.1 | 4.0 | 2.7 | 0.73 | - |
| U8-540 | 882 | - | 3.4 | 4.0 | 3.3 | 0.56 | - |
| U10-540 | 443 | 78 | 3.1 | 3.1 | 3.8 | 0.27 | 0.02 |
| Sample | (10) Peak Position [nm−1] | HWHM by SAXS [nm−1] | d [nm] | Domain Size [nm] | Reduction in Domain Size | d and HWHM by TEM [nm] |
|---|---|---|---|---|---|---|
| U2-100 | 1.74 | 0.06 | 3.61 | 103.8 | ||
| U2-540 | 1.76 | 0.05 | 3.57 | 118.6 | 0.87 | 3.22, 0.09 |
| U4-100 | 1.60 | 0.45 | 3.92 | 13.18 | ||
| U4-540 | 1.65 | 0.52 | 3.81 | 11.38 | 0.86 | |
| U6-100 | 1.78 | 0.16 | 3.53 | 36.11 | ||
| U6-540 | 1.90 | 0.18 | 3.31 | 33.22 | 0.92 | 3.02, 0.16 |
| U8-100 | 1.76 | 0.12 | 3.57 | 48.85 | ||
| U8-540 | 1.85 | 0.16 | 3.40 | 37.75 | 0.77 | |
| U10-100 | 1.80 | 0.36 | 3.49 | 16.28 | ||
| U10-540 | 1.88 | 0.47 | 3.34 | 12.58 | 0.77 | 3.05, 0.05 |
| Sample Name | σobs [1/nm] | σlattice [1/nm] | σins [1/nm] | σsize [1/nm] | Domain Size After the Correction [nm] | Domain Size Before the Correction [nm] |
|---|---|---|---|---|---|---|
| U6-540 | 0.075 | 0.018 | 0.013 | 0.072 | 34.6 | 33.2 |
| U10-540 | 0.2 | 0.005 | 0.013 | 0.199 | 13.0 | 12.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbonov, S.; Czigány, Z.; Kovács, Z.; Péter, L.; Pisárčik, M.; Lukáč, M.; Kriechbaum, M.; Ryukhtin, V.; Lacrămă, A.-M.; Almásy, L. Structural Characterization of Ordered Mesoporous Silica Prepared by a Sol–Gel Process Using Urea-Based Cationic Gemini Surfactants. Gels 2025, 11, 804. https://doi.org/10.3390/gels11100804
Kurbonov S, Czigány Z, Kovács Z, Péter L, Pisárčik M, Lukáč M, Kriechbaum M, Ryukhtin V, Lacrămă A-M, Almásy L. Structural Characterization of Ordered Mesoporous Silica Prepared by a Sol–Gel Process Using Urea-Based Cationic Gemini Surfactants. Gels. 2025; 11(10):804. https://doi.org/10.3390/gels11100804
Chicago/Turabian StyleKurbonov, Sarvarjon, Zsolt Czigány, Zoltán Kovács, László Péter, Martin Pisárčik, Miloš Lukáč, Manfred Kriechbaum, Vasyl Ryukhtin, Ana-Maria Lacrămă, and László Almásy. 2025. "Structural Characterization of Ordered Mesoporous Silica Prepared by a Sol–Gel Process Using Urea-Based Cationic Gemini Surfactants" Gels 11, no. 10: 804. https://doi.org/10.3390/gels11100804
APA StyleKurbonov, S., Czigány, Z., Kovács, Z., Péter, L., Pisárčik, M., Lukáč, M., Kriechbaum, M., Ryukhtin, V., Lacrămă, A.-M., & Almásy, L. (2025). Structural Characterization of Ordered Mesoporous Silica Prepared by a Sol–Gel Process Using Urea-Based Cationic Gemini Surfactants. Gels, 11(10), 804. https://doi.org/10.3390/gels11100804












