Laser-Based Fabrication of Hydrogel Scaffolds for Medicine: From Principles to Clinical Applications
Abstract
1. Introduction
2. Fundamentals of Hydrogels for Medicine
2.1. Hydrogel Classification
2.1.1. Natural Hydrogels
2.1.2. Synthetic Hydrogels
2.1.3. Hybrid Hydrogels
2.2. Key Properties of Resorbable Hydrogels
2.2.1. Mechanisms of Degradation
2.2.2. Tuning to Match Tissue Regeneration
2.2.3. Mechanical Properties
2.2.4. Porosity
2.2.5. Swelling Behavior
2.2.6. Biocompatibility
2.2.7. Surface Chemistry and RGD Sequence
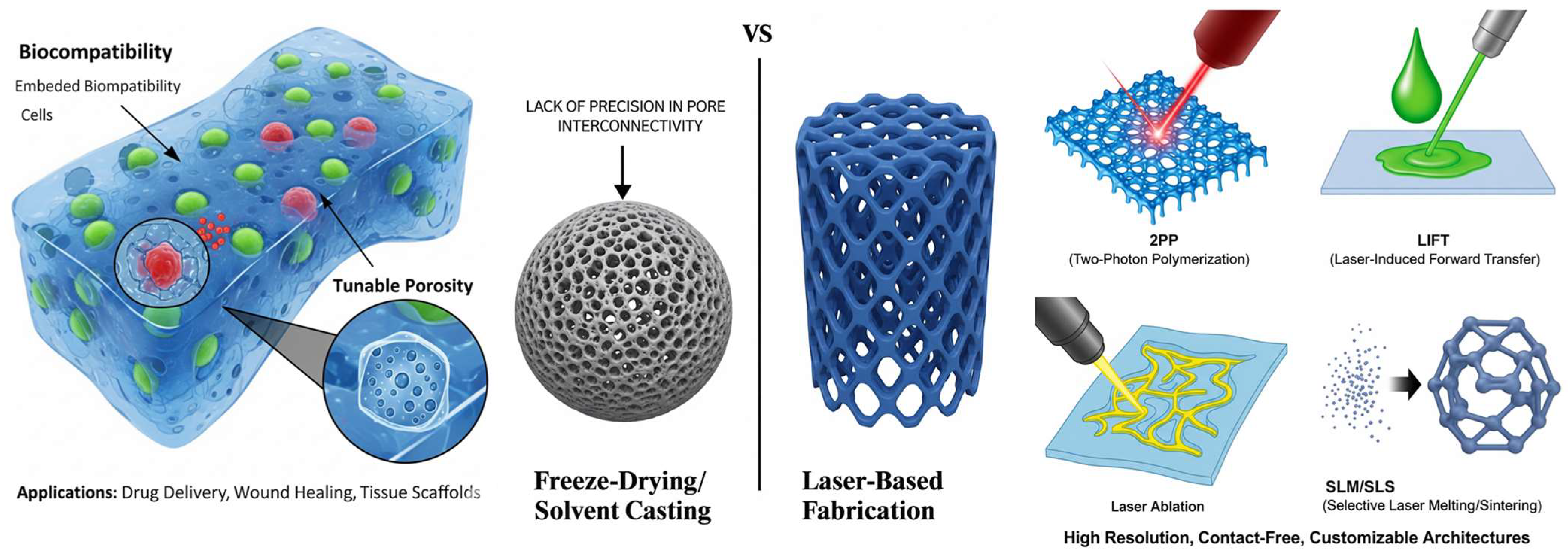
3. Advanced Laser Fabrication Techniques for Hydrogel Scaffolds
3.1. Two-Photon Polymerization/Multiphoton Lithography
3.1.1. Working Principle
3.1.2. Materials
3.1.3. Applications
3.1.4. Case Studies
Bone Tissue Engineering: 3D Hydrogel Scaffolds That Mimic Bone Structure
Cartilage Tissue Engineering: 3D Scaffolds for Articular Cartilage Regeneration
Nerve Tissue Engineering: Microstructured Scaffolds to Guide Nerve Regeneration
3.1.5. Advantages and Limitations
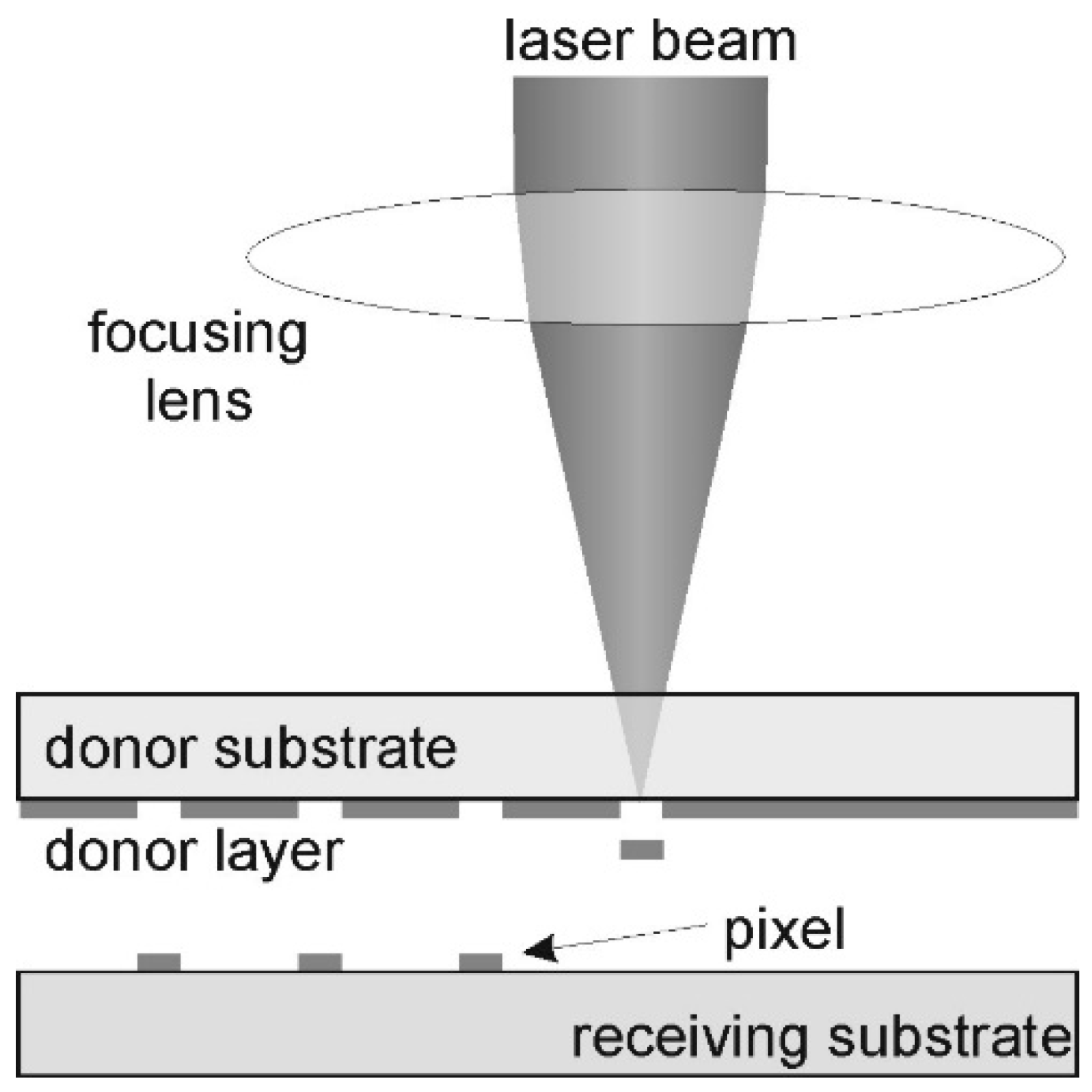
3.2. Laser Induced Forward Transfer
3.2.1. Working Principle
3.2.2. Materials
3.2.3. Applications
3.2.4. Case Studies
3.2.5. Advantages and Limitations
Advantages of LIFT
Limitations and Disadvantages of LIFT
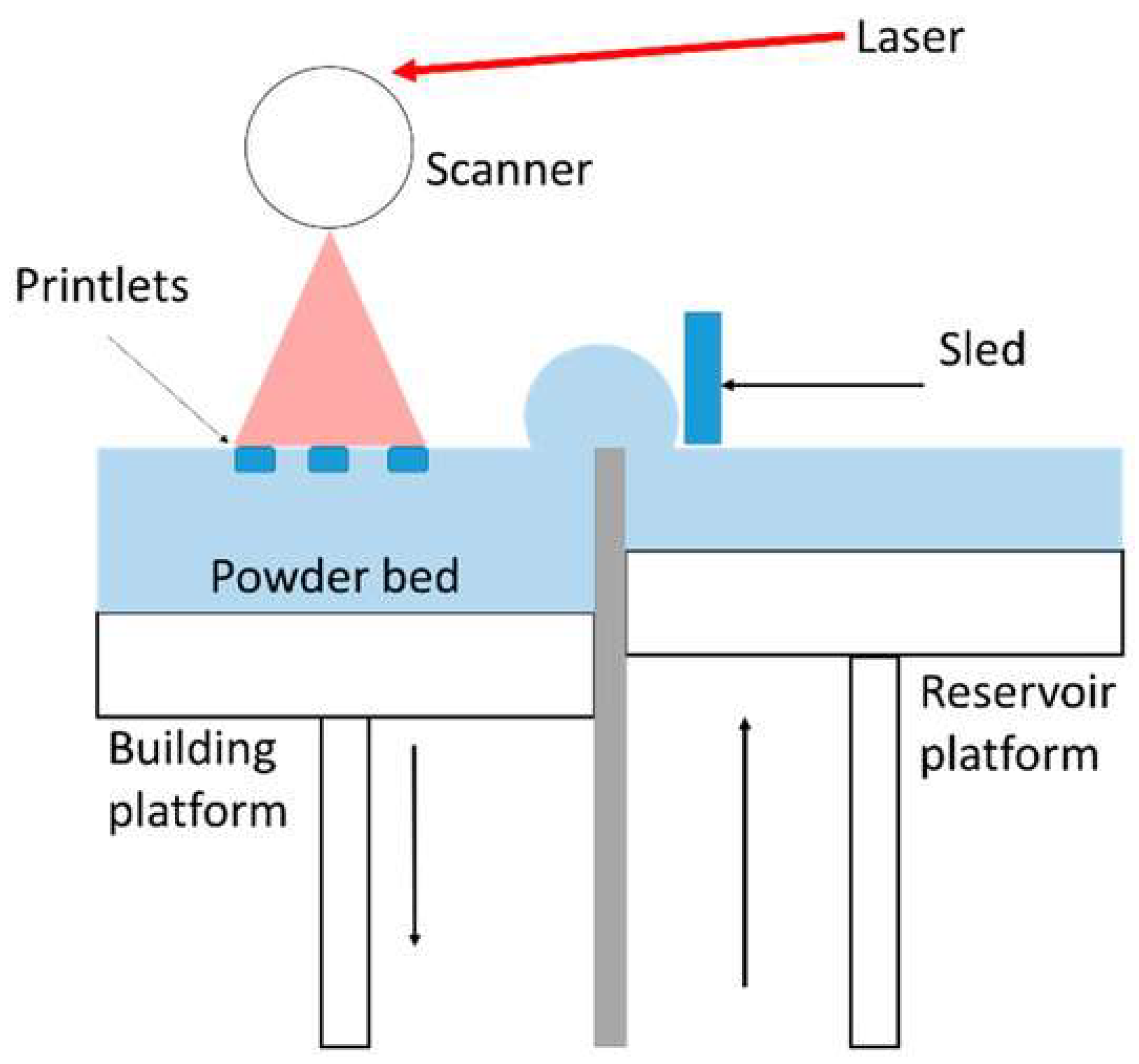
3.3. Selective Laser Sintering/Selective Laser Melting
3.3.1. Working Principle
3.3.2. Materials
3.3.3. Applications
3.3.4. Case Studies
3.3.5. Advantages and Limitations
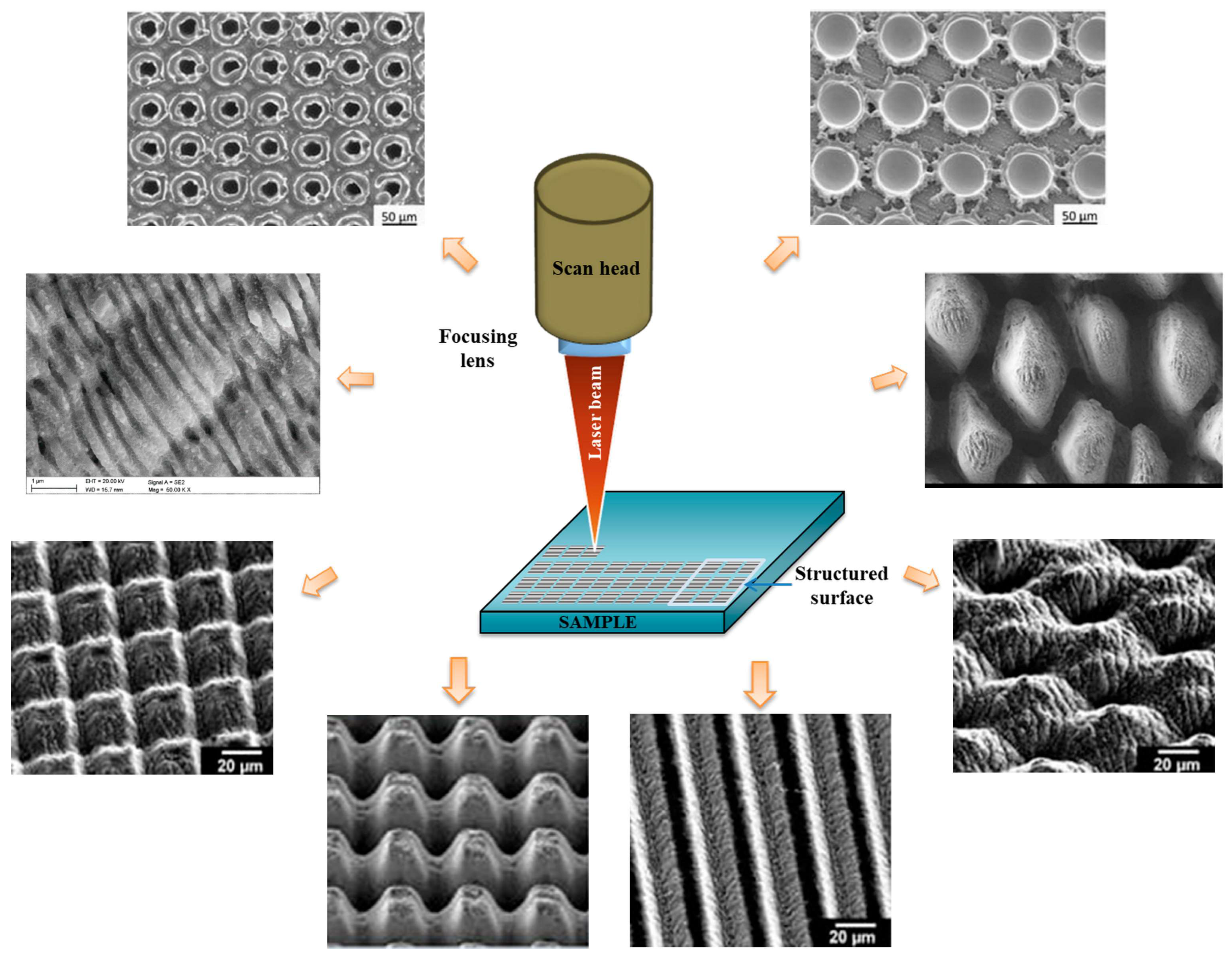
3.4. Laser Direct Writing
3.4.1. Working Principle
3.4.2. Materials
3.4.3. Applications
3.4.4. Case Studies
Case Study 1: Neural Tissue Engineering—Photoablated Microchannels to Guide Nerve Regeneration
Case Study 2: Vascular Engineering—Laser Fabricated Microchannels for Graft Pre-Vascularization
3.4.5. Advantages and Limitations
Advantages
Limitations
4. Clinical Application of Hydrogel Scaffold Products
5. Challenges
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciuciu, A.I.; Cywiński, P.J. Two-photon polymerization of hydrogels—Versatile solutions to fabricate well-defined 3D structures. RSC Adv. 2014, 4, 45504–45516. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhou, S.; Chen, X. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ.-Sci. B 2017, 18, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Sun, X. Laser-induced forward transfer based laser bioprinting in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1255782. [Google Scholar] [CrossRef]
- Phillips, L.; Valavanis, A.; Burnett, A.D.; Kay, R.; Harris, R.; Saleh, E. Process and material constraints of additive manufacturing for fabrication of terahertz quasi-optical components. Appl. Mater. Today 2025, 42, 102619. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Sadlik, J.; Kosińska, E.; Słota, D.; Niziołek, K.; Tomala, A.; Włodarczyk, M.; Piątek, P.; Skibiński, J.; Jampilek, J.; Sobczak-Kupiec, A. Bioactive Hydrogel Based on Collagen and Hyaluronic Acid Enriched with Freeze-Dried Sheep Placenta for Wound Healing Support. Int. J. Mol. Sci. 2024, 25, 1687. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Liu, S.; Zhu, W.; Li, Y.; Huang, L.; Zhang, G.; Zhang, Y. Synthesis, Characterization, Properties, and Biomedical Application of Chitosan-Based Hydrogels. Polymers 2023, 15, 2482. [Google Scholar] [CrossRef]
- Chamkouri, H.; Chamkouri, M. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Khan, S. Chitosan/Poly (vinyl alcohol) Based Hydrogels for Biomedical Applications: A Review. J. Pharm. Altern. Med. 2013, 2, 40–41. [Google Scholar]
- Mansuri, A.; Gupta, D.; Pawar, R.; Tanwar, S.S. A Comprehensive Review of Hydrogel Classification, Fabrication, and Utility. Int. J. Res. Publ. Rev. 2025, 6, 521–530. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Hu, B.; Gao, J.; Lu, Y.; Wang, Y. Applications of Degradable Hydrogels in Novel Approaches to Disease Treatment and New Modes of Drug Delivery. Pharmaceutics 2023, 15, 2370. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.P.; Guldberg, R.; Stayton, P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Ashton, M.; Dodou, K. Effect of Crosslinking Agent Concentration on the Properties of Unmedicated Hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Boaru, D.L.; Perez-Exposito, R.E.; Fraile-Martinez, O.; García-Montero, C.; Diaz, R.; Bujan, J.; García-Honduvilla, N.; Lopez-Gonzalez, L.; Álvarez-Mon, M.; et al. Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review. Gels 2023, 9, 885. [Google Scholar] [CrossRef]
- Ning, X.; Huang, J.; Y, A.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Zhou, H. Biomimetic Hydrogel Applications and Challenges in Bone, Cartilage, and Nerve Repair. Pharmaceutics 2023, 15, 2405. [Google Scholar] [CrossRef]
- Yarali, E.; Mubeen, A.A.; Cussen, K.; van Zanten, L.; Moosabeiki, V.; Zadpoor, A.A.; Accardo, A.; Mirzaali, M.J. Two-photon polymerization based 4D printing of poly(N-isopropylacrylamide) hydrogel microarchitectures for reversible shape morphing. Sci. Rep. 2025, 15, 21549. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite Hydrogels for Biomedical Applications. Biotechnol. Bioeng. 2013, 111, 441–453. [Google Scholar] [CrossRef]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef]
- Li, T.; Peng, Z.; Lv, Q.; Li, L.; Zhang, C.; Pang, L.; Zhang, C.; Li, Y.; Chen, Y.; Tang, X. SLS 3D Printing To Fabricate Poly(vinyl alcohol)/Hydroxyapatite Bioactive Composite Porous Scaffolds and Their Bone Defect Repair Property. ACS Biomater. Sci. Eng. 2023, 9, 6734–6744. [Google Scholar] [CrossRef]
- Wang, L.; Dong, S.; Liu, Y.; Ma, Y.; Zhang, J.; Yang, Z.; Jiang, W.; Yuan, Y. Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process. Polymers 2020, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Gonella, S.; Domingues, M.F.; Miguel, F.; Moura, C.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Silva, J.C. Fabrication and Characterization of Porous PEGDA Hydrogels for Articular Cartilage Regeneration. Gels 2024, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.T.; Hadley, D.J.; Kukis, D.L.; Silva, E.A. Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications. PLoS ONE 2017, 12, e0181484. [Google Scholar] [CrossRef]
- Radecki, M.; Spěváček, J.; Zhigunov, A.; Sedláková, Z.; Hanyková, L. Temperature-induced phase transition in hydrogels of interpenetrating networks of poly(N-isopropylacrylamide) and polyacrylamide. Eur. Polym. J. 2015, 68, 68–79. [Google Scholar] [CrossRef]
- Torgersen, J.; Qin, X.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for Two-Photon Polymerization: A Toolbox for Mimicking the Extracellular Matrix | Request PDF. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef]
- Yusupov, V.; Churbanov, S.; Churbanova, E.; Bardakova, K.; Antoshin, A.; Evlashin, S.; Timashev, P.; Minaev, N. Laser-induced Forward Transfer Hydrogel Printing: A Defined Route for Highly Controlled Process. Int. J. Bioprinting 2020, 6, 271. [Google Scholar] [CrossRef]
- Kryou, C.; Zergioti, I. Laser-Induced Forward Transfer on Regenerative Medicine Applications. Biomed. Mater. Devices 2023, 1, 5–20. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, X.; Wang, X.; He, E.; Zhang, Y.; Wang, C. Microfabrication of hydrogels based on femtosecond laser three-dimensional ablation. Chem. Eng. J. 2025, 520, 166034. [Google Scholar] [CrossRef]
- Pradhan, S.; Keller, K.A.; Sperduto, J.L.; Slater, J.H. Fundamentals of Laser-Based Hydrogel Degradation and Applications in Cell and Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700681. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Narayan, R.J. Two-photon polymerization for biological applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Jing, X.; Fu, H.; Yu, B.; Sun, M.; Wang, L. Two-photon polymerization for 3D biomedical scaffolds: Overview and updates. Front. Bioeng. Biotechnol. 2022, 10, 994355. [Google Scholar] [CrossRef]
- Kawata, S.; Sun, H.-B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. High-resolution two-photon polymerization: The most versatile technique for the fabrication of microneedle arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Gao, W.; Chao, H.; Zheng, Y.-C.; Zhang, W.-C.; Liu, J.; Jin, F.; Dong, X.-Z.; Liu, Y.-H.; Li, S.-J.; Zheng, M.-L. Ionic Carbazole-Based Water-Soluble Two-Photon Photoinitiator and the Fabrication of Biocompatible 3D Hydrogel Scaffold. ACS Appl. Mater. Interfaces 2021, 13, 27796–27805. [Google Scholar] [CrossRef]
- Calin, B.S.; Paun, I.A. A Review on Stimuli-Actuated 3D Micro/Nanostructures for Tissue Engineering and the Potential of Laser-Direct Writing via Two-Photon Polymerization for Structure Fabrication. Int. J. Mol. Sci. 2022, 23, 14270. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Zheng, M.-L.; Liu, J.; Jin, F.; Dong, X.-Z.; Guo, M.; Li, T. Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration. Nanomaterials 2021, 11, 2325. [Google Scholar] [CrossRef]
- Koroleva, A.; Deiwick, A.; Nguyen, A.; Schlie-Wolter, S.; Narayan, R.; Timashev, P.; Popov, V.; Bagratashvili, V.; Chichkov, B. Osteogenic Differentiation of Human Mesenchymal Stem Cells in 3-D Zr-Si Organic-Inorganic Scaffolds Produced by Two-Photon Polymerization Technique. PLoS ONE 2015, 10, e0118164. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Mustaciosu, C.C.; Zamfirescu, M.; Calin, B.S.; Mihailescu, M.; Dinescu, M.; Popescu, A.; Chioibasu, D.; Soproniy, M.; et al. Laser-direct writing by two-photon polymerization of 3D honeycomb-like structures for bone regeneration. Biofabrication 2018, 10, 025009. [Google Scholar] [CrossRef]
- Mačiulaitis, J.; Deveikytė, M.; Rekštytė, S.; Bratchikov, M.; Darinskas, A.; Šimbelytė, A.; Daunoras, G.; Laurinavičienė, A.; Laurinavičius, A.; Gudas, R.; et al. Preclinical study of SZ2080 material 3D microstructured scaffolds for cartilage tissue engineering made by femtosecond direct laser writing lithography. Biofabrication 2015, 7, 015015. [Google Scholar] [CrossRef]
- Crowe, J.A.; El-Tamer, A.; Nagel, D.; Koroleva, A.V.; Madrid-Wolff, J.; Olarte, O.E.; Sokolovsky, S.; Estevez-Priego, E.; Ludl, A.-A.; Soriano, J.; et al. Development of two-photon polymerised scaffolds for optical interrogation and neurite guidance of human iPSC-derived cortical neuronal networks. Lab. Chip 2020, 20, 1792–1806. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Nguyen, A.; Obata, K.; Koroleva, A.; Narayan, R.J.; Chichkov, B.N. Fabrication of microscale medical devices by two-photon polymerization with multiple foci via a spatial light modulator. Biomed. Opt. Express 2011, 2, 3167–3178. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Polymer–Ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Gittard, S.D.; Narayan, R.J. Laser direct writing of micro- and nano-scale medical devices. Expert Rev. Med. Devices 2010, 7, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Selimis, A.; Mironov, V.; Farsari, M. Direct laser writing: Principles and materials for scaffold 3D printing. Microelectron. Eng. 2015, 132, 83–89. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Y.; Lin, J. A review on the processing accuracy of two-photon polymerization. AIP Adv. 2015, 5, 030701. [Google Scholar] [CrossRef]
- Das, S.; Jegadeesan, J.T.; Basu, B. Gelatin Methacryloyl (GelMA)-Based Biomaterial Inks: Process Science for 3D/4D Printing and Current Status. Biomacromolecules 2024, 25, 2156–2221. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Halevi, A.E.; Nuttelman, C.R.; Bowman, C.N.; Anseth, K.S. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater. Deerfield Beach Fla 2009, 21, 5005–5010. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Shi, M.; Zhang, L.-P.; Zhang, Y.; Zhao, Y. A highly biocompatible bio-ink for 3D hydrogel scaffolds fabrication in the presence of living cells by two-photon polymerization. Eur. Polym. J. 2021, 153, 110505. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Serra, P. Laser-Induced Forward Transfer: A Method for Printing Functional Inks. Crystals 2020, 10, 651. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Minaeva, E.D.; Antoshin, A.A.; Kosheleva, N.V.; Koteneva, P.I.; Gonchukov, S.A.; Tsypina, S.I.; Yusupov, V.I.; Timashev, P.S.; Minaev, N.V. Laser Bioprinting with Cell Spheroids: Accurate and Gentle. Micromachines 2023, 14, 1152. [Google Scholar] [CrossRef]
- Karakaidos, P.; Kryou, C.; Simigdala, N.; Klinakis, A.; Zergioti, I. Laser Bioprinting of Cells Using UV and Visible Wavelengths: A Comparative DNA Damage Study. Bioengineering 2022, 9, 378. [Google Scholar] [CrossRef]
- Gruene, M.; Unger, C.; Koch, L.; Deiwick, A.; Chichkov, B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed. Eng. OnLine 2011, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Rémy, M.; Bellance, S.; et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef]
- Guillemot, F.; Guillotin, B.; Fontaine, A.; Ali, M.; Catros, S.; Kériquel, V.; Fricain, J.-C.; Rémy, M.; Bareille, R.; Amédée-Vilamitjana, J. Laser-assisted bioprinting to deal with tissue complexity in regenerative medicine. MRS Bull. 2011, 36, 1015–1019. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser bioprinting of human induced pluripotent stem cells—The effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Laser-Functionalized Aptamer-Based Photonic Biosensors. Available online: https://www.spie.org/news/6410-laser-functionalized-aptamer-based-photonic-biosensors (accessed on 22 September 2025).
- Chatzipetrou, M.; Tsekenis, G.; Tsouti, V.; Chatzandroulis, S.; Zergioti, I. Biosensors by means of the laser induced forward transfer technique. Appl. Surf. Sci. 2013, 278, 250–254. [Google Scholar] [CrossRef]
- MacAdam, A.; Chaudry, E.; McTiernan, C.D.; Cortes, D.; Suuronen, E.J.; Alarcon, E.I. Development of in situ bioprinting: A mini review. Front. Bioeng. Biotechnol. 2022, 10, 940896. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.L.; Sklare, S.C.; Bowser, D.A.; Saksena, J.; Moore, M.J.; Chrisey, D.B. Isolated node engineering of neuronal systems using laser direct write. Biofabrication 2016, 8, 015013. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and Proliferative Ability of Various Living Cell Types after Laser-Induced Forward Transfer. Tissue Eng. 2005, 11, 1817–1823. [Google Scholar] [CrossRef]
- Kim, M.H.; Singh, Y.P.; Celik, N.; Yeo, M.; Rizk, E.; Hayes, D.J.; Ozbolat, I.T. High-throughput bioprinting of spheroids for scalable tissue fabrication. Nat. Commun. 2024, 15, 10083. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Morenza, J.L. Novel laser printing technique for miniaturized biosensors preparation. Sens. Actuators B Chem. 2010, 145, 596–600. [Google Scholar] [CrossRef]
- Koch, L.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser-assisted bioprinting at different wavelengths and pulse durations with a metal dynamic release layer: A parametric study. Int. J. Bioprinting 2017, 3, 001. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.; Li, Z.; Wang, Z.; Yan, X. Powder-Based 3D Printed Porous Structure and Its Application as Bone Scaffold. Front. Mater. 2020, 7, 150. [Google Scholar] [CrossRef]
- Wu, G.-H.; Hsu, S. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef]
- Yao, H.; Zhu, W.; Zhu, X.; Yi, X.; Yao, J.; Yuan, X.; Chen, F.; Han, X. Development of Hydroxyapatite/Polycaprolactone Composite Biomaterials for Laser Powder Bed Fusion: Evaluation of Powder Characteristics, Mechanical Properties and Biocompatibility. Polymers 2024, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Staufer, U.; Accardo, A. Engineered 3D Polymer and Hydrogel Microenvironments for Cell Culture Applications. Bioengineering 2019, 6, 113. [Google Scholar] [CrossRef]
- Yehia, H.M.; Hamada, A.; Sebaey, T.A.; Abd-Elaziem, W. Selective Laser Sintering of Polymers: Process Parameters, Machine Learning Approaches, and Future Directions. J. Manuf. Mater. Process. 2024, 8, 197. [Google Scholar] [CrossRef]
- Wiria, F.E.; Chua, C.K.; Leong, K.F.; Quah, Z.Y.; Chandrasekaran, M.; Lee, M.W. Improved biocomposite development of poly(vinyl alcohol) and hydroxyapatite for tissue engineering scaffold fabrication using selective laser sintering. J. Mater. Sci. Mater. Med. 2008, 19, 989–996. [Google Scholar] [CrossRef]
- Hsieh, Y.-K.; Chen, S.-C.; Huang, W.-L.; Hsu, K.-P.; Gorday, K.A.V.; Wang, T.; Wang, J. Direct Micromachining of Microfluidic Channels on Biodegradable Materials Using Laser Ablation. Polymers 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Shyu, V.B.-H.; Chen, J.-P.; Lee, M.-Y. Selective laser sintered poly-ε-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication 2014, 6, 015004. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Tsai, W.-W.; Chen, H.-J.; Chen, J.-P.; Chen, C.-H.; Yeh, W.-L.; An, J. Laser sintered porous polycaprolacone scaffolds loaded with hyaluronic acid and gelatin-grafted thermoresponsive hydrogel for cartilage tissue engineering. Bio-Medical Mater. Eng. 2013, 23, 533–543. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Mercelis, P.; van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding Mechanisms in Selective Laser Sintering and Selective Laser Melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Pande, S.; Agrawal, S.; Bobade, S.M. Selection of selective laser sintering materials for different applications. Rapid Prototyp. J. 2015, 21, 630–648. [Google Scholar] [CrossRef]
- Saghir, S.; Imenes, K.; Schiavone, G. Integration of hydrogels in microfabrication processes for bioelectronic medicine: Progress and outlook. Front. Bioeng. Biotechnol. 2023, 11, 1150147. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Mirmalek-Sani, S.H.; Hanley, N.A.; Ivanov, A.L.; Barry, J.J.; Upton, C.; Shakesheff, K.M.; Howdle, S.M.; Antonov, E.N.; Bagratashvili, V.N.; et al. Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomater. 2009, 5, 2063–2071. [Google Scholar] [CrossRef]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. Laser Photoablation of Guidance Microchannels into Hydrogels Directs Cell Growth in Three Dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, Y.; Zheng, Z.; Khan, M.T.A.; Lyu, X.; Byun, J.; Giessen, H.; Sitti, M. Micro- and nanofabrication of dynamic hydrogels with multichannel information. Nat. Commun. 2023, 14, 8208. [Google Scholar] [CrossRef] [PubMed]
- Aravind, M.; Saxena, A.; Mishra, D.; Singh, K.; George, S.D. Microfluidic contact lens: Fabrication approaches and applications. Microsyst. Nanoeng. 2025, 11, 59. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.F. Advanced Laser Techniques for the Development of Nature-Inspired Biomimetic Surfaces Applied in the Medical Field. Coatings 2024, 14, 1290. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Scudder, L.L.; Halvorson, R.T.; Tresback, J.; Ferrier, J.P.; Sheehy, S.P.; Cho, A.; Kannan, S.; Sunyovszki, I.; Goss, J.A.; et al. Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications. Biofabrication 2018, 10, 025004. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019, 97, 141–153. [Google Scholar] [CrossRef]
- Ma, J.; Wu, J.; Lin, Z.; Wang, J.; Yao, W.; Zhang, Y.; Zhang, X.; Zhu, L.; Hayasaki, Y.; Zhang, H. Femtosecond-Laser Preparation of Hydrogel with Micro/Nano-Structures and their Biomedical Applications. Small Sci. 2025, 5, 2400400. [Google Scholar] [CrossRef]
- Won, D.; Kim, J.; Choi, J.; Kim, H.; Han, S.; Ha, I.; Bang, J.; Kim, K.K.; Lee, Y.; Kim, T.-S.; et al. Digital selective transformation and patterning of highly conductive hydrogel bioelectronics by laser-induced phase separation. Sci. Adv. 2022, 8, eabo3209. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, D.H.; Yoon, J.K.; Park, D.B.; Kim, H.S.; Shin, Y.M.; Baek, W.; Kang, M.L.; Kim, H.J.; Sung, H.J. Microchannel network hydrogel induced ischemic blood perfusion connection. Nat. Commun. 2020, 11, 615. [Google Scholar] [CrossRef]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef]
- Song, J.; Michas, C.; Chen, C.S.; White, A.E.; Grinstaff, M.W. From Simple to Architecturally Complex Hydrogel Scaffolds for Cell and Tissue Engineering Applications: Opportunities Presented by Two-Photon Polymerization. Adv. Healthc. Mater. 2020, 9, e1901217. [Google Scholar] [CrossRef] [PubMed]
- Menges, J.; Klingel, S.; Oesterschulze, E.; Bart, H.-J. Exploiting Direct Laser Writing for Hydrogel Integration into Fragile Microelectromechanical Systems. Sensors 2019, 19, 2494. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Spargo, B.J.; Ringeisen, B.R. Biological laser printing of three dimensional cellular structures. Appl. Phys. Mater. Sci. Process. 2004, 79, 1027–1030. [Google Scholar] [CrossRef]
- Colina, M.; Serra, P.; Fernández-Pradas, J.M.; Sevilla, L.; Morenza, J.L. DNA deposition through laser induced forward transfer. Biosens. Bioelectron. 2005, 20, 1638–1642. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- FDA Approves Collagen-Based DermiSphere Hydrogel. Dermatology Times. Available online: https://www.dermatologytimes.com/view/fda-approves-collagen-based-dermisphere-hydrogel (accessed on 22 September 2025).
- Kim, E.H.; Lee, S.H. Efficacy of Cultured Allogenic Keratinocytes in Treatment of Deep Second-Degree Burn. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2021, 42, 533–537. [Google Scholar] [CrossRef]
- KeraHeal-Allo [WWW Document], n.d. Available online: https://www.kobia.kr (accessed on 9 August 2025).
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef]
- Bioprinted Dermo-Epidermal Subsitute-Poieskin®. Cellbox Solutions. Bioprinted Dermo-Epidermal Subsitute-Poieskin®. Cellbox Solutions. Available online: https://cellbox-solutions.com/usecases/bioprinted-dermo-epidermal-subsitute-poieskin (accessed on 22 September 2025).
- Burnshield Dressing 200 mm × 200 mm (8″ × 8″)-Emergency Burncare. [Online]. Available online: https://www.burnshield.com/product/burnshield-dressing-200mm-x-200mm/ (accessed on 22 September 2025).
- Convatec-Pioneering Trusted Medical Solutions to Improve the Lives We Touch. Available online: https://www.convatec.com/en-gb/ (accessed on 22 September 2025).
- Coloplast UK. Developing Products and Services That Make Life Easier for People with Intimate Healthcare Needs. Available online: https://www.coloplast.co.uk/ (accessed on 22 September 2025).
- The Only FDA-Approved Vaginal Insert. Available online: https://bit.ly/2rpVwqE (accessed on 22 September 2025).
- Home. Endo. Available online: https://www.endo.com/ (accessed on 22 September 2025).
- Loh, J.M.; Lim, Y.J.L.; Tay, J.T.; Cheng, H.M.; Tey, H.L.; Liang, K. Design and fabrication of customizable microneedles enabled by 3D printing for biomedical applications. Bioact. Mater. 2024, 32, 222–241. [Google Scholar] [CrossRef]
- Khiari, Z. Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting. Mar. Drugs 2024, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Shorakaie, A.; Nahvifard, E.; Shirkavand, A.; Fashtami, L.A.; Mohajerani, E. CO2 Fractional Laser Induced Skin Micro-Tunnel Thermal Damage Patterns: A Simulation Study. J. Lasers Med. Sci. 2024, 15, e63. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D bioprinting in tissue engineering and regenerative medicine: Current progress and challenges. Int. J. Bioprinting 2023, 9, 759. [Google Scholar] [CrossRef]
- Gehre, C.; Qiu, W.; Jäger, P.K.; Wang, X.; Correira Marques, F.; Nelson, B.; Müller, R.; Qin, X.-H. Guiding bone cell network formation in 3D via photosensitized two-photon ablation. Acta Biomater. 2024, 174, 141–152. [Google Scholar] [CrossRef]
- Loterie, D.; Delrot, P.; Moser, C. High-resolution tomographic volumetric additive manufacturing. Nat. Commun. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Regehly, M.; Garmshausen, Y.; Reuter, M.; König, N.F.; Israel, E.; Kelly, D.P.; Chou, C.-Y.; Koch, K.; Asfari, B.; Hecht, S. Xolography for linear volumetric 3D printing. Nature 2020, 588, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. Deerfield Beach Fla 2019, 31, e1904209. [Google Scholar] [CrossRef]


| Hydrogel Type | Advantages | Disadvantages | References |
|---|---|---|---|
| Natural hydrogels |
|
| [6,7,8,9,10,11,12] |
| Synthetic hydrogels |
|
| [6,7,8,9,10,11,12] |
| Hybrid hydrogels |
|
| [6,7,8,9,10,11,12] |
| Hydrogel Type | Relevant Properties and Characteristics | Young’s Modulus (Elasticity Modulus) Range | Associated Tissue Mimicry | Key References |
|---|---|---|---|---|
| Chemically Crosslinked Hydrogels (e.g., PEGDA, Alginate) | Tunable stiffness and mechanical resistance via crosslink density, polymer concentration, and molecular weight. Softer hydrogels are suitable for soft tissue, while more rigid ones can support hard tissue. | ~0.1 kPa to >100 kPa (for soft tissues); can reach into the low MPa range for hard tissue mimics. | General soft (e.g., brain, fat, muscle) and hard tissues (e.g., bone). | [25,26] |
| Photo-crosslinked Gelatin Methacrylate (GelMA) | Excellent bioactivity (contains cell-adhesive RGD motifs). Supports chondrocytes and new ECM production. Stiffness is tuned by UV exposure time, photoinitiator concentration, and methacrylation degree. | ~1 kPa to 100 kPa is most common for cell culture. The statement “a few MPa” is high but possible with very high polymer concentration and crosslinking, though it exceeds typical values for cartilage. | Cartilage (modulus ~0.1–1 MPa), but also widely used for various soft tissues like blood vessels and muscle. | [27,28] |
| Double Network (DN) Hydrogels | Comprise two interpenetrating polymer networks: a rigid, brittle first network and a soft, ductile second network. This structure dissipates energy under load, leading to high toughness and fracture resistance. | Compressive strength can indeed reach tens of MPa (e.g., 10–40 MPa). Elastic modulus typically ranges from ~0.1 MPa to 1.0 MPa, which is a direct match for native cartilage. | Cartilage tissue (articular cartilage modulus: ~0.2–1.0 MPa in compression). Excellent mimic due to high water content and toughness. | [29] |
| Polymer–Ceramic Composites (e.g., GelMA-HAp, PEG-HAp) | Created by mixing polymers with inorganic materials like hydroxyapatite (HAp) or calcium carbonate to increase rigidity and provide osteoinductive signals for bone tissue engineering. | Wide range: 10 MPa to 2 GPa+. The stiffness depends heavily on the ceramic content (e.g., from 10% to 70% by weight). The trade-off with water absorption is important, as ceramics are hydrophilic but not swellable. | Hard tissues, such as bone (cortical bone modulus: ~15–20 GPa; trabecular bone modulus: ~0.1–2 GPa). Composites aim to approach the lower end of this range. | [30] |
| Porous PVA Hydrogels (via SLS) | Created via selective laser sintering (SLS), a 3D printing technique that creates a periodic-porous structure. This porosity is critical for nutrient waste exchange and cell migration. PVA is biocompatible and supports osteoblastic cell adhesion. | The modulus is not specified in your text, which is common, as it varies greatly with porosity and structure. However, for bone applications, targets are typically in the MPa to low GPa range. The key property is often the compressive strength and the scaffold’s architecture. | Bone tissue, specifically for creating scaffolds that mimic the microporosity and mechanical function of trabecular bone. | [31,32] |
| Method | Principles |
|---|---|
| Two-photon polymerization | It uses two-photon absorption of near-infrared radiation to induce polymerization in a photosensitive material only at the laser’s focal point. This allows for direct 3D writing without a mask and at a sub-diffraction limit resolution. |
| Laser-induced forward transfer | A pulsed laser is focused on an absorbing layer, creating a pressure wave that propels a droplet of a donor material (bio-ink) toward a collector substrate. It is a nozzle-free, direct deposition technique. |
| Selective laser sintering/melting | A high-power laser selectively fuses fine powder particles, layer by layer, to create a 3D structure. The heat consolidates the powder physically without chemical additives. |
| Laser ablation | A focused laser is used to precisely remove material from a hydrogel with high precision, creating patterns, grooves, or internal channels. |
| Parameter | Summary | References |
|---|---|---|
| Resolution |
| [56,57] |
| Speed |
| [58,59] |
| Suitable Materials |
| [28,60] |
| Cell Compatibility |
| [61,62] |
| Key Advantages/Limitations |
| [56] |
| Parameter | Summary | References |
|---|---|---|
| Resolution |
| [68] |
| Speed |
| [81] |
| Suitable Materials |
| [68,82] |
| Cell Compatibility |
| [82,83] |
| Key Advantages/Limitations |
| [68,82] |
| Parameter | Summary | References |
|---|---|---|
| Resolution |
| [96,97] |
| Speed |
| [97,98] |
| Suitable Materials |
| [97,99] |
| Cell Compatibility |
| [96,100] |
| Key Advantages/Limitations |
| [96,97] |
| Parameter | Summary | References |
|---|---|---|
| Resolution |
| [113] |
| Speed |
| [114] |
| Suitable Materials |
| [113,115] |
| Cell Compatibility |
| [113,115] |
| Key Advantages/Limitations |
| [113,114] |
| Technique | Primary Limitations | Key References |
|---|---|---|
| Two-Photon Polymerization (2PP) |
| [56,58,131] |
| Laser-Induced Forward Transfer (LIFT) |
| [96,97] |
| Selective Laser Sintering (SLS) |
| [31,96,97] |
| Laser Direct Writing (LDW) |
| [113,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoliu, D.S.; Zagar, C.; Negut, I.; Visan, A.I. Laser-Based Fabrication of Hydrogel Scaffolds for Medicine: From Principles to Clinical Applications. Gels 2025, 11, 811. https://doi.org/10.3390/gels11100811
Manoliu DS, Zagar C, Negut I, Visan AI. Laser-Based Fabrication of Hydrogel Scaffolds for Medicine: From Principles to Clinical Applications. Gels. 2025; 11(10):811. https://doi.org/10.3390/gels11100811
Chicago/Turabian StyleManoliu, Dan Stefan, Cristian Zagar, Irina Negut, and Anita Ioana Visan. 2025. "Laser-Based Fabrication of Hydrogel Scaffolds for Medicine: From Principles to Clinical Applications" Gels 11, no. 10: 811. https://doi.org/10.3390/gels11100811
APA StyleManoliu, D. S., Zagar, C., Negut, I., & Visan, A. I. (2025). Laser-Based Fabrication of Hydrogel Scaffolds for Medicine: From Principles to Clinical Applications. Gels, 11(10), 811. https://doi.org/10.3390/gels11100811








