Understanding the Colloidal and Hydration Control in Rheological Evolution of 3D Printed MgO-SiO2-K2HPO4 Gel System
Abstract
1. Introduction
2. Results and Discussion
2.1. Setting Time Results
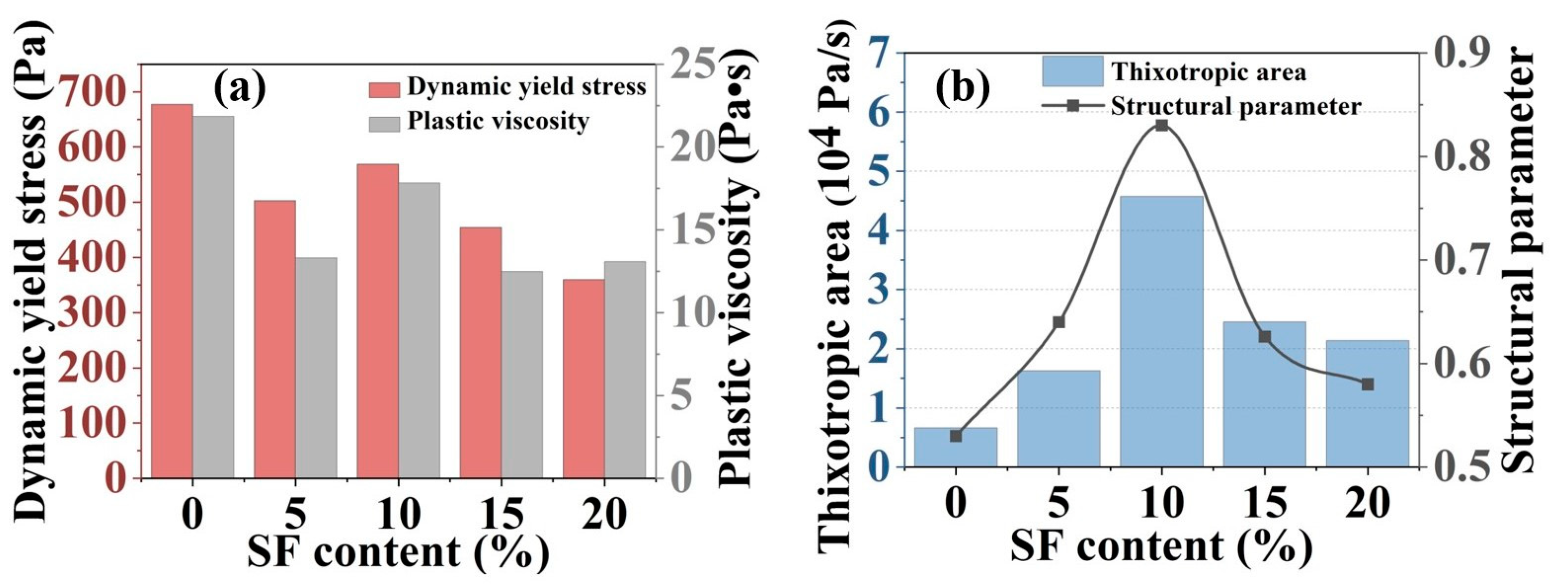
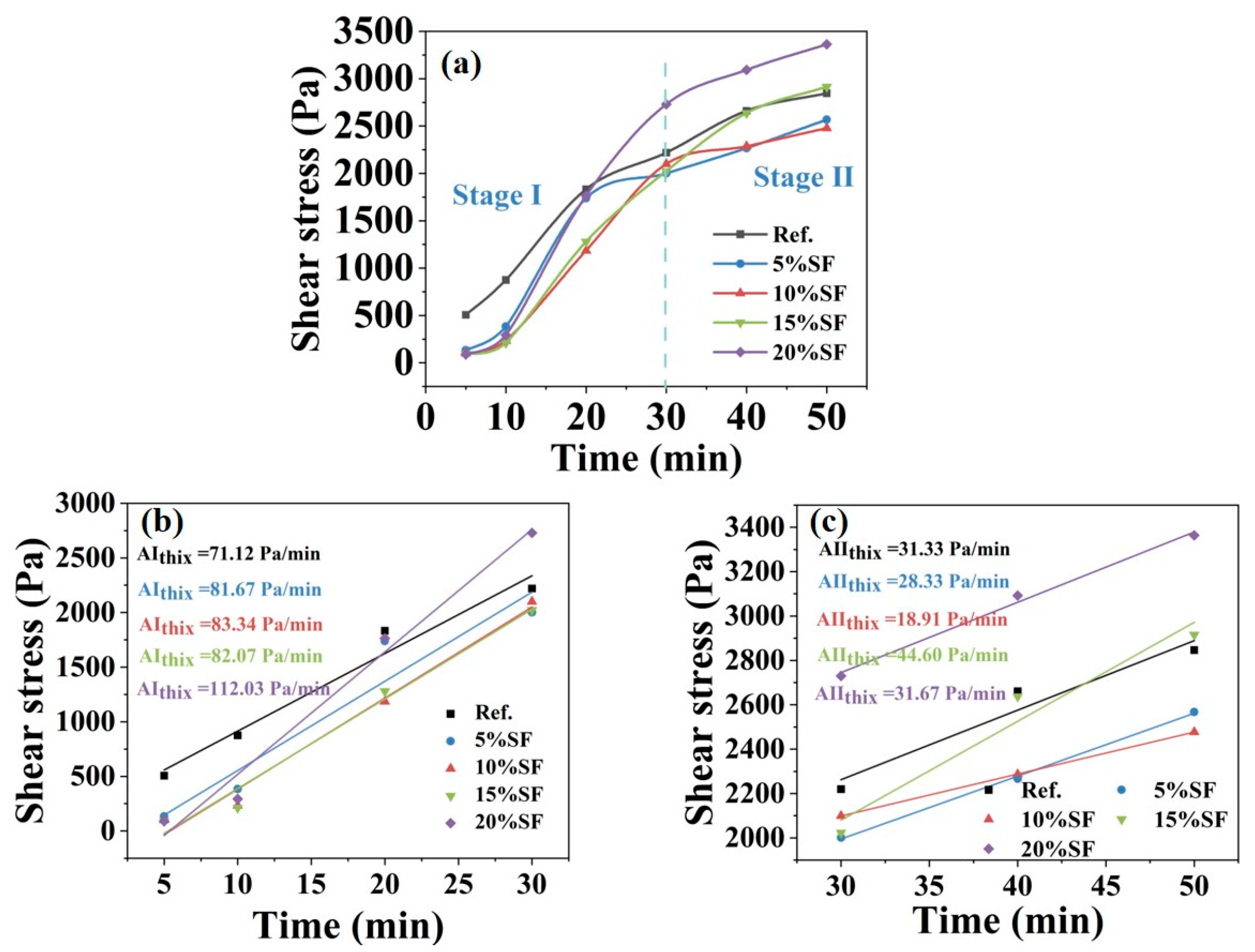
2.2. Rheological Property Results
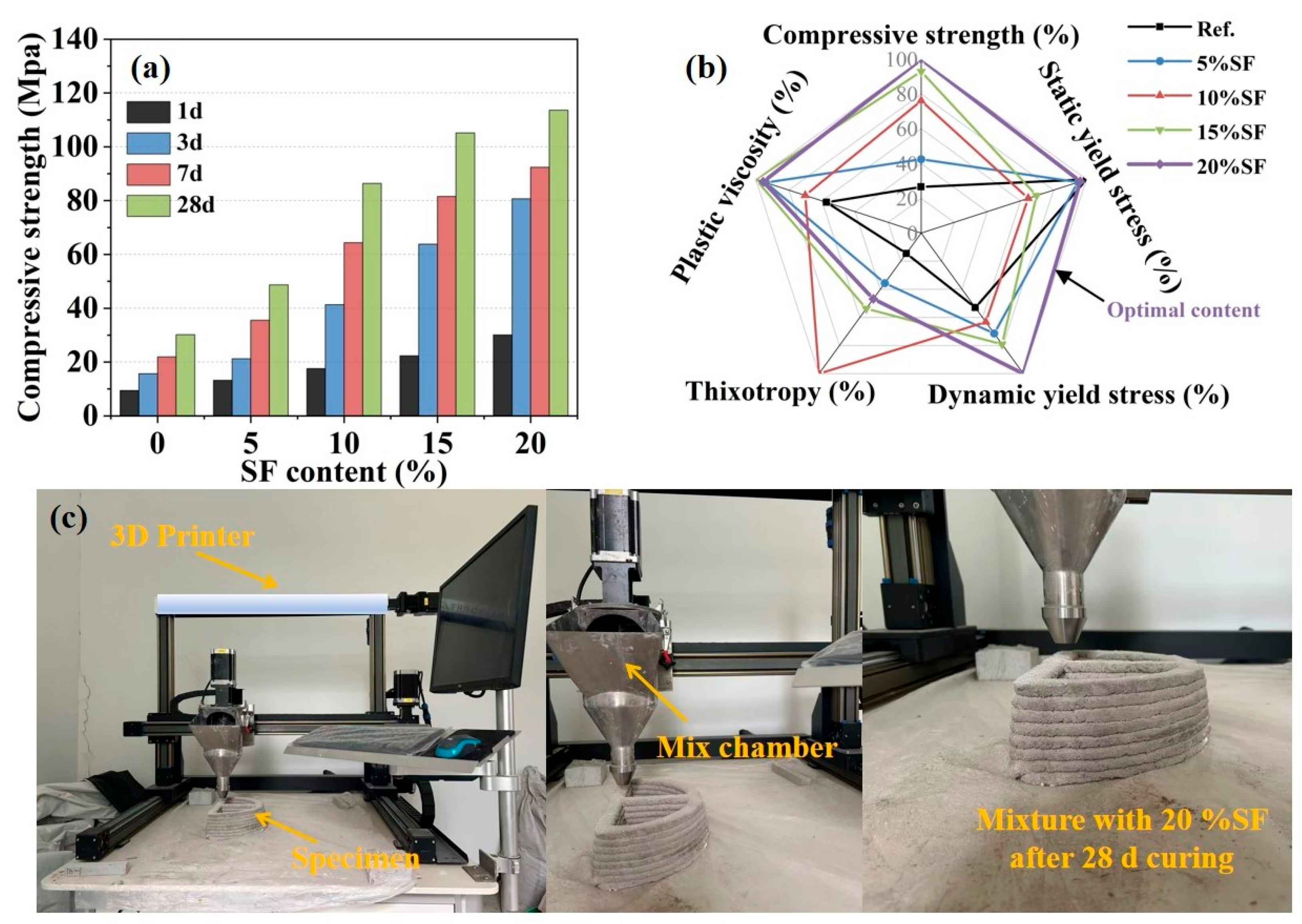
2.3. Mechanical Property and 3D Printing Results
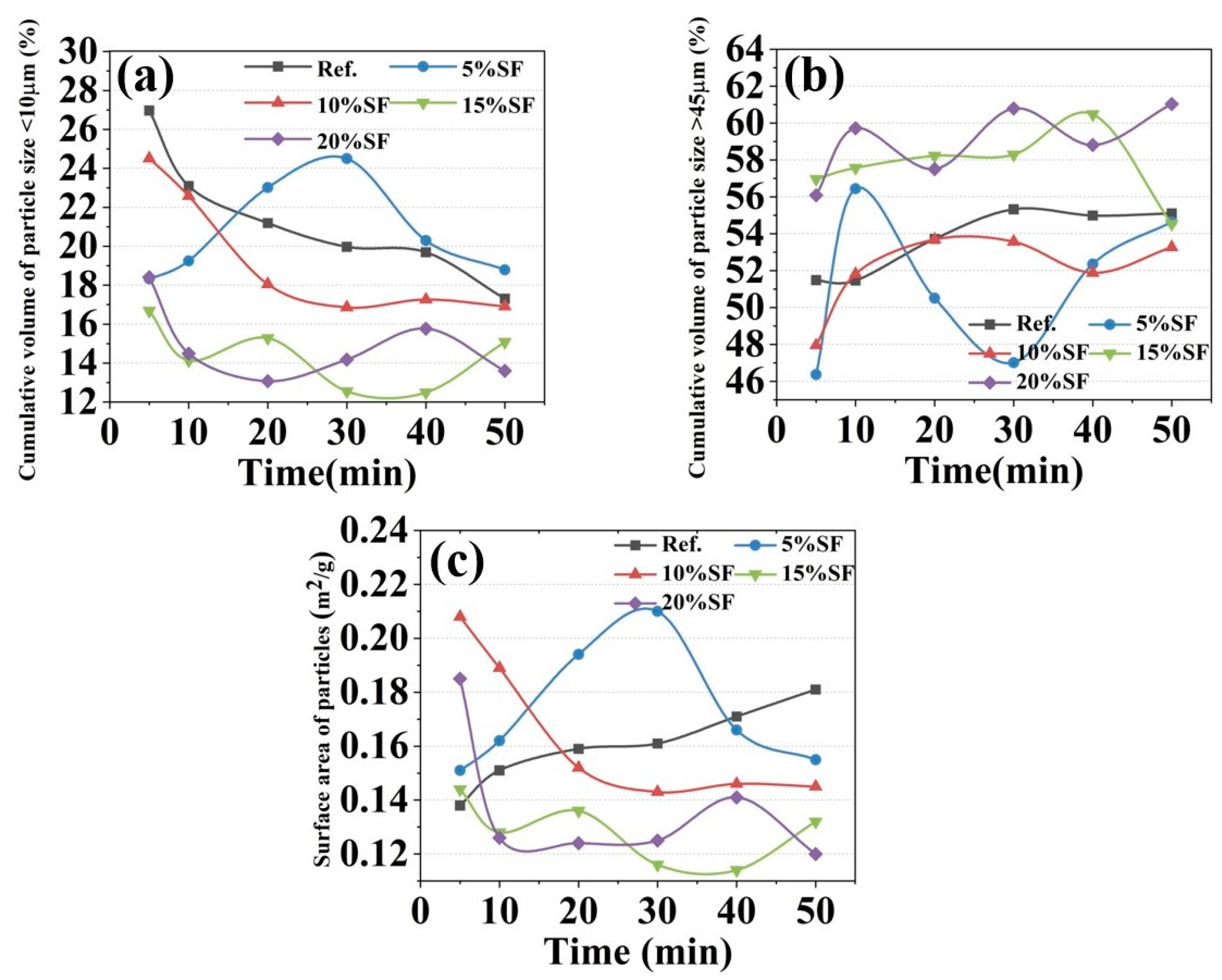
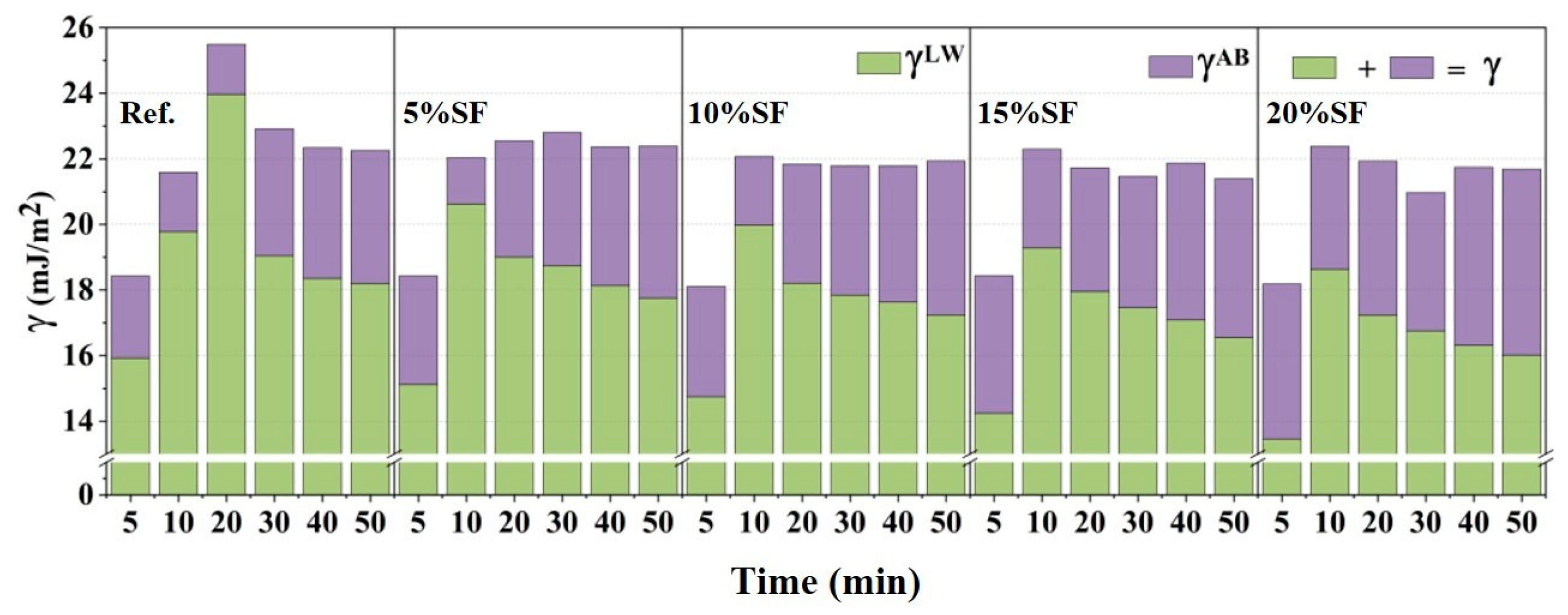
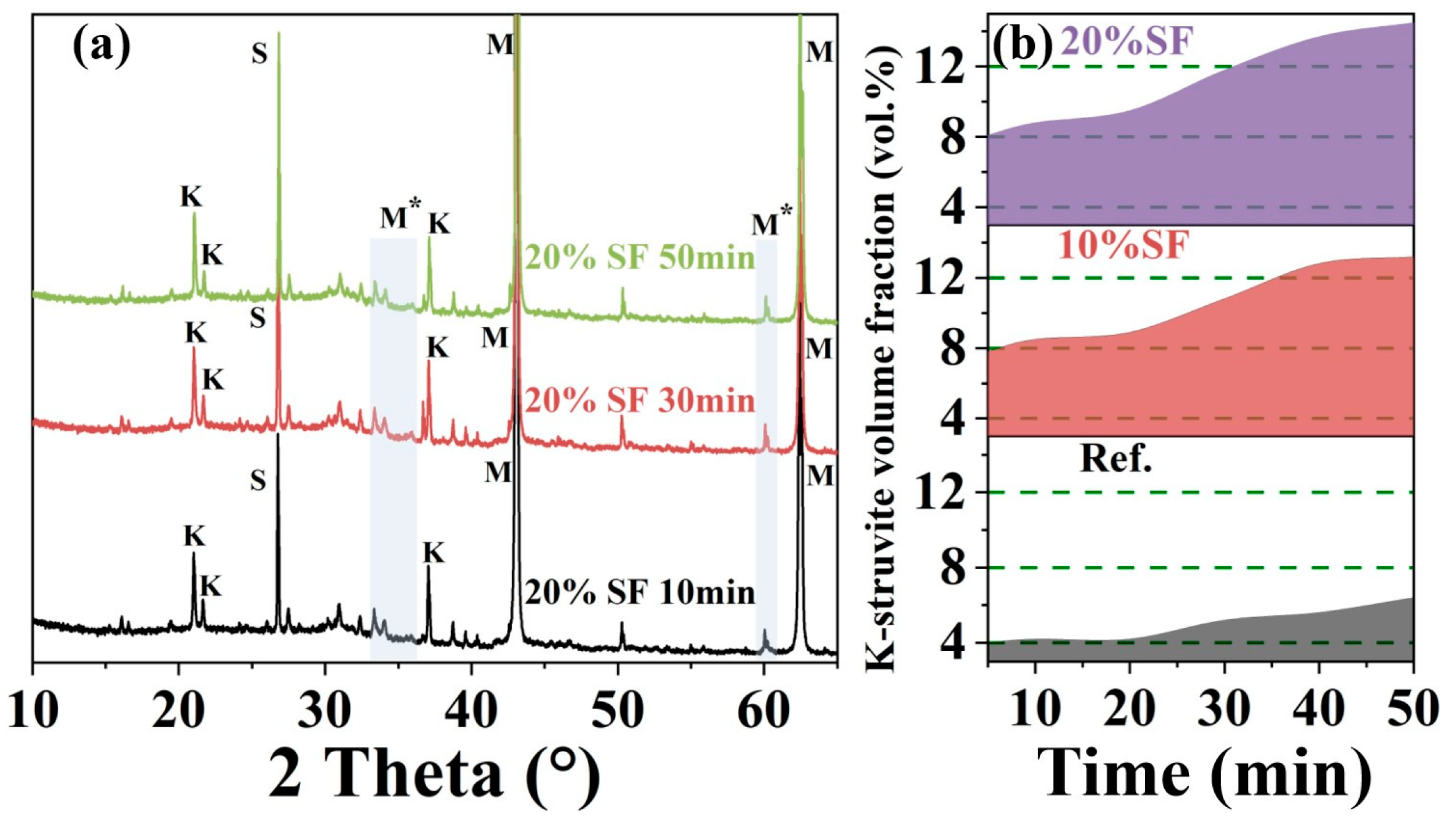
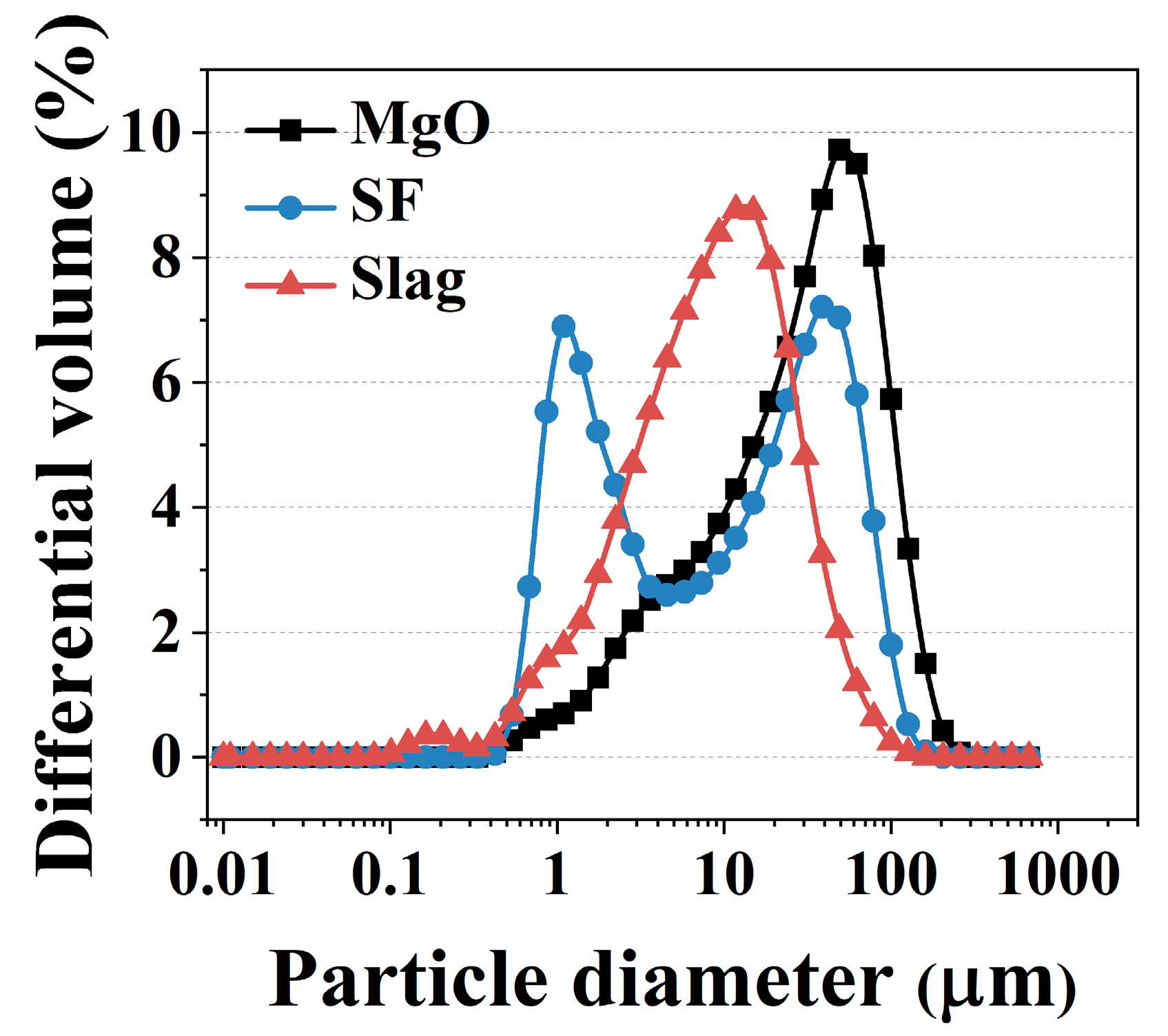
2.4. Particle Physical Properties Results
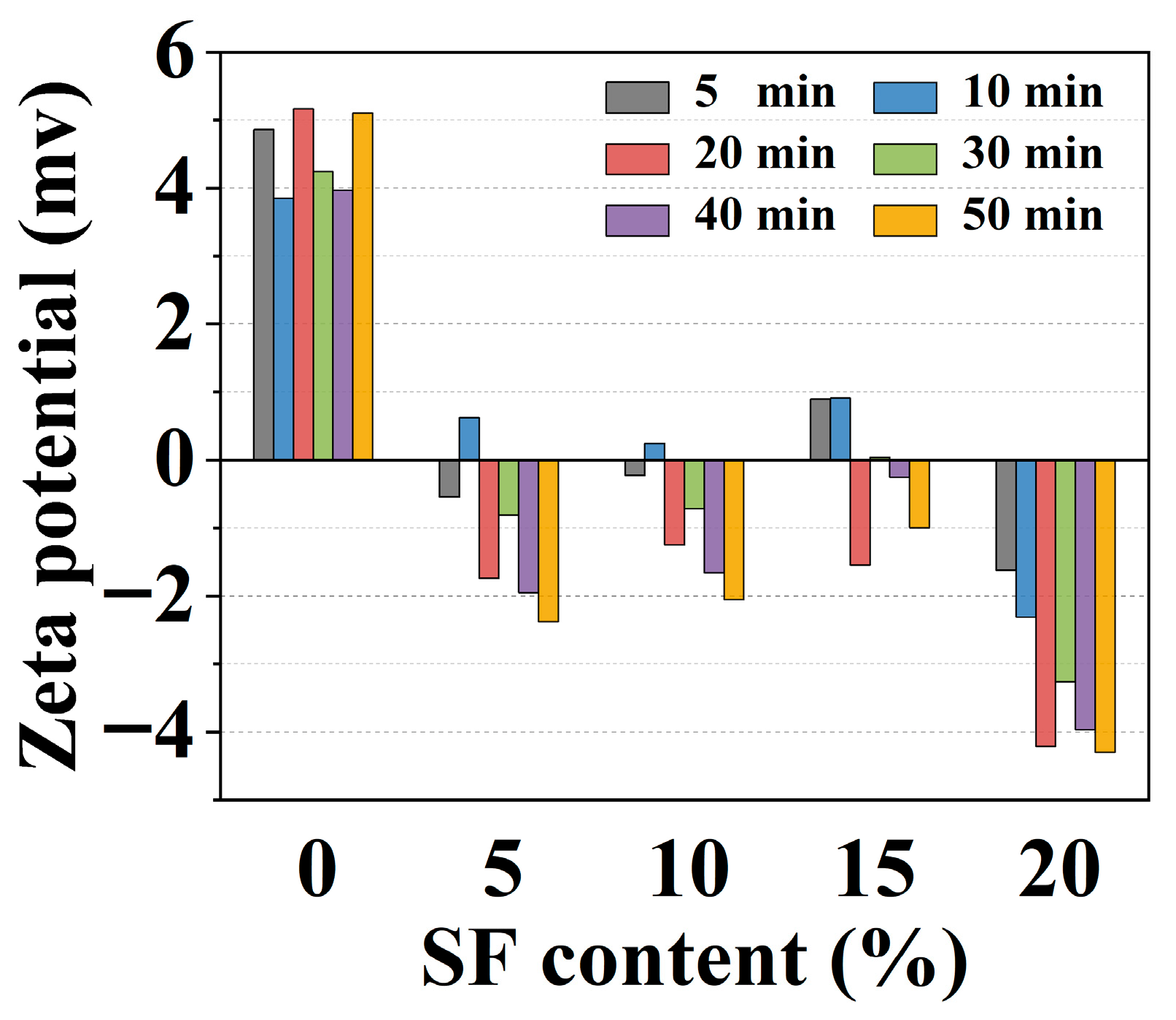
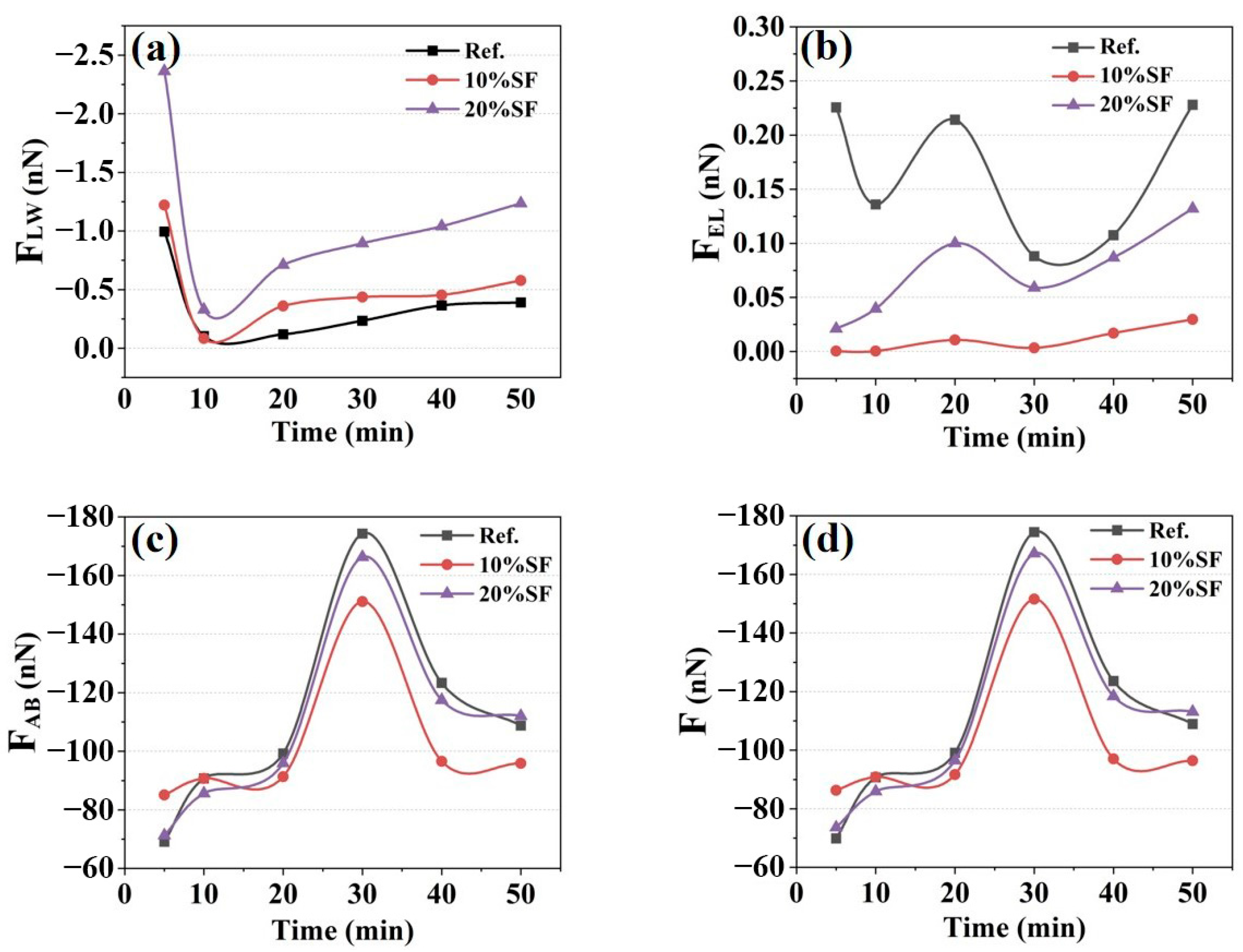
2.5. Electrodynamic Parameter Results
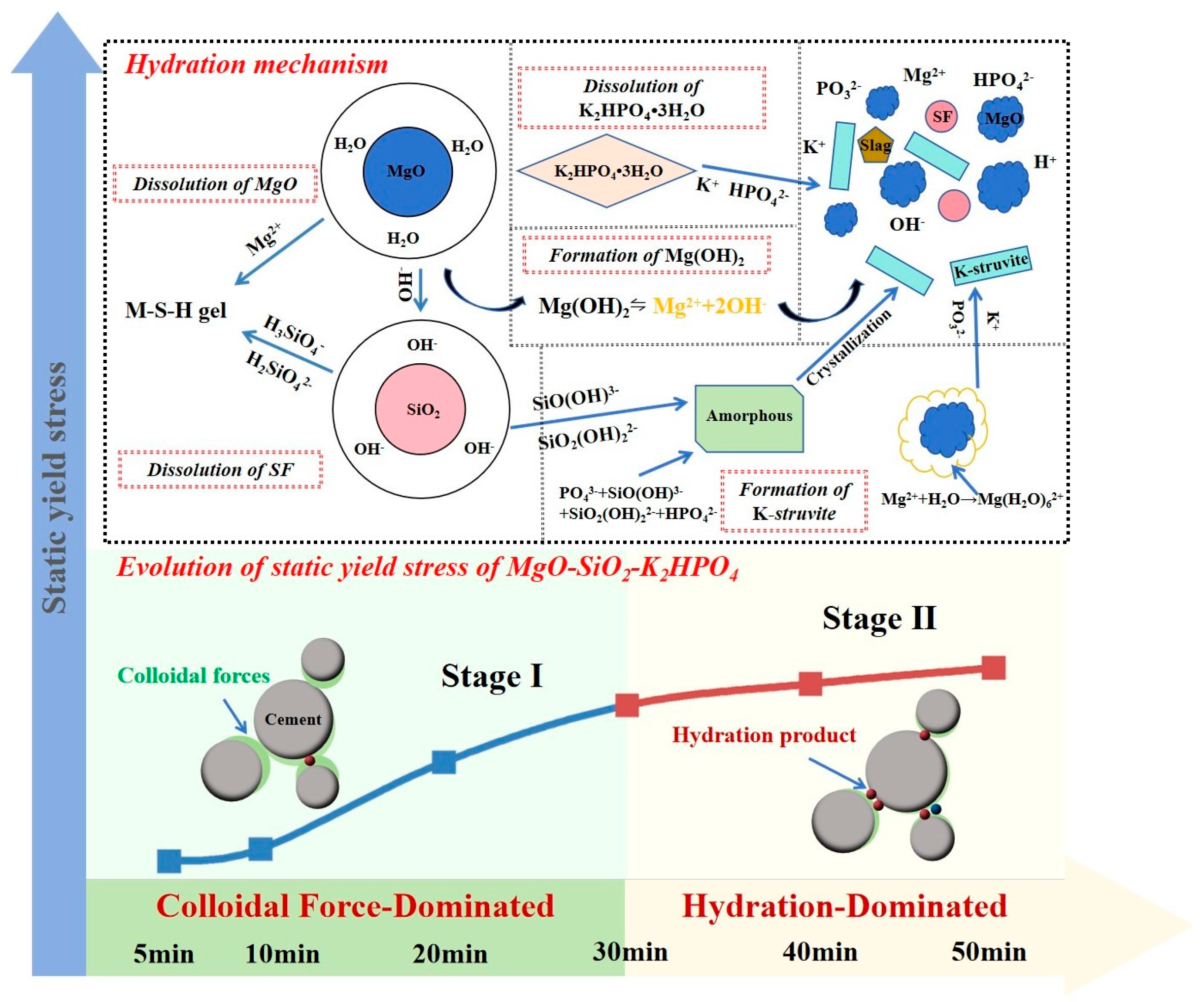
2.6. Discussion
3. Conclusions
- (1)
- The MgO-SiO2-K2HPO4 system with 20% SF achieved synergistic rheo-mechanical balance without triggering excessively rapid setting time, resolving the trade-off between early-stage rheological properties and long-term strength, demonstrating superior applicability for 3D printing.
- (2)
- The evolution of static yield stress within 50 min consisted of two stages, which corresponded to rapid linear growth in 0–30 min (Stage I) and slow increased plateau in 30–50 min (Stage II). Colloidal forces driven by EDLVO interactions dominated Stage I, while hydration network strength controlled Stage II by K-struvite crystallization.
- (3)
- The main driving force of increased static yield stress in Stage II was related to crystallization pathways of K-struvite, including direct precipitation from soluble Mg2+, K+, PO43− coordination and non-classical crystallization of transformation from the potassium-deficient phosphate amorphous phase.
- (4)
- The incorporation of SF influenced static yield stress through enhancing the colloidal forces by M-S-H gel formation which altered interparticle forces and system stability, and increased hydration network strength by accelerating hydration.
4. Materials and Methods
4.1. Raw Materials and Sample Preparation
4.2. Rheological Property Tests
4.3. Setting Time Tests
4.4. Sample Preparation
4.5. Particle Properties Tests
4.6. Electrodynamic Parameter Tests
4.7. Microstructure Characterization
4.8. Interparticle Forces Calculation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, Y.; Li, M.; Tan, M.J.; Qian, S. Design 3D printing cementitious materials via Fuller Thompson theory and Marson-Percy model. Constr. Build. Mater. 2018, 163, 600–610. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Dos Anjos, M.A.S.; Cabral, K.C.; Dias, L.S. Development of composites for 3D printing with reduced cement consumption. Constr. Build. Mater. 2022, 341, 127775. [Google Scholar] [CrossRef]
- Tay, Y.W.D.; Qian, Y.; Tan, M.J. Printability region for 3D concrete printing using slump and slump flow test. Compos. Part B Eng. 2019, 174, 106968. [Google Scholar] [CrossRef]
- Roussel, N. Rheological requirements for printable concretes. Cem. Concr. Res. 2018, 112, 76–85. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Yuan, L.; Zhao, P.; Li, Q.; Lu, L.; Wang, L. Use of creep and recovery protocol to assess the printability of fibre-reinforced 3D printed white Portland cement composites. Virtual Phys. Prototyp. 2024, 19, e2331201. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; Zhong, X.; Huang, Y.; Yang, L.; Zhao, P.; Wang, S.; Lu, L.; Cheng, X. Effects of bentonite, diatomite and metakaolin on the rheological behavior of 3D printed magnesium potassium phosphate cement composites. Addit. Manuf. 2021, 46, 102184. [Google Scholar] [CrossRef]
- Yang, J.; Qian, C. Effect of borax on hydration and hardening properties of magnesium and pottassium phosphate cement pastes. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 613–618. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Chen, B. Experimental Study of Dipotassium Hydrogen Phosphate Influencing Properties of Magnesium Phosphate Cement. J. Mater. Civ. Eng. 2016, 28, 04015170. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Kang, A.; Cai, X.; Chen, M.; Lu, L. Rheology and buildability of sustainable 3D printed magnesium potassium phosphate cement composites incorporating MgO-SiO2-K2HPO4. Constr. Build. Mater. 2024, 446, 138048. [Google Scholar] [CrossRef]
- Xu, X.; Lin, X.; Pan, X.; Ji, T.; Liang, Y.; Zhang, H. Influence of silica fume on the setting time and mechanical properties of a new magnesium phosphate cement. Constr. Build. Mater. 2020, 235, 117544. [Google Scholar] [CrossRef]
- Lin, C.; Lin, X.; Li, X.; Lin, X. Properties of MgO–SiO2–K2HPO4 cement modified with ground blast furnace slag. Ceram. Int. 2022, 48, 23618–23629. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Investigation of the properties of alkali-activated slag mixes involving the use of nanoclay and nucleation seeds for 3D printing. Compos. Part B Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Zhang, C.; Nerella, V.N.; Krishna, A.; Wang, S.; Zhang, Y.; Mechtcherine, V.; Banthia, N. Mix design concepts for 3D printable concrete: A review. Cem. Concr. Compos. 2021, 122, 104155. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Yao, P.; Lin, X.; Wu, Y.; Ji, T.; Liang, Y. Influence of slag on water resistance of magnesium silicon potassium phosphate cement. J. Build. Eng. 2024, 97, 110862. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Zhou, S.; Chen, E.; Tang, S. Hydration, mechanical property and C-S-H structure of early-strength low-heat cement-based materials. Mater. Lett. 2018, 217, 151–154. [Google Scholar] [CrossRef]
- Kanchanason, V.; Plank, J. Role of pH on the structure, composition and morphology of C-S-H–PCE nanocomposites and their effect on early strength development of Portland cement. Cem. Concr. Res. 2017, 102, 90–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Z.; Shi, J.; Jiang, Y.; Banthia, N.; Zhang, Y. Clarifying and quantifying the driving force for the evolution of static yield stress of cement pastes. Cem. Concr. Res. 2023, 167, 107129. [Google Scholar] [CrossRef]
- Roussel, N.; Bessaies-Bey, H.; Kawashima, S.; Marchon, D.; Vasilic, K.; Wolfs, R. Recent advances on yield stress and elasticity of fresh cement-based materials. Cem. Concr. Res. 2019, 124, 105798. [Google Scholar] [CrossRef]
- Flatt, R.J. Dispersion forces in cement suspensions. Cem. Concr. Res. 2004, 34, 399–408. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, X.; Pan, X.; Ji, T. Preliminary investigation of the hydration mechanism of MgO-SiO2-K2HPO4 cement. Constr. Build. Mater. 2020, 235, 117471. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Chen, Z.; Liu, Z.; Li, J.; Lu, Z.; Lai, Z. Influence of Mg(OH)2 on the Hydration Process and Properties of Magnesium Phosphate Cement. Mater. Rep. 2024, 38, 77–83. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; Xu, J.; Li, L.; Huang, Y.; Yang, L.; Zhao, P.; Lu, L. Mix design and rheological properties of magnesium potassium phosphate cement composites based on the 3D printing extrusion system. Constr. Build. Mater. 2021, 284, 122797. [Google Scholar] [CrossRef]
- Li, T.; Xu, X.; Yang, J.; Hu, X.; Xue, J.; He, Y.; Tang, Y. Effect of fly ash on the rheological properties of potassium magnesium phosphate cement paste. Case Stud. Constr. Mater. 2022, 17, e01650. [Google Scholar] [CrossRef]
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Ye, H.; Gao, X.; Wang, R.; Wang, H. Relationship among particle characteristic, water film thickness and flowability of fresh paste containing different mineral admixtures. Constr. Build. Mater. 2017, 153, 193–201. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Taylor, P.; Morcous, G. Assessing particle packing based self-consolidating concrete mix design method. Constr. Build. Mater. 2014, 70, 439–452. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. Research progresses on magnesium phosphate cement: A review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Lin, W.; Sun, W.; Li, Z. Study on the effects of fly ash in magnesium phosphate cement. Mater. Rep. 2024, 38, 29–35. [Google Scholar] [CrossRef]
- Mao, Y.; Jiao, D.; Hu, X.; Jiang, Z.; Shi, C. Dispersion behavior of silica fume in cementitious suspensions. Cem. Concr. Compos. 2024, 151, 105605. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Tian, Y.; Wang, Y. Determination of dynamic contact angles of cement and mineral admixtures based on thin-layer wicking technique. Dongnan Daxue Xuebao (Ziran Kexue Ban)/J. Southeast Univ. (Nat. Sci. Ed.) 2013, 43, 1074–1079. [Google Scholar] [CrossRef]
- Mehdipour, I.; Razzaghi, M.S.; Amini, K.; Shekarchi, M. Effect of mineral admixtures on fluidity and stability of self-consolidating mortar subjected to prolonged mixing time. Constr. Build. Mater. 2013, 40, 1029–1037. [Google Scholar] [CrossRef]
- Tasci, E.; Yilmaz, B. Charge and surface energy interface among particles in cement and pozzolan suspensions. Adv. Cem. Res. 2014, 26, 213–221. [Google Scholar] [CrossRef]
- Li, Z. Reaction Mechanisms and Application Study of MgO-SiO2-H2O Cementitious System; South China University of Technology: Guangzhou, China, 2015. [Google Scholar]
- Bernard, E. Magnesium Silicate Hydrate (MSH) Characterization: Temperature, Calcium, Aluminium and Alkali. Ph.D. Thesis, Université Bourgogne Franche-Comté, Dijon, France, 2017. [Google Scholar]
- Srinivasan, S.; Barbhuiya, S.A.; Charan, D.; Pandey, S.P. Characterising cement–superplasticiser interaction using zeta potential measurements. Constr. Build. Mater. 2010, 24, 2517–2521. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Yahia, A. Physico-chemical kinetics of structural build-up of neat cement-based suspensions. Cem. Concr. Res. 2017, 97, 11–27. [Google Scholar] [CrossRef]
- Chen, Z. Colloid and Interface Chemistry; Higher Education Press: Beijing, China, 2001. [Google Scholar]
- Siddique, R. Utilization of silica fume in concrete: Review of hardened properties. Resour. Conserv. Recycl. 2011, 55, 923–932. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, B.; He, F.; Zhou, J.; Chen, C.; He, Z.; Wang, L. Understanding hydration process and paste viscosity evolution of magnesium potassium phosphate cement. Cem. Concr. Res. 2023, 169, 107177. [Google Scholar] [CrossRef]
- Ghosh, P. Colloid and Interface Science; PHI Learning Pvt. Ltd.: New Delhi, India, 2009. [Google Scholar]
- Elimelech, M.; Jia, X.; Gregory, J. Particle Deposition and Aggregation Measurement, Modelling, and Simulation; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Zhang, Z.; Jia, Z.; Shi, J.; Xiong, L.; Banthia, N.; Gao, Y.; Zhang, Y. Understanding the dynamic rheological property of cement paste blended with steel slag powder: From interparticle force and physico-chemical parameters of view. Constr. Build. Mater. 2024, 422, 135826. [Google Scholar] [CrossRef]
- Wagh, A.S. Magnesium phosphate ceramics. In Chemically Bonded Phosphate Ceramics; Elsevier Science: Oxford, UK, 2004. [Google Scholar]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of k-struvite formation in magnesium phosphate cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction mechanism of magnesium potassium phosphate cement with high magnesium-to-phosphate ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Sun, H.; Sun, X.; Kang, S.; Lin, X.; Qian, J.; Wu, W. New magnesium cement optimized in MgO-K2HPO4-SiO2 system and its hardening performance. Constr. Build. Mater. 2024, 446, 137942. [Google Scholar] [CrossRef]
- Jiang, S.P.; Mutin, J.C.; Nonat, A. Studies on mechanism and physico-chemical parameters at the origin of the cement setting. I. The fundamental processes involved during the cement setting. Cem. Concr. Res. 1995, 25, 779–789. [Google Scholar] [CrossRef]
- Chibowski, E.; Holysz, L.; Szczes, A. Application of thin-layer wicking method for surface free energy determination. Surf. Innov. 2017, 5, 9–20. [Google Scholar] [CrossRef]
- Neirinck, B.; van Deursen, J.; Van der Biest, O.; Vleugels, J. Wettability Assessment of Submicrometer Alumina Powder Using a Modified Washburn Method. J. Am. Ceram. Soc. 2010, 93, 2515–2518. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, J.; Han, K.; Wang, J.; Hu, X. Study on the structural build-up of cement-ground limestone pastes and its micro-mechanism. Constr. Build. Mater. 2020, 263, 120656. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Electrostatic repulsion between particles in cement suspensions: Domain of validity of linearized Poisson–Boltzmann equation for nonideal electrolytes. Cem. Concr. Res. 2003, 33, 781–791. [Google Scholar] [CrossRef]





| EDS Zone | Atom Counts (%) | |||
|---|---|---|---|---|
| O | Mg | P | K | |
| Point1 | 34.49 | 47.20 | 3.39 | 9.74 |
| Point2 | 53.62 | 40.48 | 2.04 | 2.61 |
| Point3 | 52.11 | 28.83 | 2.99 | 12.27 |
| Point4 | 54.51 | 37.29 | 1.38 | 2.93 |
| Point5 | 62.94 | 23.68 | 4.16 | 7.39 |
| Average | 51.53 | 35.50 | 2.79 | 6.99 |
| Material | MgO | CaO | SiO2 | Al2O3 | P2O5 | Fe2O3 | K2O | SO3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Magnesia | 90.27 | 0.95 | 3.47 | 0.60 | 0.23 | 1.74 | 0.10 | 0.09 | 2.55 |
| SF | 0.71 | 0.02 | 96.72 | 1.33 | 0.63 | 0.01 | 0.09 | 0.03 | 0.46 |
| Slag | 10.68 | 41.52 | 24.99 | 17.28 | 0.09 | 0.73 | 0.38 | 1.57 | 2.76 |
| MgO | K2HPO4·3H2O | SF | Slag | W/B | S/B | |
|---|---|---|---|---|---|---|
| Ref. | 67.5 | 22.5 | 0 | 10 | 0.14 | 0.4 |
| 5%SF | 63.75 | 21.25 | 5 | 10 | ||
| 10%SF | 60 | 20 | 10 | 10 | ||
| 15%SF | 56.25 | 18.75 | 15 | 10 | ||
| 20%SF | 52.5 | 17.5 | 20 | 10 | ||
| 25%SF | 48.75 | 16.25 | 25 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Chen, F.; Zhao, Z.; Xiao, P.; Zhang, Y. Understanding the Colloidal and Hydration Control in Rheological Evolution of 3D Printed MgO-SiO2-K2HPO4 Gel System. Gels 2025, 11, 827. https://doi.org/10.3390/gels11100827
Cai X, Chen F, Zhao Z, Xiao P, Zhang Y. Understanding the Colloidal and Hydration Control in Rheological Evolution of 3D Printed MgO-SiO2-K2HPO4 Gel System. Gels. 2025; 11(10):827. https://doi.org/10.3390/gels11100827
Chicago/Turabian StyleCai, Xianhuan, Fan Chen, Zhihui Zhao, Peng Xiao, and Yujuan Zhang. 2025. "Understanding the Colloidal and Hydration Control in Rheological Evolution of 3D Printed MgO-SiO2-K2HPO4 Gel System" Gels 11, no. 10: 827. https://doi.org/10.3390/gels11100827
APA StyleCai, X., Chen, F., Zhao, Z., Xiao, P., & Zhang, Y. (2025). Understanding the Colloidal and Hydration Control in Rheological Evolution of 3D Printed MgO-SiO2-K2HPO4 Gel System. Gels, 11(10), 827. https://doi.org/10.3390/gels11100827







