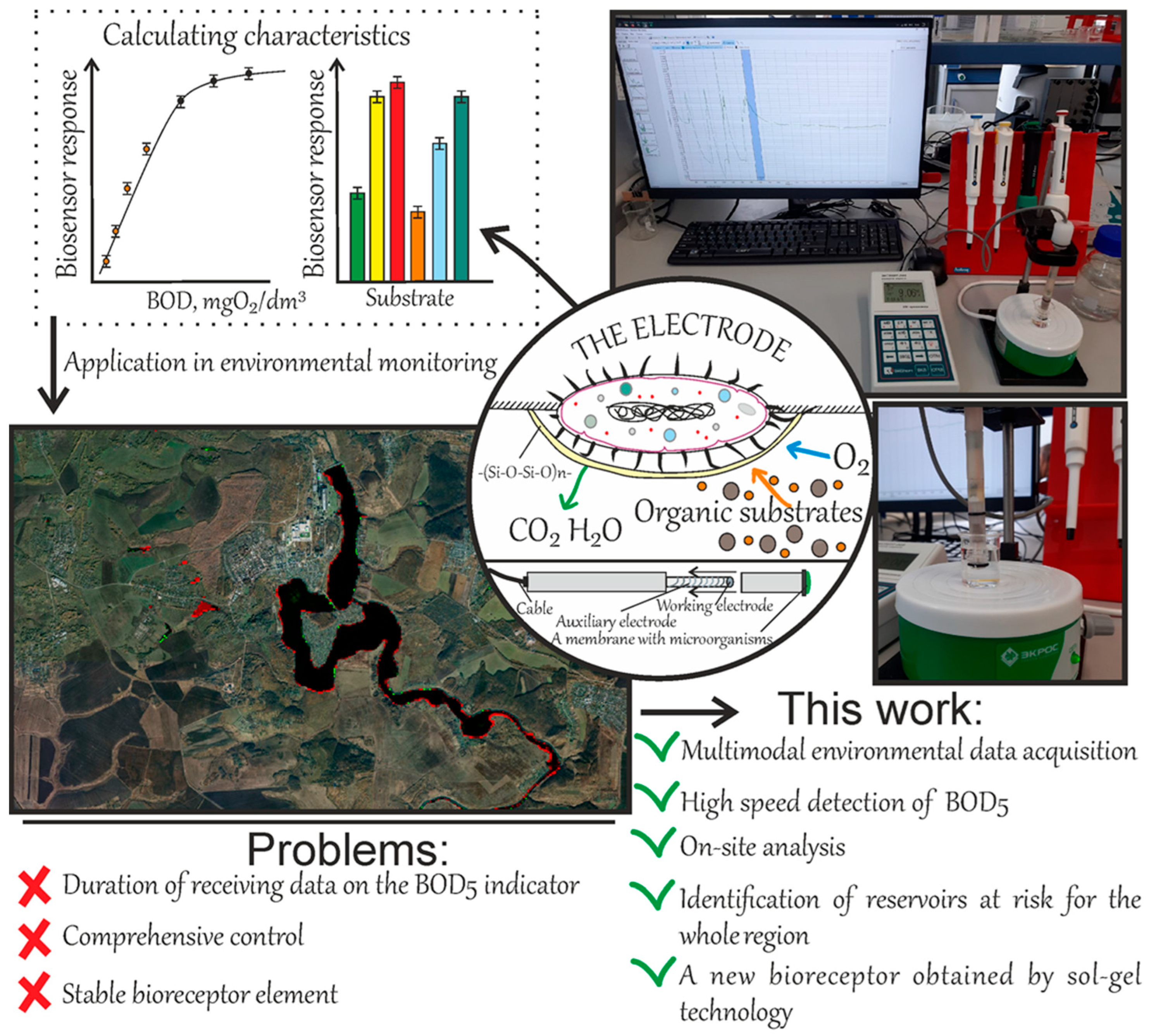
A Polyhydroxybutyrate-Supported Xerogel Biosensor for Rapid BOD Mapping and Integration with Satellite Data for Regional Water Quality Assessment
Abstract
1. Introduction
2. Results and Discussion
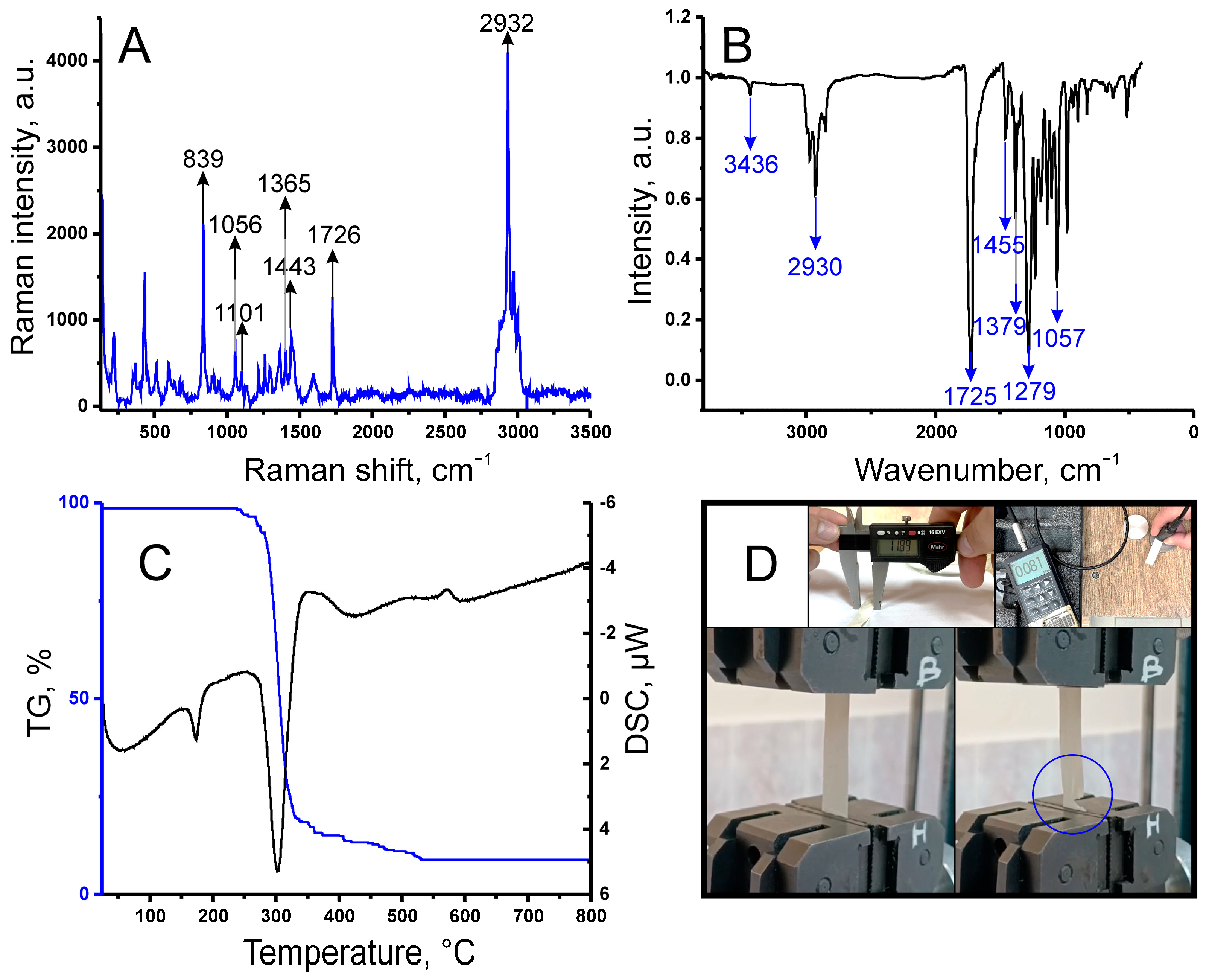
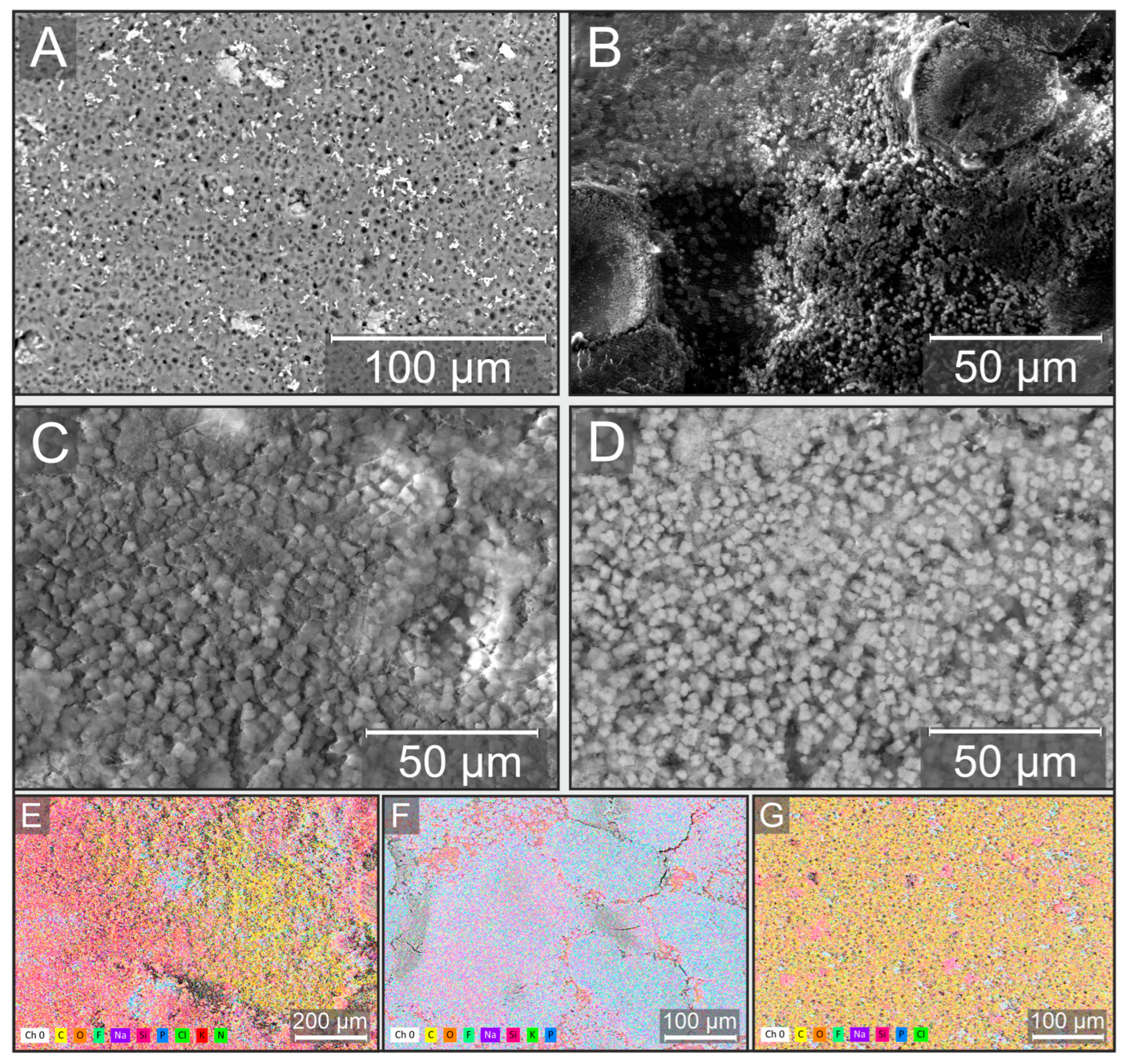
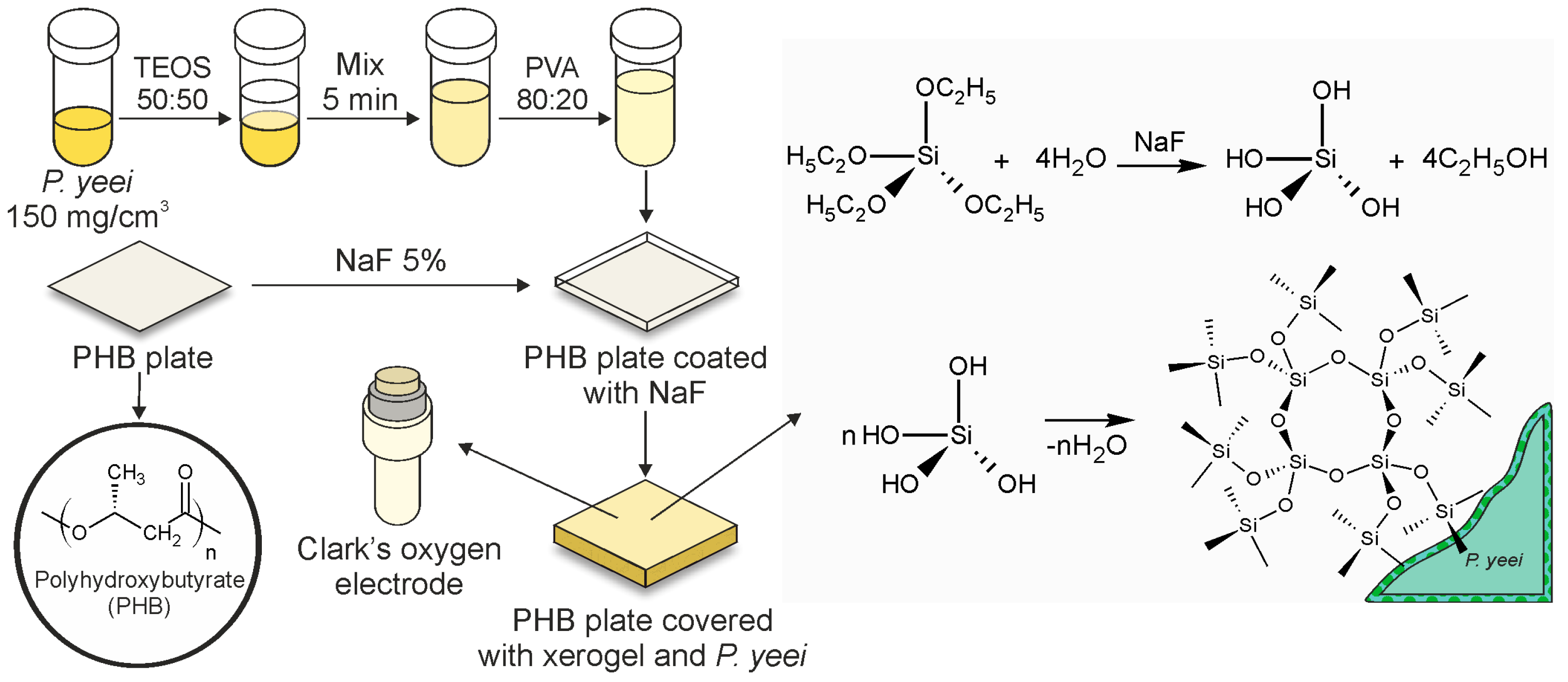
2.1. Fabrication and Characterization of the Bioreceptor Element
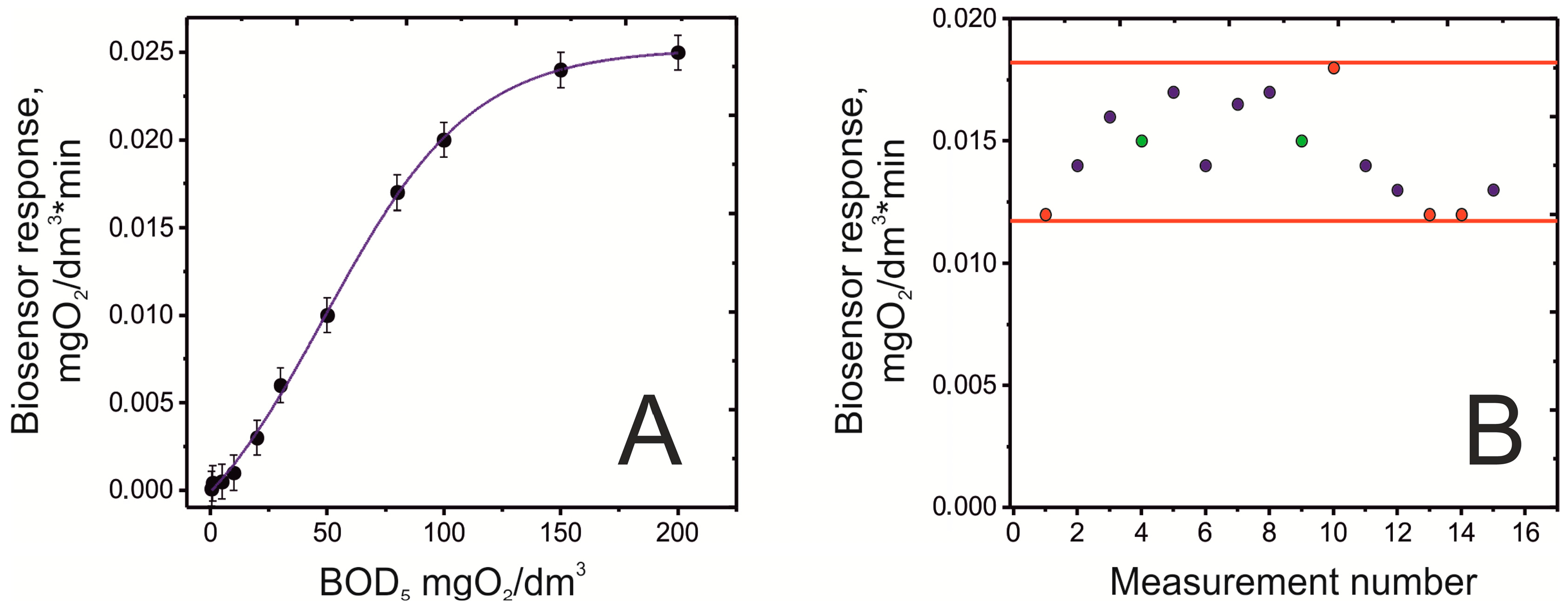
2.2. Evaluation of the Performance of the BOD Biosensor
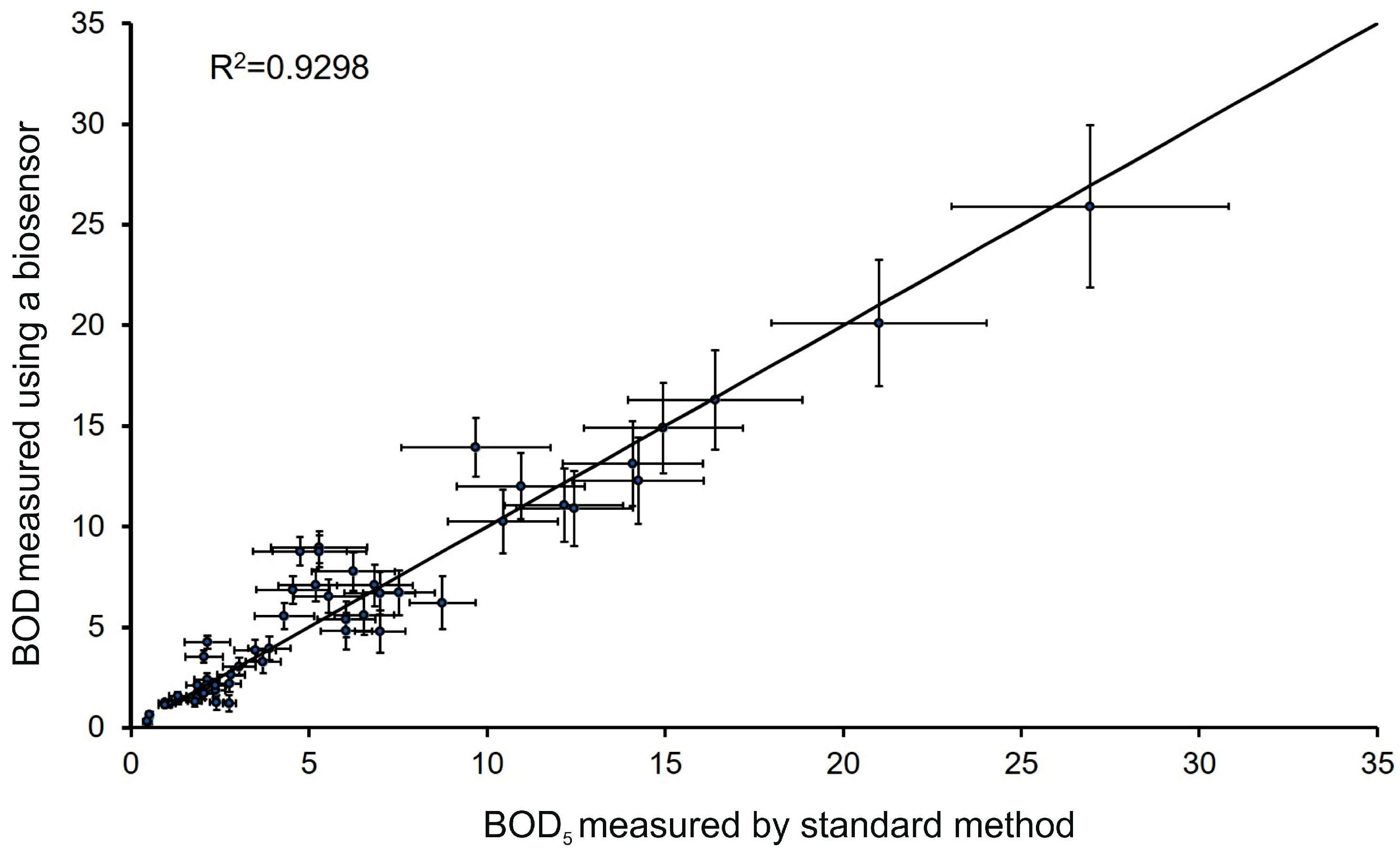
2.3. Biosensor Validation with Real Water Samples
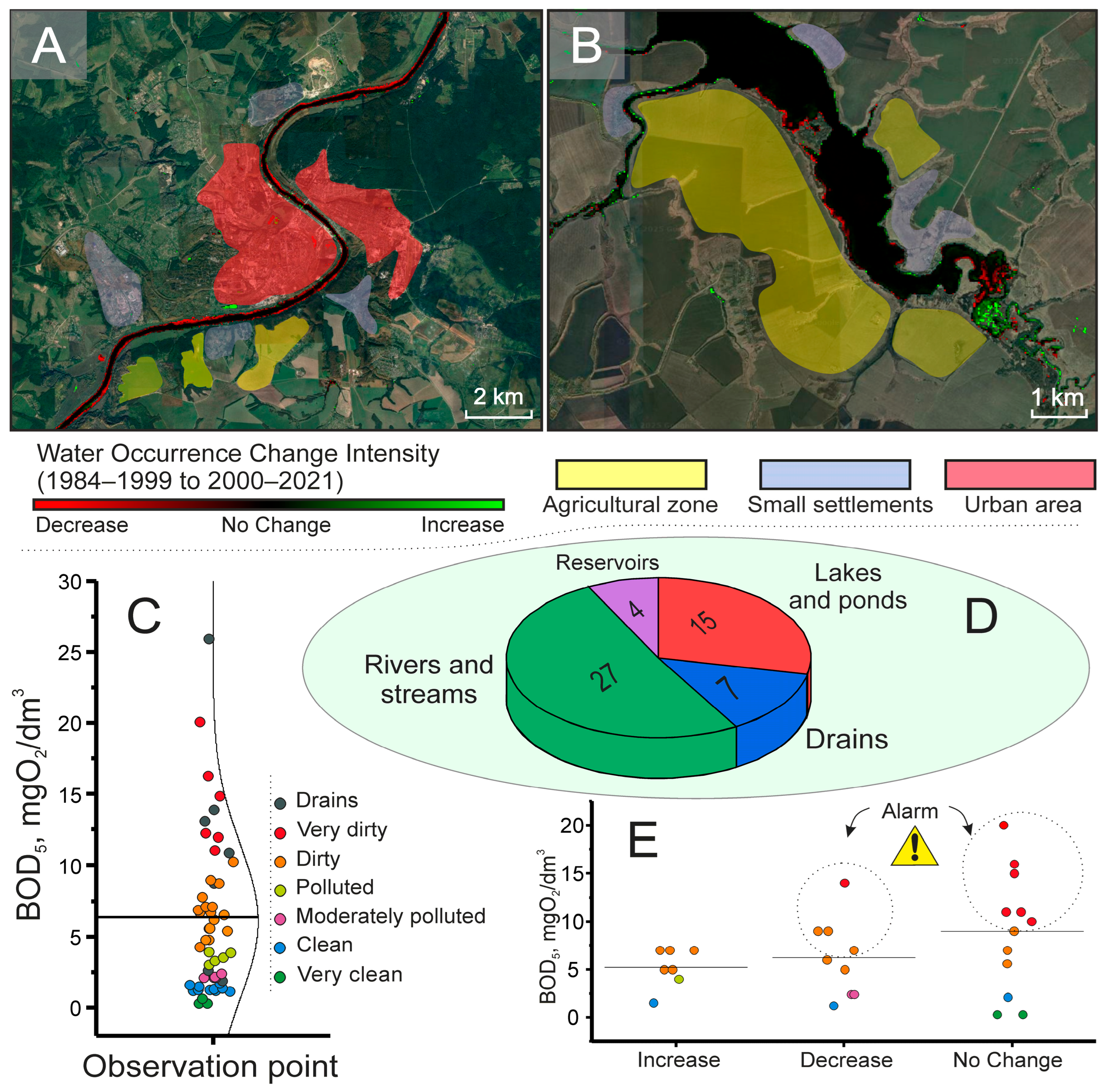
2.4. Using Global Surface Water Maps in Combination with BOD5 Indicators to Identify Environmentally Critical Situations in the Region
3. Conclusions
4. Materials and Methods
4.1. Cultivation of Microorganisms
4.1.1. Separation of P. yeei Bacterial Biomass
4.1.2. Cultivation of Cupriavidus necator VKM B-3386, PHB Extraction, and Film Fabrication
4.2. The Formation of a Bioreceptor Element Using a Xerogel Created Through Sol–Gel Technology on a Poly(3-hydroxybutyrate) Substrate
4.3. Calibration of the Dissolved Oxygen Sensor
4.4. Scanning Electron Microscopy with Energy Dispersive X-Ray Analysis (SEM-EDX)
4.5. Raman Spectroscopy
4.6. IR Spectroscopy
4.7. NMR Spectroscopy of PHB Films
4.8. Investigation of the Thermal and Physicomechanical Properties of the Produced PHB Film
4.9. Objects of Environmental Research
4.10. The Standard Method for Determining the BOD5 Indicator
4.11. Biosensory Measurements
4.12. Analyzing Changes in the Coastline and Identifying Potential Sources of Pollution
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide Cases of Water Pollution by Emerging Contaminants: A Review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; Van Vliet, M.T.H.; Strokal, M. A Triple Increase in Global River Basins with Water Scarcity Due to Future Pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Livneh, B.; Rajagopalan, B.; Wang, J.; Crétaux, J.-F.; Wada, Y.; Berge-Nguyen, M. Satellites Reveal Widespread Decline in Global Lake Water Storage. Science 2023, 380, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Chapra, S.C.; Camacho, L.A.; McBride, G.B. Impact of Global Warming on Dissolved Oxygen and BOD Assimilative Capacity of the World’s Rivers: Modeling Analysis. Water 2021, 13, 2408. [Google Scholar] [CrossRef]
- Buta, B.; Wiatkowski, M.; Gruss, Ł.; Tomczyk, P.; Kasperek, R. Spatio-Temporal Evolution of Eutrophication and Water Quality in the Turawa Dam Reservoir, Poland. Sci. Rep. 2023, 13, 9880. [Google Scholar] [CrossRef]
- Cheliukanov, M.; Gurkin, G.; Perchikov, R.; Medvedeva, A.; Lavrova, T.; Belousova, T.; Titova, A.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; et al. Whole Cells of Microorganisms—A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review. Biosensors 2025, 15, 290. [Google Scholar] [CrossRef]
- Thakur, A.; Devi, P. A Comprehensive Review on Water Quality Monitoring Devices: Materials Advances, Current Status, and Future Perspective. Crit. Rev. Anal. Chem. 2024, 54, 193–218. [Google Scholar] [CrossRef]
- Zainurin, S.N.; Wan Ismail, W.Z.; Mahamud, S.N.I.; Ismail, I.; Jamaludin, J.; Ariffin, K.N.Z.; Wan Ahmad Kamil, W.M. Advancements in Monitoring Water Quality Based on Various Sensing Methods: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14080. [Google Scholar] [CrossRef]
- Lacalamita, D.; Mongioví, C.; Crini, G. Chemical Oxygen Demand and Biochemical Oxygen Demand Analysis of Discharge Waters from Laundry Industry: Monitoring, Temporal Variability, and Biodegradability. Front. Environ. Sci. 2024, 12, 1387041. [Google Scholar] [CrossRef]
- Jouanneau, S.; Recoules, L.; Durand, M.J.; Boukabache, A.; Picot, V.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.; Thouand, G. Methods for Assessing Biochemical Oxygen Demand (BOD): A Review. Water Res. 2014, 49, 62–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yang, X.; Wang, Z.; Liu, X. Biochemical Oxygen Demand Prediction Based on Three-Dimensional Fluorescence Spectroscopy and Machine Learning. Sensors 2025, 25, 711. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Kamani, P.; Hosseini, H.R.M.; Sadrnezhaad, S.K. SPR-Based Assay Kit for Rapid Determination of Pb2+. Anal. Chim. Acta 2022, 1220, 340030. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, G.; Sun, M.; Cai, Z.; Yin, Z.; Cai, Y. Bacterial Cellulose Immobilized S. Cerevisiae as Microbial Sensor for Rapid BOD Detection. Fibers Polym. 2021, 22, 1208–1217. [Google Scholar] [CrossRef]
- Kurbanalieva, S.; Arlyapov, V.; Kharkova, A.; Perchikov, R.; Kamanina, O.; Melnikov, P.; Popova, N.; Machulin, A.; Tarasov, S.; Saverina, E.; et al. Electroactive Biofilms of Activated Sludge Microorganisms on a Nanostructured Surface as the Basis for a Highly Sensitive Biochemical Oxygen Demand Biosensor. Sensors 2022, 22, 6049. [Google Scholar] [CrossRef]
- Perchikov, R.; Cheliukanov, M.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; Butusov, D.; Arlyapov, V.; Nakamura, H.; Reshetilov, A. Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices. Biosensors 2024, 14, 302. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Qin, W. Electrochemical Biochemical Oxygen Demand Biosensors and Their Applications in Aquatic Environmental Monitoring. Sens. Bio-Sens. Res. 2024, 44, 100642. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K.; Yashtulov, N.A. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 22, 7626. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-Based Oxygen (O2) Sensors for On-Chip Monitoring of Cell, Tissue and Organ Metabolismsoftware. Biosensors 2021, 12, 6. [Google Scholar] [CrossRef]
- Mummaleti, G.; Kong, F. Fabrication, Properties and Applications of Xerogels in Food Processing. J. Agric. Food Res. 2023, 11, 100506. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Liu, L.; Wu, Y.; Li, W.; Liang, J.; Shen, H.; Fang, S.; Huang, X.; Chu, Z.; et al. Gelatin Methacryloyl Xerogel Puncture Implants Loaded with Cu0.5Mn2.5O4 Nanoparticles Synergizes Cuproptosis and STING Activation for Enhanced Breast Cancer Immunotherapy. ACS Nano 2025, 19, 27902–27918. [Google Scholar] [CrossRef]
- Arkas, M.; Kythreoti, G.; Favvas, E.; Giannakopoulos, K.; Mouti, N.; Arvanitopoulou, M.; Athanasiou, A.; Douloudi, M.; Nikoli, E.; Vardavoulias, M.; et al. Hydrophilic Antimicrobial Coatings for Medical Leathers from Silica-Dendritic Polymer-Silver Nanoparticle Composite Xerogels. Textiles 2022, 2, 464–485. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Wang, Z.; Liu, Y.; Guo, J.; Zhu, Y.; Shao, J.; Li, J.; Wang, L.; Wang, K. Antibacterial, Antioxidant and Biocompatible Nanosized Quercetin-PVA Xerogel Films for Wound Dressing. Colloids Surf. B Biointerfaces 2022, 209, 112175. [Google Scholar] [CrossRef] [PubMed]
- Rößler, S.; Brückner, A.; Kruppke, I.; Wiesmann, H.-P.; Hanke, T.; Kruppke, B. 3D Plotting of Silica/Collagen Xerogel Granules in an Alginate Matrix for Tissue-Engineered Bone Implants. Materials 2021, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; El-Naggar, M.E.; Abu-Saied, M.A.; Khattab, T.A.; Saleh, D.I. Preparation of Biosensor Based on Triarylmethane Loaded Cellulose Acetate Xerogel for the Detection of Urea. Mater. Chem. Phys. 2022, 276, 125377. [Google Scholar] [CrossRef]
- Yu, M.; Ye, R.; Zeng, T.; Tan, L.; Zhao, Z.; Gao, W.; Chen, X.; Lian, Z.; Ma, Y.; Li, A.; et al. Constructing an Ultra-Rapid Nanoconfinement-Enhanced Fluorescence Clinical Detection Platform by Using Machine Learning and Tunable DNA Xerogel “Probe”. Anal. Chem. 2023, 95, 15690–15699. [Google Scholar] [CrossRef]
- Wemple, A.H.; Kaplan, J.S.; Leopold, M.C. Mechanistic Elucidation of Nanomaterial-Enhanced First-Generation Biosensors Using Probe Voltammetry of an Enzymatic Reaction. Biosensors 2023, 13, 798. [Google Scholar] [CrossRef]
- Cruz-Quesada, G.; Espinal-Viguri, M.; López-Ramón, M.V.; Garrido, J.J. Hybrid Xerogels: Study of the Sol-Gel Process and Local Structure by Vibrational Spectroscopy. Polymers 2021, 13, 2082. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Sun, S.; Tan, Y.; Cheng, Q.; Cai, Y.; Zheng, J.; Wang, W.; Xu, L.; Li, G.; Wang, D.; Zhang, L.; et al. Thermoplastic PHB-Reinforced Chitosan Piezoelectric Films for Biodegradable Pressure Sensors. ACS Appl. Bio Mater. 2024, 7, 6823–6831. [Google Scholar] [CrossRef]
- Kossieris, S.; Tsiakos, V.; Tsimiklis, G.; Amditis, A. Inland Water Level Monitoring from Satellite Observations: A Scoping Review of Current Advances and Future Opportunities. Remote Sens. 2024, 16, 1181. [Google Scholar] [CrossRef]
- Batina, A.; Krtalić, A. Integrating Remote Sensing Methods for Monitoring Lake Water Quality: A Comprehensive Review. Hydrology 2024, 11, 92. [Google Scholar] [CrossRef]
- Papa, F.; Crétaux, J.-F.; Grippa, M.; Robert, E.; Trigg, M.; Tshimanga, R.M.; Kitambo, B.; Paris, A.; Carr, A.; Fleischmann, A.S.; et al. Water Resources in Africa under Global Change: Monitoring Surface Waters from Space. Surv. Geophys. 2023, 44, 43–93. [Google Scholar] [CrossRef]
- Su, P.-W.; Lo, S.-L. Satellite Imagery: A Way to Monitor Water Quality for the Future? Environ. Sci. Pollut. Res. 2022, 29, 57022–57029. [Google Scholar] [CrossRef]
- Adjovu, G.E.; Stephen, H.; James, D.; Ahmad, S. Overview of the Application of Remote Sensing in Effective Monitoring of Water Quality Parameters. Remote Sens. 2023, 15, 1938. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-Resolution Mapping of Global Surface Water and Its Long-Term Changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Albariño, R.; Larrañaga, A.; LeRoy, C.J.; Masese, F.O.; Moretti, M.S. Ecosystem Services Provided by Small Streams: An Overview. Hydrobiologia 2023, 850, 2501–2535. [Google Scholar] [CrossRef]
- Ma, J.; Ma, R.; Pan, Q.; Liang, X.; Wang, J.; Ni, X. A Global Review of Progress in Remote Sensing and Monitoring of Marine Pollution. Water 2023, 15, 3491. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Edmunds, P.; Bussi, G.; O’Donnell, S.; Futter, M.; Groom, S.; Rampley, C.; Szweda, C.; Johnson, D.; Triggs Hodge, A.; et al. Real-Time Water Quality Forecasting in Rivers Using Satellite Data and Dynamic Models: An Online System for Operational Management, Control and Citizen Science. Front. Environ. Sci. 2024, 12, 1331783. [Google Scholar] [CrossRef]
- Curnick, D.J.; Davies, A.J.; Duncan, C.; Freeman, R.; Jacoby, D.M.P.; Shelley, H.T.E.; Rossi, C.; Wearn, O.R.; Williamson, M.J.; Pettorelli, N. SmallSats: A New Technological Frontier in Ecology and Conservation? Remote Sens. Ecol. Conserv. 2022, 8, 139–150. [Google Scholar] [CrossRef]
- Mucheye, T.; Haro, S.; Papaspyrou, S.; Caballero, I. Water Quality and Water Hyacinth Monitoring with the Sentinel-2A/B Satellites in Lake Tana (Ethiopia). Remote Sens. 2022, 14, 4921. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Gill, L.; Regan, S.; Waldren, S.; Ghosh, B. A Nested Drone-Satellite Approach to Monitoring the Ecological Conditions of Wetlands. ISPRS J. Photogramm. Remote Sens. 2021, 174, 151–165. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD Using Paracoccus Yeei Bacteria Isolated from Activated Sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef]
- Alferov, S.V.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Biofuel Cell Based on Bacteria of the Genus Gluconobacter as a Sensor for Express Analysis of Biochemical Oxygen Demand. Appl. Biochem. Microbiol. 2018, 54, 689–694. [Google Scholar] [CrossRef]
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Podsevalova, E.; Ponamoreva, O. Application of Organosilicate Matrix Based on Methyltriethoxysilane, PVA and Bacteria Paracoccus Yeei to Create a Highly Sensitive BOD. 3 Biotech 2021, 11, 331. [Google Scholar] [CrossRef]
- Asgharnejad, H.; Khorshidi Nazloo, E.; Madani Larijani, M.; Hajinajaf, N.; Rashidi, H. Comprehensive Review of Water Management and Wastewater Treatment in Food Processing Industries in the Framework of Water-food-environment Nexus. Comp. Rev. Food Sci. Food Safe 2021, 20, 4779–4815. [Google Scholar] [CrossRef]
- Cao, S.T.; Tran, H.P.; Le, H.T.T.; Bui, H.P.K.; Nguyen, G.T.H.; Nguyen, L.T.; Nguyen, B.T.; Luong, A.D. Impacts of Effluent from Different Livestock Farm Types (Pig, Cow, and Poultry) on Surrounding Water Quality: A Comprehensive Assessment Using Individual Parameter Evaluation Method and Water Quality Indices. Environ. Sci. Pollut. Res. 2021, 28, 50302–50315. [Google Scholar] [CrossRef]
- ISO 5815–1: 2003; ISO Water Quality: Determination of Biochemical Oxygen Demand After n Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition. ISO: Geneva, Switzerland, 2003.
- Kraus, E.; Orf, L.; Starostina, I.; Stoyanov, O. Improvement of Polymeric Material Adhesion by UV Laser and Plasma Pretreatment; Shaker: Düren, Germany, 2017; ISBN 3-8440-5451-0. [Google Scholar]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Noda, I.; Ochiai, S.; Ozaki, Y. Raman Microspectroscopy Study of Structure, Dispersibility, and Crystallinity of Poly(Hydroxybutyrate)/Poly(l-Lactic Acid) Blends. Polymer 2006, 47, 3132–3140. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Ramachandran, D.; Kanagasabai, V.; Gogoi, M.; Hazarika, S.; Koch, P.J.; Dutta, N.; Maadurshni, G.B.; Manivannan, J.; et al. Polyhydroxybutyrate (PHB)-Based Sustainable Bioplastic Derived from Bacillus sp. KE4 Isolated from Kitchen Waste Effluent. Sustain. Chem. Pharm. 2024, 39, 101507. [Google Scholar] [CrossRef]
- Hou, J.; Cheng, L.; Zhang, S.; Zhang, X.; Zheng, X.; Zhang, Q. Production of Polyhydroxyalkanoate from New Isolated Bacteria of Acidovorax Diaphorobacter ZCH-15 Using Orange Peel and Its Underlying Metabolic Mechanisms. Bioresour. Technol. 2025, 418, 131949. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.C.; Araújo, E.S.; Ramos, L.C.; Jesus, A.A.; Leite, S.P.; Bjerk, T.R.; López, J.A.; Hernández-Macedo, M.L. Optimized Bioconversion of Cheese Whey to Poly(Hydroxybutyrate) (PHB) by Mangrove-Isolated Bacillus Cereus. J. Polym. Environ. 2025, 33, 1881–1896. [Google Scholar] [CrossRef]
- Thanh, N.H.; Olekhnovich, R.O.; Uspenskaya, M.V.; Sitnikova, V.E.; Elangwe, C.N. Effect of Polymer Ratio on Thermal Properties of Polyhydroxybutyrate/Polyhydroxyhexanoate. Bull. St. Petersburg State Inst. Technol. (Tech. Univ.) 2023, 66, 27–30. [Google Scholar] [CrossRef]
| Parameter | This Work | [45] | [15] | [45] | [46] |
|---|---|---|---|---|---|
| Range of Determined Concentrations, mgO2/dm3 | 0.5–50 | 0.05–5.0 | 10–220 | 0.34–9.6 | 0.1–21 |
| Time of a single biosensor measurement, min | ~5 | 4–6 | 20 | 30–130 | 4–6 |
| RSD, % | <15 | 7 | 3.1 | 4.1 | 7 |
| Long-term stability, days | 20 | 45 | - | 7 | 11 |
| Number of oxidizable substrates | 22 of 25 | 32 of 33 | - | 12 of 14 | 20 of 24 |
| Parameter | This Work | [44] | [15] | [46] |
|---|---|---|---|---|
| The sensitive element | P. yeei in a xerogel matrix on PHB | P. yeei in modified PVA hydrogel | S. cerevisiae on bacterial cellulose | P. yeei in a xerogel matrix |
| R2 | 0.929 | 0.999 | 0.986 | 0.998 |
| Overestimation or underestimation of biosensor parameters compared to the standard method for measuring BOD, %. | Underestimation by 5.4% | - | Overestimation by 13.9% | - |
| The number of real samples examined | 53 | 9 | 8 | 9 |
| Rank | The Influence Factor | The Magnitude of the Impact on BOD |
|---|---|---|
| 1 | Wastewater treatment plants | Very high |
| 2 | Industrial enterprises (food, livestock) | High |
| 3 | Private settlements | Moderate/Average |
| 4 | Recreational areas (beaches, recreation centers, parks) | Weak |
| 5 | Graveyard | Weak/Background |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurkin, G.; Efremov, A.; Koryakina, I.; Perchikov, R.; Kharkova, A.; Medvedeva, A.; Fabiano, B.; Reverberi, A.P.; Arlyapov, V. A Polyhydroxybutyrate-Supported Xerogel Biosensor for Rapid BOD Mapping and Integration with Satellite Data for Regional Water Quality Assessment. Gels 2025, 11, 849. https://doi.org/10.3390/gels11110849
Gurkin G, Efremov A, Koryakina I, Perchikov R, Kharkova A, Medvedeva A, Fabiano B, Reverberi AP, Arlyapov V. A Polyhydroxybutyrate-Supported Xerogel Biosensor for Rapid BOD Mapping and Integration with Satellite Data for Regional Water Quality Assessment. Gels. 2025; 11(11):849. https://doi.org/10.3390/gels11110849
Chicago/Turabian StyleGurkin, George, Alexey Efremov, Irina Koryakina, Roman Perchikov, Anna Kharkova, Anastasia Medvedeva, Bruno Fabiano, Andrea Pietro Reverberi, and Vyacheslav Arlyapov. 2025. "A Polyhydroxybutyrate-Supported Xerogel Biosensor for Rapid BOD Mapping and Integration with Satellite Data for Regional Water Quality Assessment" Gels 11, no. 11: 849. https://doi.org/10.3390/gels11110849
APA StyleGurkin, G., Efremov, A., Koryakina, I., Perchikov, R., Kharkova, A., Medvedeva, A., Fabiano, B., Reverberi, A. P., & Arlyapov, V. (2025). A Polyhydroxybutyrate-Supported Xerogel Biosensor for Rapid BOD Mapping and Integration with Satellite Data for Regional Water Quality Assessment. Gels, 11(11), 849. https://doi.org/10.3390/gels11110849









