The Diketopyrrolopyrrole (DPP) Core as a Gel-Forming Material: Current Status and Untapped Potential
Abstract
:1. Introduction
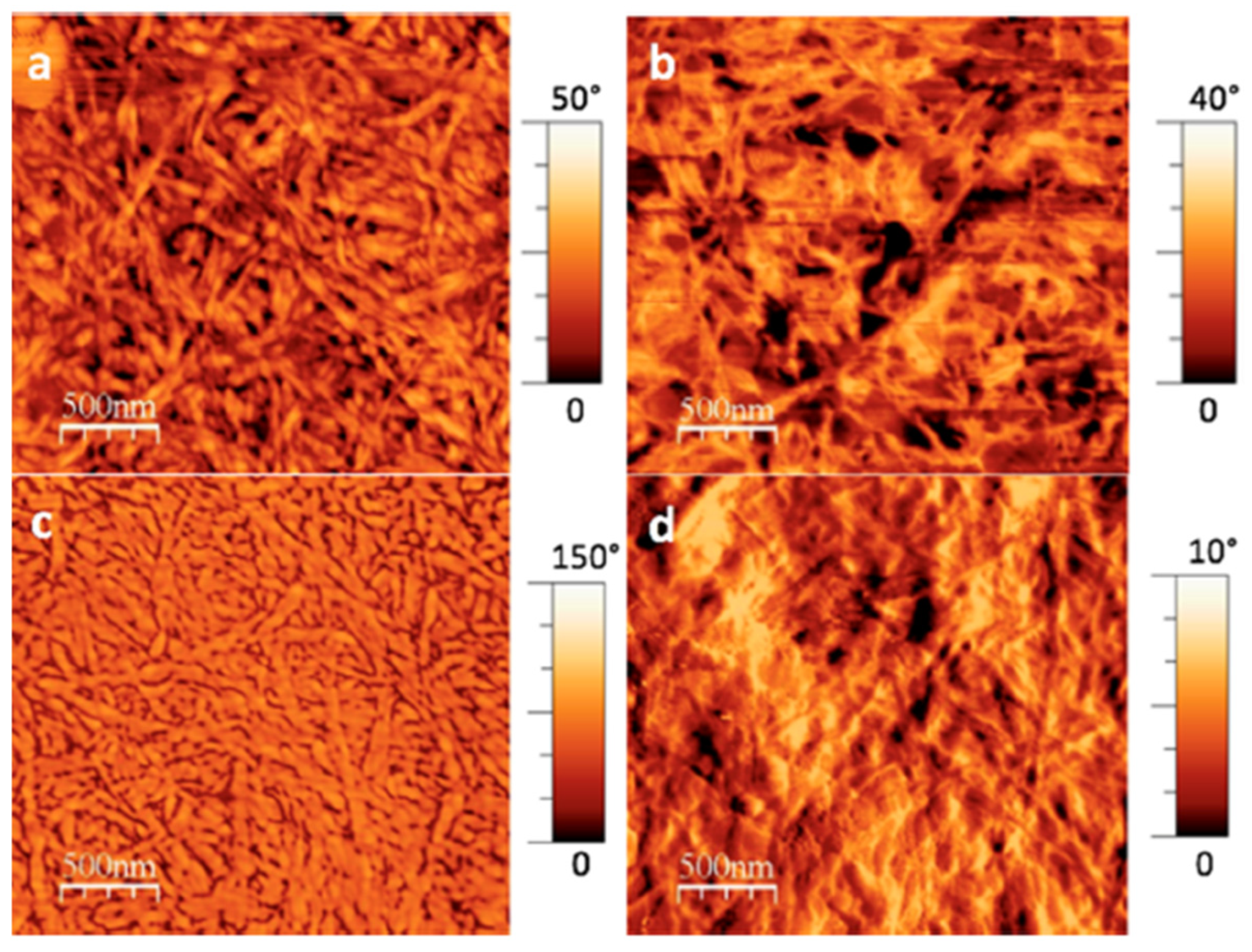
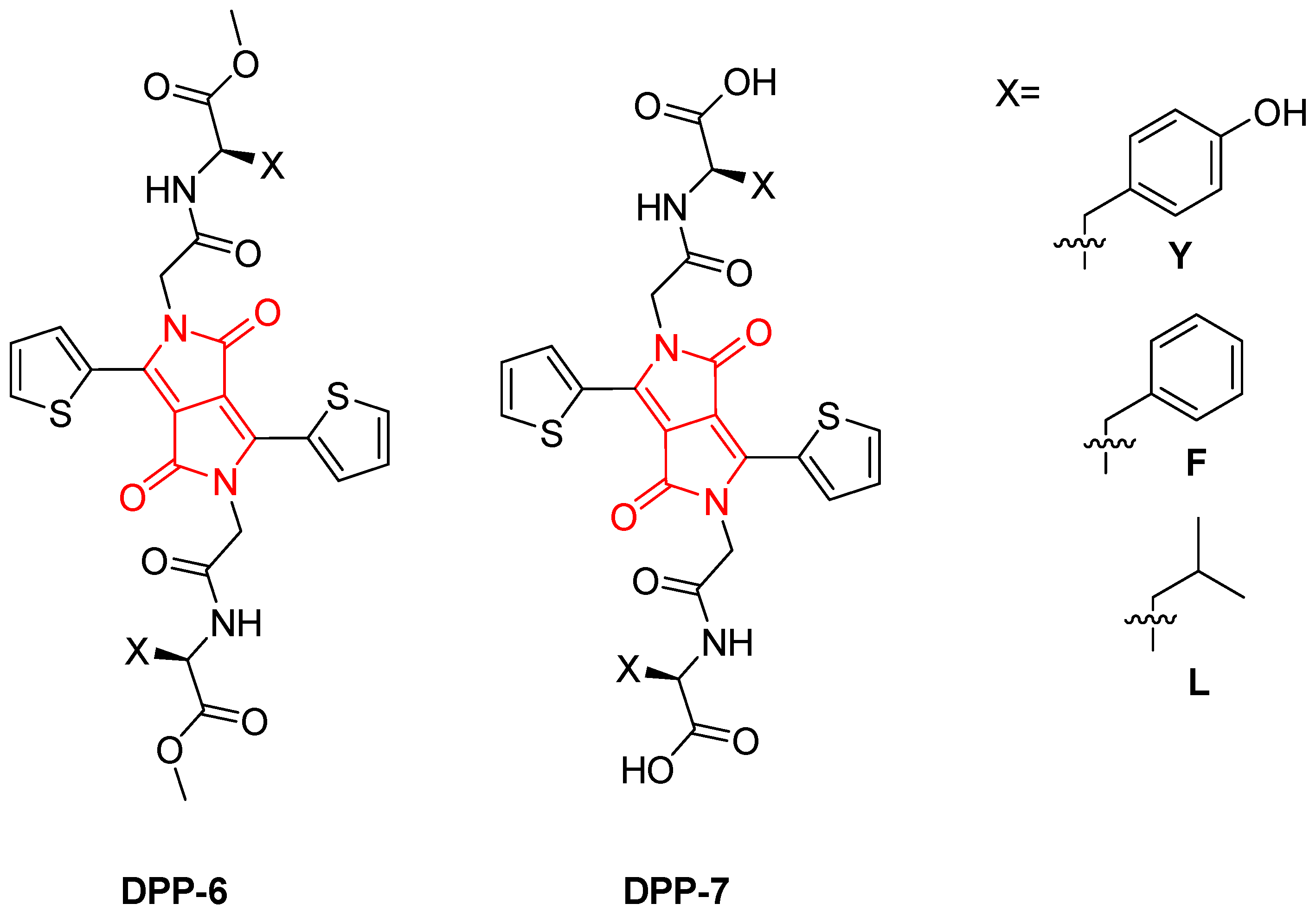
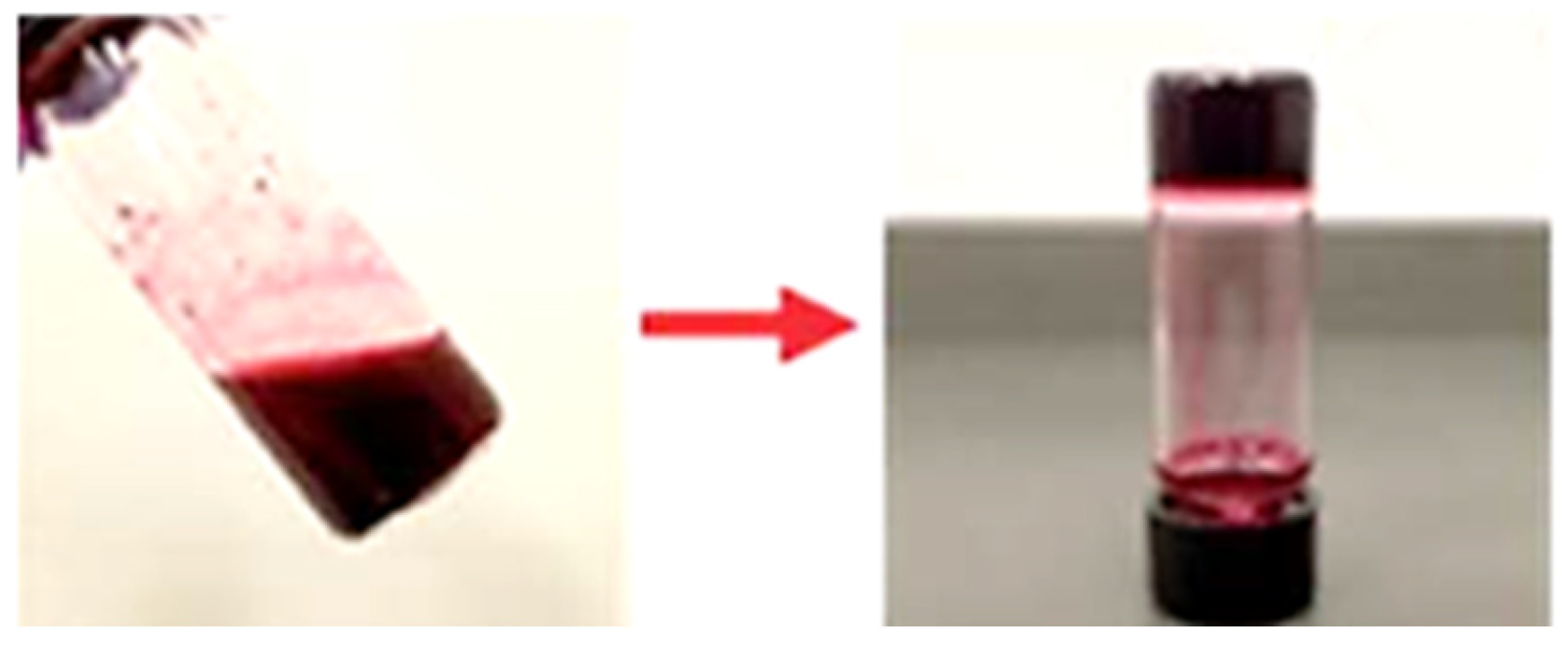
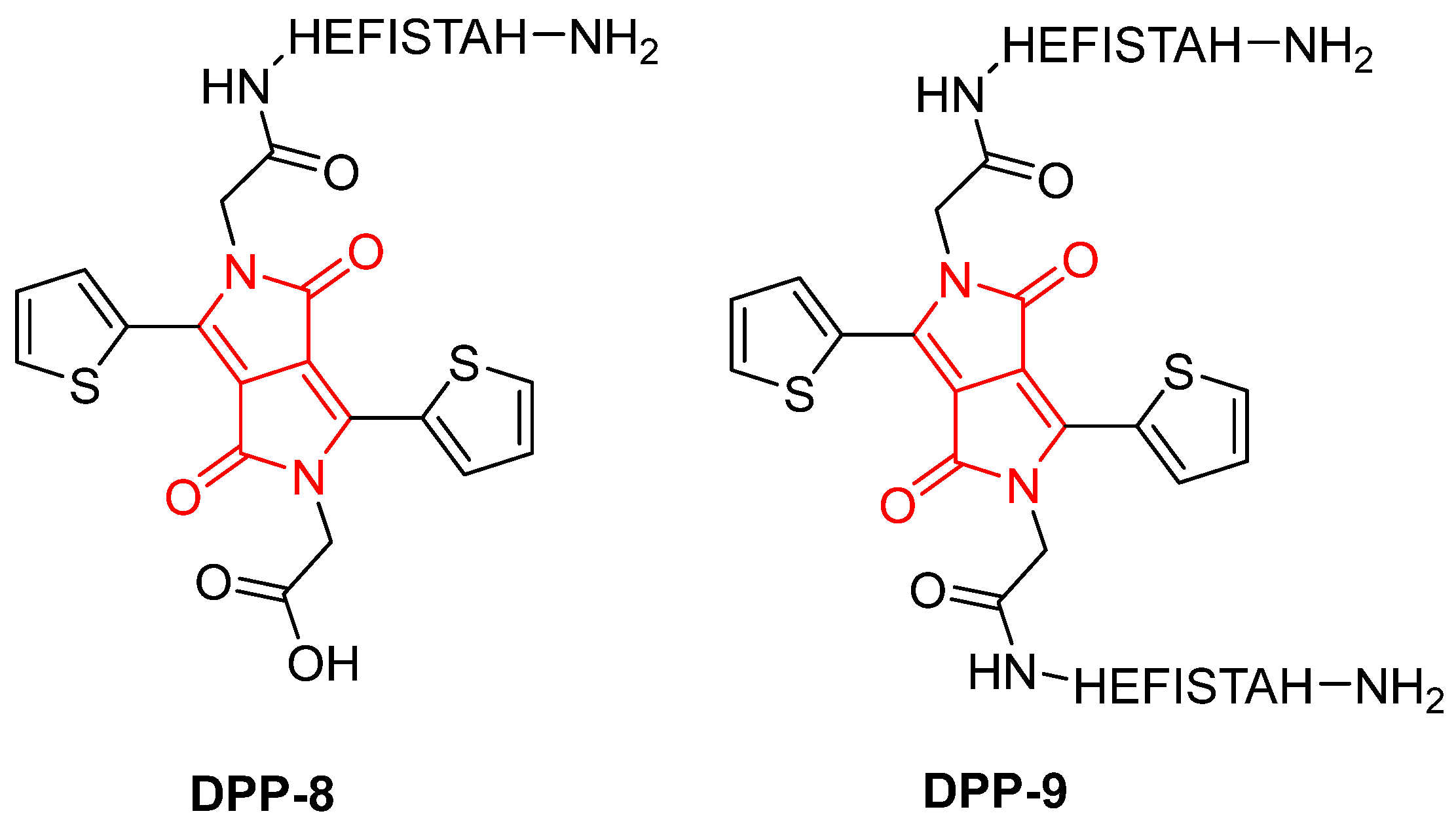
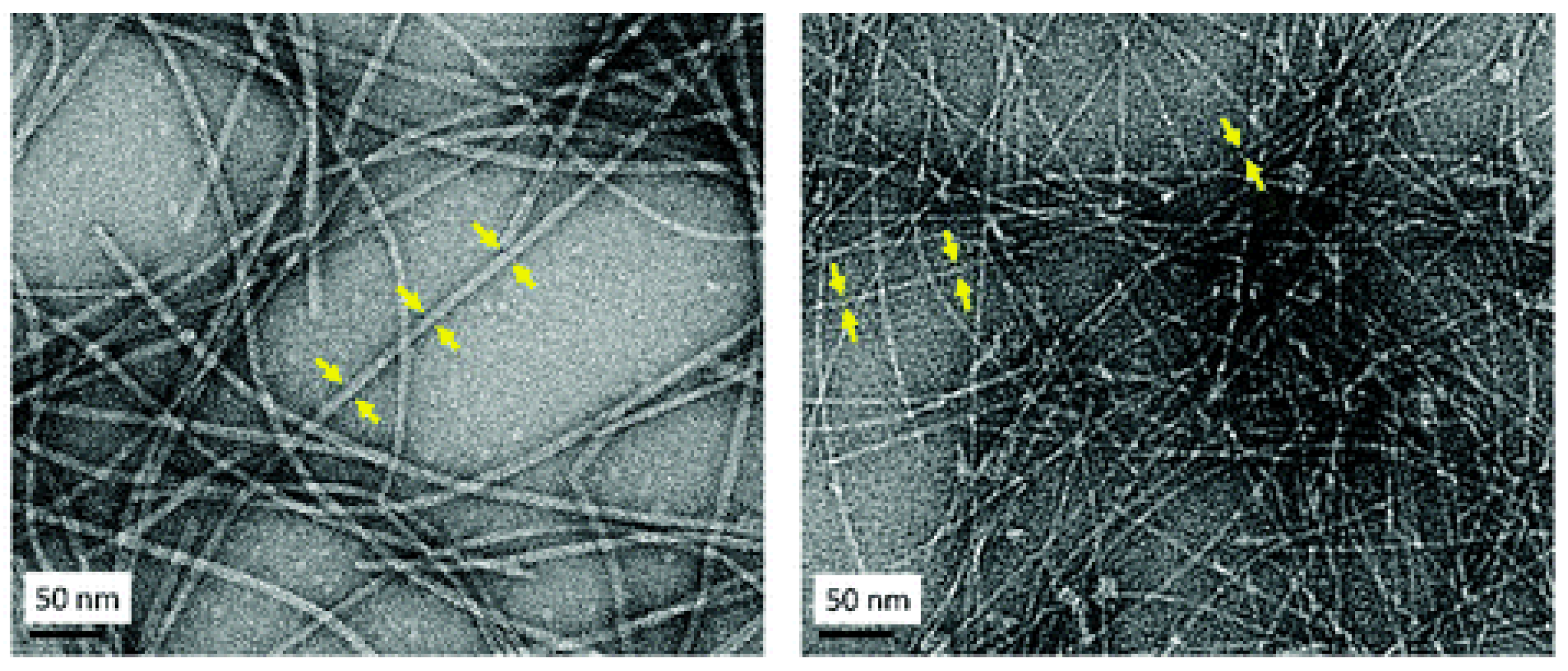
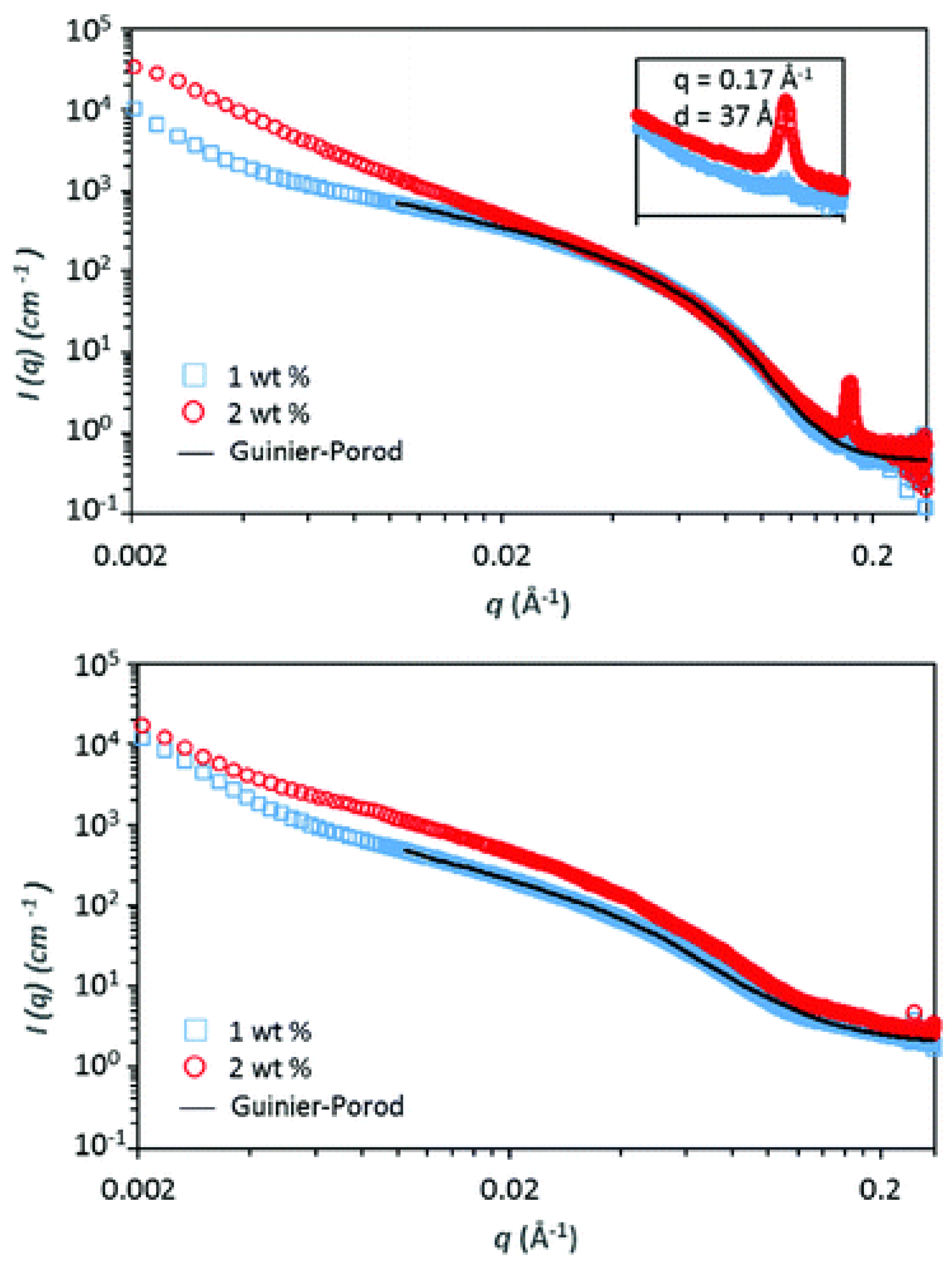
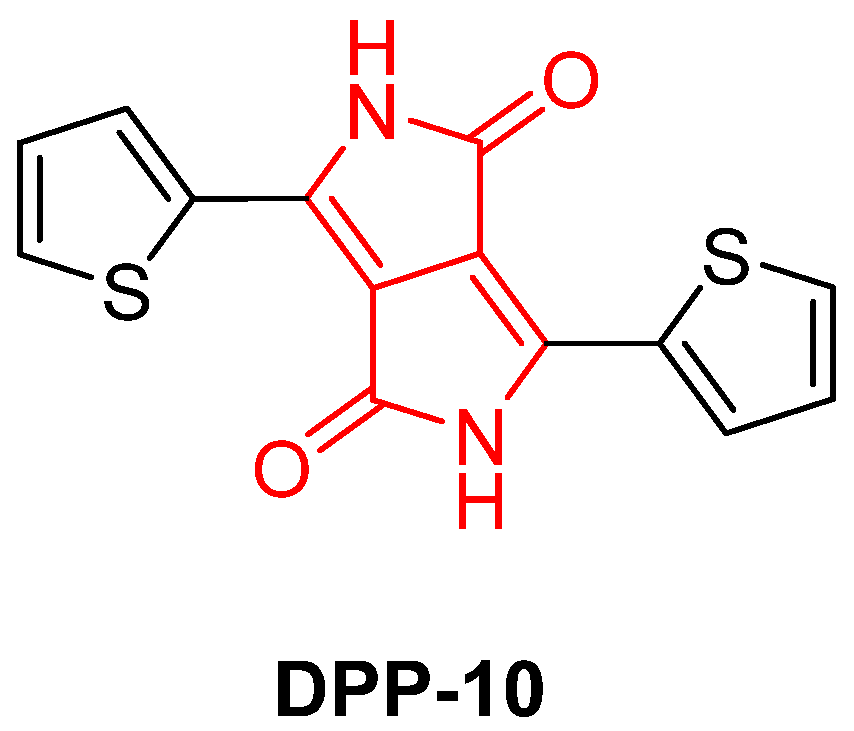
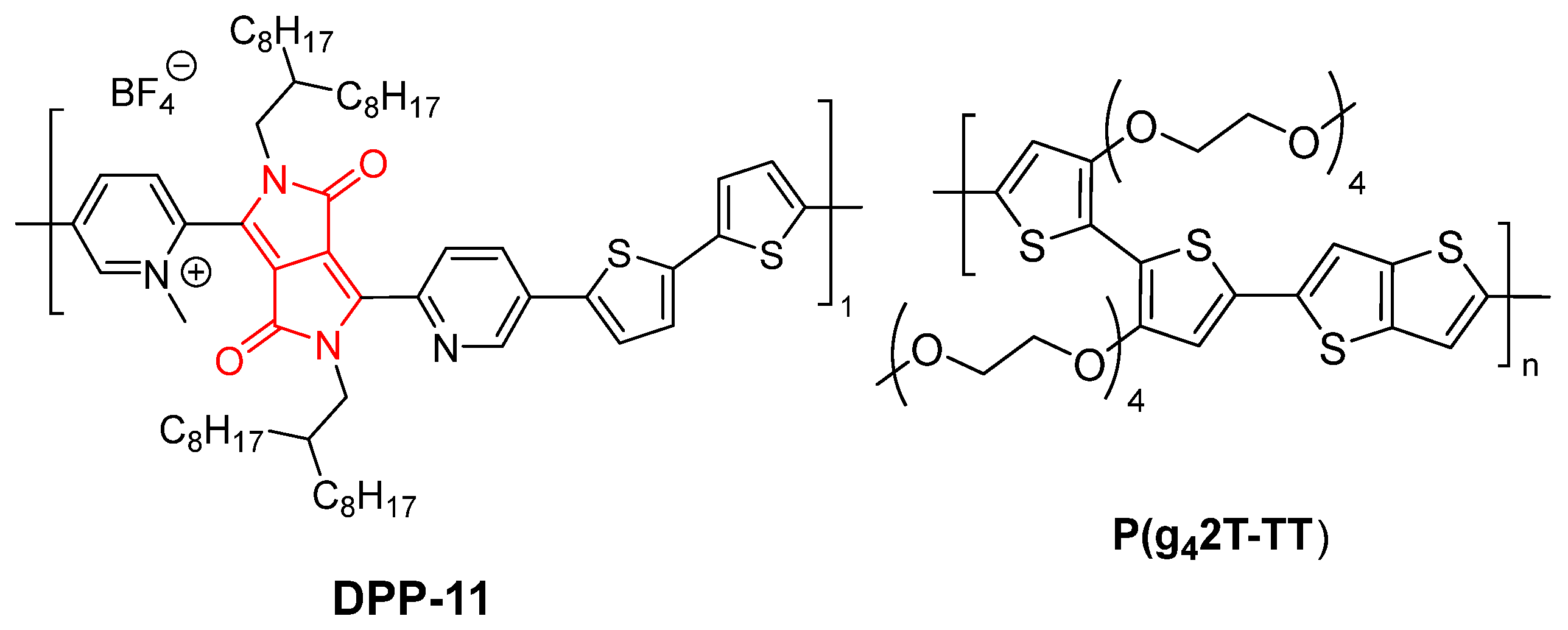
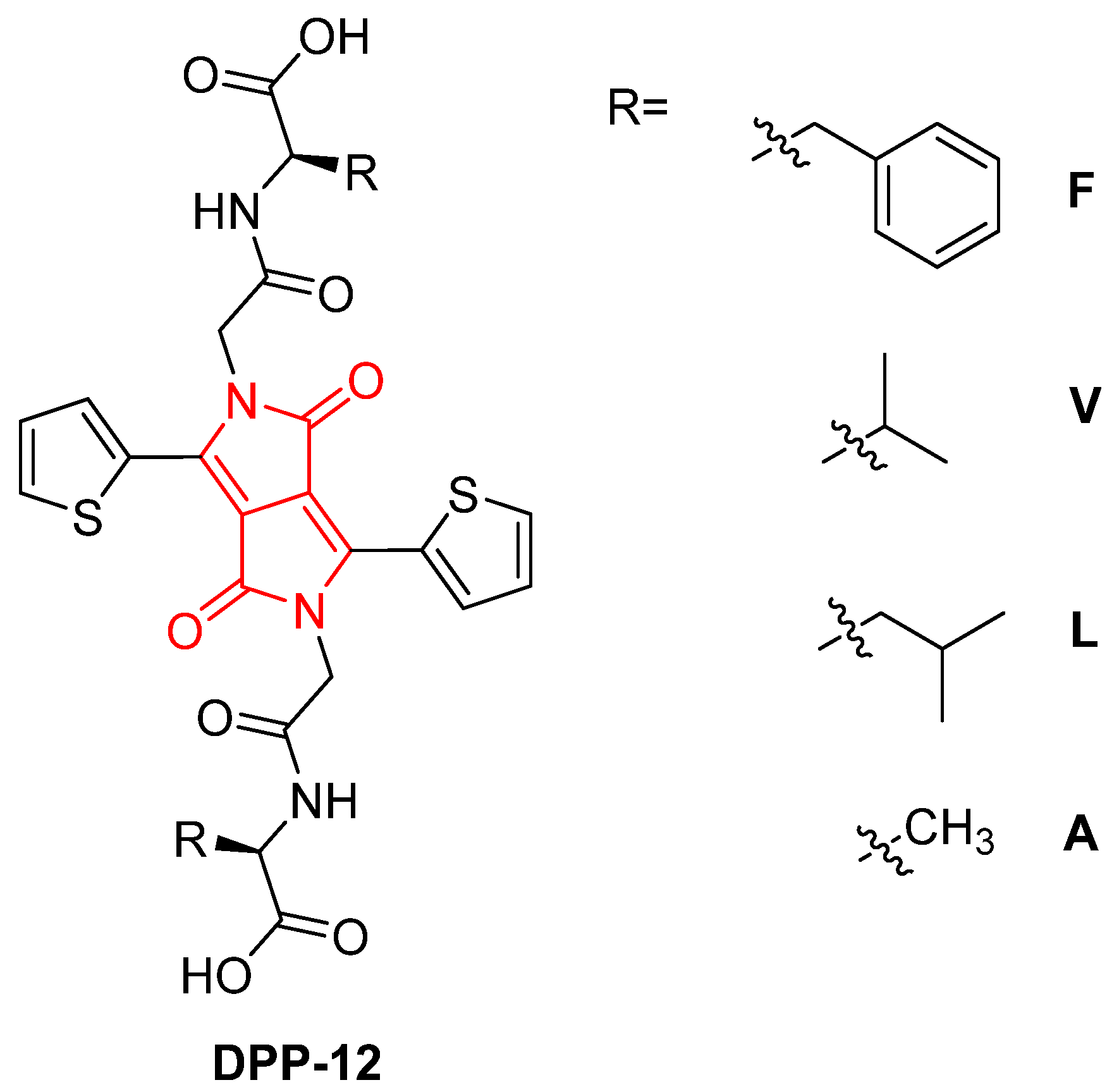
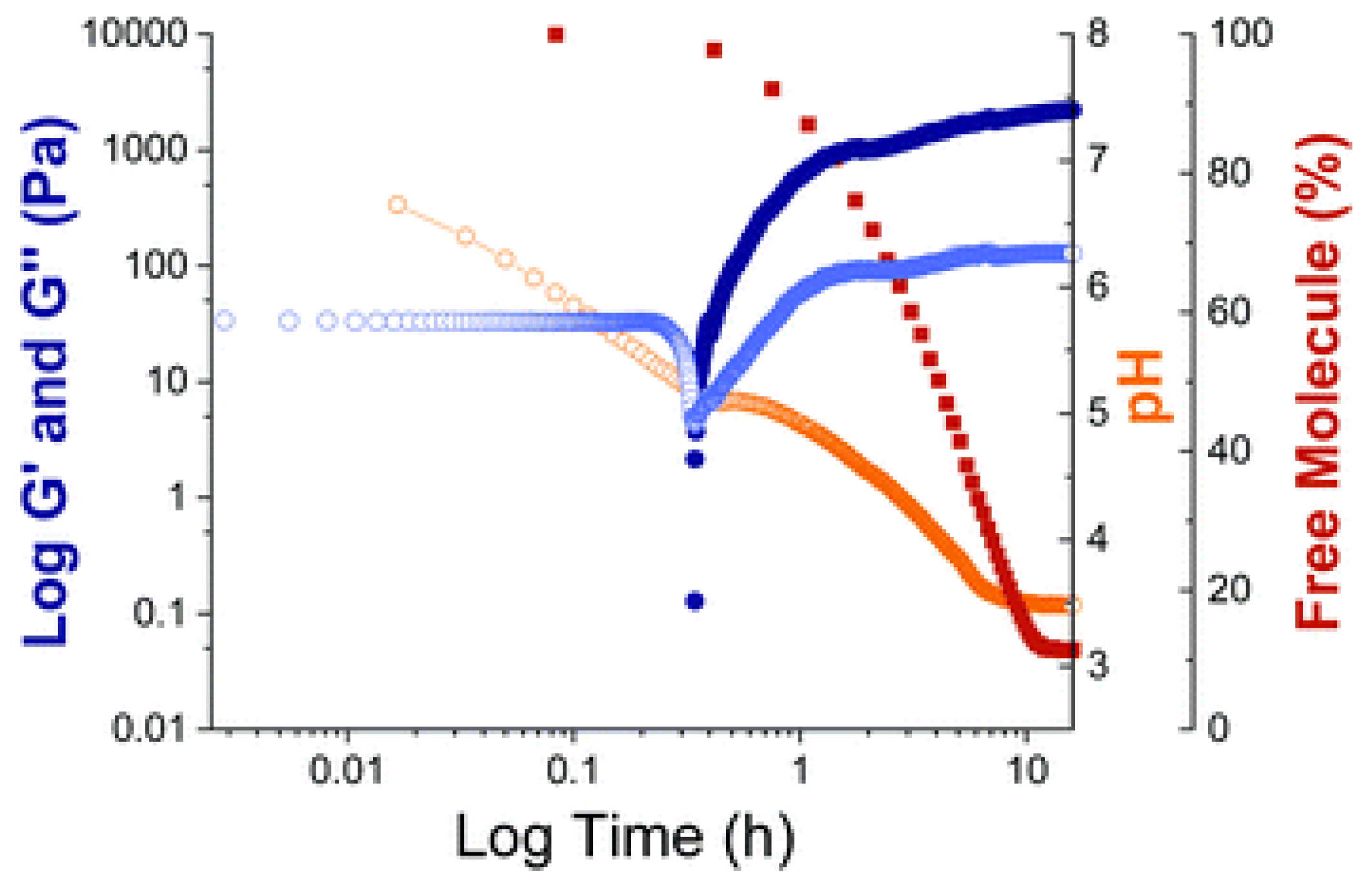
2. Results and Discussion
3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Qing, G.; Shan, X.; Chen, W.; Lv, Z.; Xiong, P.; Sun, T. Solvent-driven Chiral-interaction Reversion for Organogel Formation. Angew. Chem. Int. Ed. Engl. 2014, 53, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, B.; Zhang, Y.; Li, Y.; Cheng, R.; Wei, C.; Ning, C.; Dong, K.; Wang, Z.L. Ultrastretchable Organogel/Silicone Fiber-Helical Sensors for Self-Powered Implantable Ligament Strain Monitoring. ACS Nano 2022, 16, 10958–10967. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Shan, J.; Guo, C.; Wang, Y. Selective Colorimetric and Fluorometric Organogel Sensors for the Detection of F− and ClO− Based on Chiral Glutamic and Phenothiazine Derivatives. Colloid. Polym. Sci. 2023, 301, 107–115. [Google Scholar] [CrossRef]
- Ge, J.; Dai, S.; Dong, X.; Li, M.; Xu, Y.; Jiang, Y.; Yuan, N.; Ding, J. A Wide-Temperature-Range Sensor Based on Wide-Strain-Range Self-Healing and Adhesive Organogels. New J. Chem. 2022, 46, 4334–4342. [Google Scholar] [CrossRef]
- Koo, J.; Lim, S.-I.; Jang, J.; Oh, M.; Jeong, K.-U. From Polymer Gels to 3D Actuators: Transformation of Programmed 2D Structures to 3D Objects. J. Chem. Educ. 2020, 97, 1396–1401. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Li, Y. Recent Advances in Plasticized PVC Gels for Soft Actuators and Devices: A Review. J. Mater. Chem. C Mater. Opt. Electron. Devices 2019, 7, 12991–13009. [Google Scholar] [CrossRef]
- Hwang, T.; Frank, Z.; Neubauer, J.; Kim, K.J. High-Performance Polyvinyl Chloride Gel Artificial Muscle Actuator with Graphene Oxide and Plasticizer. Sci. Rep. 2019, 9, 9658. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel Based Tissue Engineering and Its Future Applications in Personalized Disease Modeling and Regenerative Therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L. Long-Acting Gel Formulations: Advancing Drug Delivery across Diverse Therapeutic Areas. Pharmaceuticals 2024, 17, 493. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical Polymorph Control in a Drug-Mimetic Supramolecular Gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef]
- Aparicio, F.; Matesanz, E.; Sánchez, L. Cooperative Self-Assembly of Linear Organogelators. Amplification of Chirality and Crystal Growth of Pharmaceutical Ingredients. Chem. Commun. 2012, 48, 5757. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Sánchez, A.; Saikia, B.; Yufit, D.S.; Prieto, P.; Carrillo, J.R.; Steed, J.W. Highly Thermally Resistant Bisamide Gelators as Pharmaceutical Crystallization Media. Gels 2022, 9, 26. [Google Scholar] [CrossRef]
- Kaliaraj, G.; Shanmugam, D.; Dasan, A.; Mosas, K. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Silva, P.M.; Martins, A.J.; Fasolin, L.H.; Vicente, A.A. Modulation and Characterization of Wax-Based Olive Oil Organogels in View of Their Application in the Food Industry. Gels 2021, 7, 12. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. An Overview of the Past, Present, and Future of Organogels. In Edible Oleogels; Marangoni, A.G., Garti, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–17. ISBN 9780983079118. [Google Scholar]
- Mehta, C.; Bhatt, G.; Kothiyal, P. A Review on Organogel for Skin Aging. Indian J. Pharm. Biol. Res. 2016, 4, 28–37. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Nandi, A.K.; Chatterjee, D.P. Hybrid Polymer Gels for Energy Applications. J. Mater. Chem. A Mater. Energy Sustain. 2023, 11, 12593–12642. [Google Scholar] [CrossRef]
- Lal, J.; Biswas, P.; Singh, S.K.; Debbarma, R.; Mehta, N.K.; Deb, S.; Sharma, S.; Waikhom, G.; Patel, A.B. Moving towards Gel for Fish Feeding: Focus on Functional Properties and Its Acceptance. Gels 2023, 9, 305. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Tajarudin, H.A.; Balakrishnan, V.; Nasution, H. Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications. Gels 2022, 8, 334. [Google Scholar] [CrossRef]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main Features and Applications of Organogels in Cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P. Preparation, Characterization and Evaluation of Organogel-Based Lipstick Formulations: Application in Cosmetics. Gels 2021, 7, 97. [Google Scholar] [CrossRef]
- Mosquera Narvaez, L.E.; Ferreira, L.M.D.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef]
- Galindo, J.M.; Tardío, C.; Saikia, B.; Van Cleuvenbergen, S.; Torres-Moya, I. Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels 2023, 9, 875. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef] [PubMed]
- Pokusaev, B.; Vyazmin, A.; Karlov, S.; Zakharov, N.; Reznik, V.; Nekrasov, D. Agar Gels: Kinetics of Formation and Structure. Chem. Eng. Trans. 2017, 57, 1327–1332. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-Based Gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Parker, A.; Normand, V. Glassy Dynamics of Gelatin Gels. Soft Matter 2010, 6, 4916. [Google Scholar] [CrossRef]
- Geonzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation Mechanism and Network Structure in Gels of Carrageenans and Their Mixtures Viewed at Different Length Scales—A Review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Yamagishi, R.; Miura, S.; Yabu, K.; Ando, M.; Hachikubo, Y.; Yokoyama, Y.; Yasuda, K.; Takei, S. Fabrication Technology of Self-Dissolving Sodium Hyaluronate Gels Ultrafine Microneedles for Medical Applications with UV-Curing Gas-Permeable Mold. Gels 2024, 10, 65. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A Comprehensive Review of Polyacrylamide Polymer Gels for Conformance Control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-Isopropylacrylamide)-Based Smart Hydrogels: Design, Properties and Applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Takeno, H.; Kimura, Y.; Nakamura, W. Mechanical, Swelling, and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, K.; Suzuki, M. Development of Low-Molecular-Weight Gelators and Polymer-Based Gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Raphaelides, S.N. Rheological Studies of Starch—Fatty Acid Gels. Food Hydrocoll. 1993, 7, 479–495. [Google Scholar] [CrossRef]
- Hoffmann, H.; Ulbricht, W. Surfactant Gels. Curr. Opin. Colloid Interface Sci. 1996, 1, 726–739. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A Review on Recent Advances in Polymer and Peptide Hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Okihara, M.; Matsuda, A.; Kawamura, A.; Miyata, T. Design of Dual Stimuli-Responsive Gels with Physical and Chemical Properties That Vary in Response to Light and Temperature and Cell Behavior on Their Surfaces. Polym. J. 2024, 56, 193–204. [Google Scholar] [CrossRef]
- Ahn, S.-K.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-Responsive Polymer Gels. Soft Matter 2008, 4, 1151. [Google Scholar] [CrossRef]
- Hu, L.; Gao, Y.; Serpe, M.J.; Wiley, J. (Eds.) Smart Stimuli-Responsive Polymers, Films, and Gels; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Smith, D.K. Supramolecular Gels—A Panorama of Low-Molecular-Weight Gelators from Ancient Origins to next-Generation Technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Cui, S.; Li, J.; Wei, X.; Zheng, M. Diketopyrrolopyrrole Based Organic Semiconductor Materials for Field-Effect Transistors. Front. Chem. 2021, 9, 671294. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Sun, H.; Lee, J.; Noh, Y.-Y. High Performance Solution Processed Organic Field Effect Transistors with Novel Diketopyrrolopyrrole-Containing Small Molecules. Sci. Rep. 2017, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Heinrichová, P.; Pospíšil, J.; Stříteský, S.; Vala, M.; Weiter, M.; Toman, P.; Rais, D.; Pfleger, J.; Vondráček, M.; Šimek, D.; et al. Diketopyrrolopyrrole-Based Organic Solar Cells Functionality: The Role of Orbital Energy and Crystallinity. J. Phys. Chem. C Nanomater. Interfaces 2019, 123, 11447–11463. [Google Scholar] [CrossRef]
- Li, W.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Buccheri, N.; Rooney, M.; Botta, C.; Bruni, F.; Giovanella, U.; Brovelli, S.; Beverina, L. Near-Infrared Roll-off-Free Electroluminescence from Highly Stable Diketopyrrolopyrrole Light Emitting Diodes. Sci. Rep. 2016, 6, 34096. [Google Scholar] [CrossRef]
- Shukla, A.; Entoma, V.; McGregor, S.K.M.; Hasan, M.; Mamada, M.; Moore, E.G.; Adachi, C.; Lo, S.-C.; Namdas, E.B. Low Light Amplification Threshold and Reduced Efficiency Roll-off in Thick Emissive Layer OLEDs from a Diketopyrrolopyrrole Derivative. Macromol. Rapid Commun. 2022, 43, 2200115. [Google Scholar] [CrossRef]
- Matsuo, T.; Kuwabara, J.; Kanbara, T.; Hayashi, S. Flexible and Red-Emissive Organic Single-Crystal Microresonator for Efficient Active Waveguides. J. Phys. Chem. Lett. 2023, 14, 6577–6582. [Google Scholar] [CrossRef]
- Sánchez-Oliva, A.; Tardío, C.; Pinilla-Peñalver, E.; Saikia, B.; Torres-Moya, I. Advanced Photonic Circuits Using a Mechanofluorochromic Diketopyrrolopyrrole Derivative. Dyes Pigm. 2025, 235, 112593. [Google Scholar] [CrossRef]
- Thool, G.S.; Narayanaswamy, K.; Venkateswararao, A.; Naqvi, S.; Gupta, V.; Chand, S.; Vivekananthan, V.; Koner, R.R.; Krishnan, V.; Singh, S.P. Highly Directional 1D Supramolecular Assembly of New Diketopyrrolopyrrole-Based Gel for Organic Solar Cell Applications. Langmuir 2016, 32, 4346–4351. [Google Scholar] [CrossRef]
- Draper, E.R.; Dietrich, B.; Adams, D.J. Self-Assembly, Self-Sorting, and Electronic Properties of a Diketopyrrolopyrrole Hydrogelator. Chem. Commun. 2017, 53, 1864–1867. [Google Scholar] [CrossRef] [PubMed]
- Nyayachavadi, A.; Mason, G.T.; Nazir Tahir, M.; Ocheje, M.U.; Rondeau-Gagné, S. Covalent Cross-Linking of Diketopyrrolopyrrole-Based Organogels with Polydiacetylenes. Langmuir 2018, 34, 12126–12136. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumar, M.; Levine, A.M.; Jimenez, I.; Ulijn, R.V.; Braunschweig, A.B. Visible-Light Photooxidation in Water by 1O2-Generating Supramolecular Hydrogels. Chem. Sci. 2020, 11, 4239–4245. [Google Scholar] [CrossRef]
- Rani, A.; Kavianinia, I.; Hume, P.; De Leon-Rodriguez, L.M.; Kihara, S.; Williams, D.E.; McGillivray, D.J.; Plank, N.O.V.; Gerrard, J.; Hodgkiss, J.M.; et al. Directed Self-Assembly of Peptide–Diketopyrrolopyrrole Conjugates—A Platform for Bio-Organic Thin Film Preparation. Soft Matter 2020, 16, 6563–6571. [Google Scholar] [CrossRef]
- Kumar, S.; Panigrahi, P.; Mohanty, S.; Nayak, S.K.; Palai, A.K. Tuning up the Photovoltaic Performances upon the Utility of Diketopyrrolopyrrole in PEO-Based Gel Polymer Electrolytes. Dalton Trans. 2021, 50, 7647–7655. [Google Scholar] [CrossRef]
- Stegerer, D.; Pracht, M.; Günther, F.; Sun, H.; Preis, K.; Zerson, M.; Maftuhin, W.; Tan, W.L.; Kroon, R.; McNeill, C.R.; et al. Organogels from Diketopyrrolopyrrole Copolymer Ionene/Polythiophene Blends Exhibit Ground-State Single Electron Transfer in the Solid State. Macromolecules 2022, 55, 4979–4994. [Google Scholar] [CrossRef]
- Gauci, V.; Seddon, A.; Adams, D.J. Synthesis and Characterisation of Diketopyrrolopyrrole-Based Hydrogels. Soft Matter 2022, 18, 3756–3761. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Oliva, A.; Torres-Moya, I. The Diketopyrrolopyrrole (DPP) Core as a Gel-Forming Material: Current Status and Untapped Potential. Gels 2025, 11, 134. https://doi.org/10.3390/gels11020134
Sánchez-Oliva A, Torres-Moya I. The Diketopyrrolopyrrole (DPP) Core as a Gel-Forming Material: Current Status and Untapped Potential. Gels. 2025; 11(2):134. https://doi.org/10.3390/gels11020134
Chicago/Turabian StyleSánchez-Oliva, Abelardo, and Iván Torres-Moya. 2025. "The Diketopyrrolopyrrole (DPP) Core as a Gel-Forming Material: Current Status and Untapped Potential" Gels 11, no. 2: 134. https://doi.org/10.3390/gels11020134
APA StyleSánchez-Oliva, A., & Torres-Moya, I. (2025). The Diketopyrrolopyrrole (DPP) Core as a Gel-Forming Material: Current Status and Untapped Potential. Gels, 11(2), 134. https://doi.org/10.3390/gels11020134







