Modified Nanocellulose Hydrogels and Applications in Sensing Fields
Abstract
1. Introduction
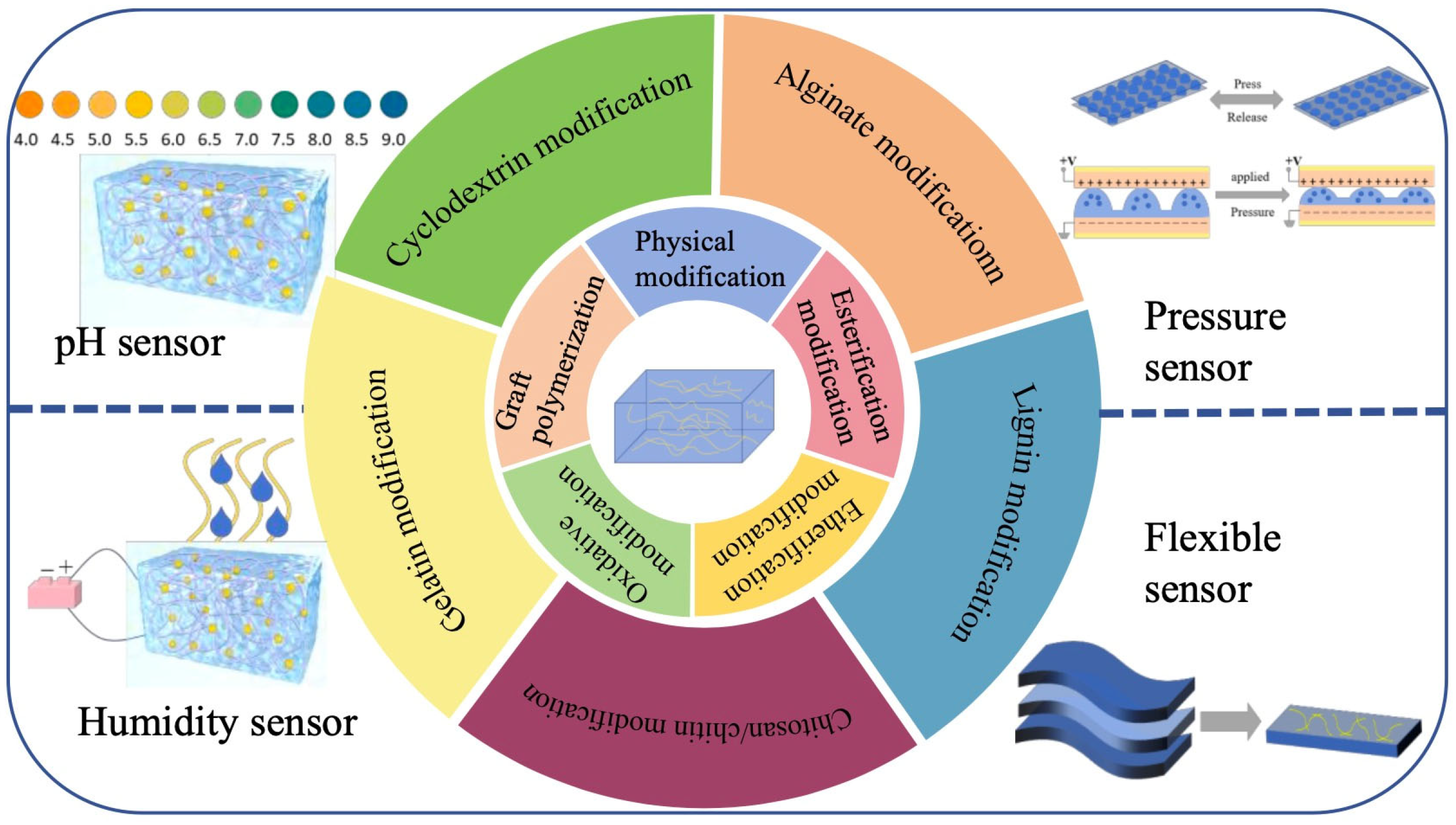
2. Cellulose Modification
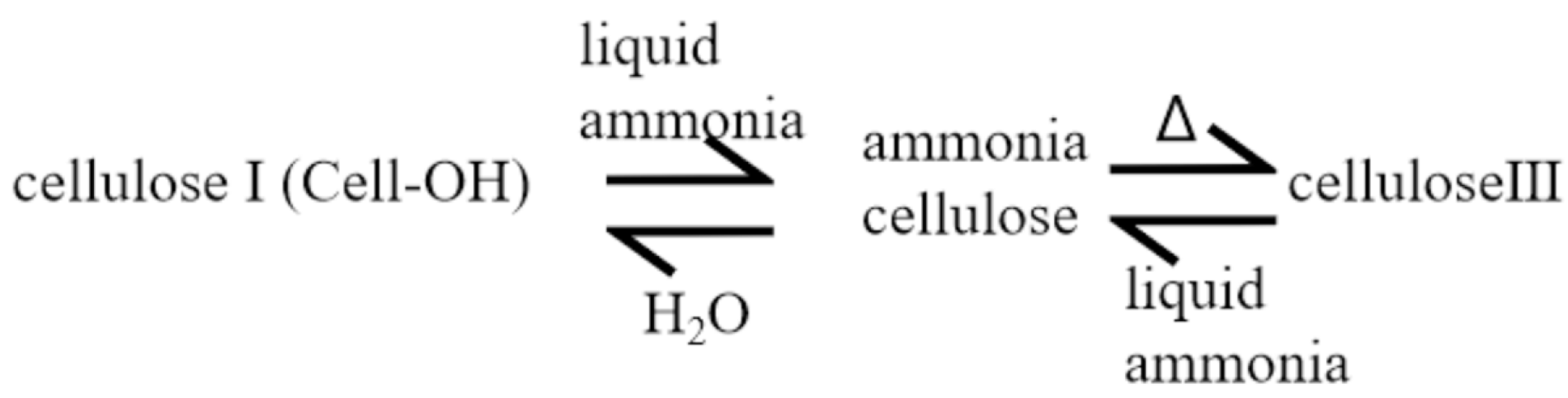
2.1. Physical Modification of Cellulose
2.2. Chemical Modification of Cellulose
2.2.1. Etherification Modification
2.2.2. Oxidative Modification
2.2.3. Esterification Modification
2.2.4. Grafting Polymerization
| Chemically Modified Types | Cellulose Type | Method of Modification | Ref. |
|---|---|---|---|
| Etherification modification | Microcrystalline cellulose | Cationic etherification modification with NAOH. | [36] |
| Etherification modification | Cellulose nanocrystals | The addition of NaNO2 to an aqueous solution causes the cellulose to deprotonate, forming a sodium salt. Step 2: Add NaHCO3 to introduce the carbonic acid group, which opens through the bond to produce the anionic oxygen group on the surface of the material. | [37] |
| Etherification modification | Hydroxypropyl cellulose | Etherification of alkali cellulose with propylene oxide. | [38] |
| Oxidative modification | Cellulose fibers | Ferrous ions are preloaded into the fiber cell wall, and after the introduction of hydrogen peroxide, the catalytic oxidation of cellulose is initiated in the fiber cell wall structure. | [43] |
| Oxidative modification | Natural cellulose | Natural cellulose is oxidized using sodium hypochlorite and different catalytic amounts of tetramethylpiperidin-1-oxy radical and sodium bromide. | [44] |
| Oxidative modification | Cellulose nanocrystals | Periodate is used to oxidize cellulose nanocrystals. | [45] |
| Esterification modification | Cotton cellulose | Cotton cellulose is esterified using a mixture of sulfuric and nitric acid. | [46] |
| Esterification modification | Cellulose nanocrystals | Cellulose nanocrystals are esterified using citric acid as catalyst and acetic anhydride as a reagent and reaction medium. | [47] |
| Grafting polymerization | Cellulose nanocrystals | Acrylyl ethyl trimethyl chloride is grafted with ammonium cerium nitrate through initiator polymerization. | [48] |
| Grafting polymerization | Cellulose nanocrystals | Poly (dimethylaminoethyl methacrylate), poly (diethyl methacrylate) and poly (diisopropylaminoethyl methacrylate) are grafted onto cellulose’s surface. | [49] |
| Grafting polymerization | Cellulose fibers | The thiocarbon H2O2 REDOX system grafts methylacryloyl hydroxybenzyl dimethyl ammonium chloride onto cellulose fibers. | [50] |
3. Modified Cellulose Hydrogel
3.1. Cyclodextrin
3.2. Alginate
3.3. Lignin
3.4. Chitin/Chitosan
3.5. Gelatin
| Biomass | Method | Cellulose | Ref. |
|---|---|---|---|
| Cyclodextrin | In situ crosslinking using epichlorohydrin as a crosslinker | Bamboo shoot cellulose | [53] |
| Cyclodextrin | In situ crosslinking using citric acid as a crosslinker | Carboxymethyl cellulose | [54] |
| Alginate | The single water-in-water emulsion gel method | Methylcellulose | [57] |
| Alginate | Ion crosslinking and supramolecular interaction methods | Nanocrystalline cellulose | [58] |
| Lignin | Cellulose and lignin are co-dissolved in 1-ethyl-3-methylimidazolium acetate and then reconstructed with distilled water | Cellulose | [60] |
| Lignin | Bacterial cellulose is combined with coniferol dehydrogenation polymer | Bacterial cellulose | [63] |
| Chitin/chitosan | Two natural polyelectrolytes, chitosan and carboxymethyl cellulose solution, are mixed and crosslinked with glutaraldehyde | Carboxymethyl cellulose | [65] |
| Chitin/chitosan | Methylene bisacrylamide, as a crosslinking agent, is irradiated in acetic acid/aqueous solution to synthesize the interpolymer complex of chitosan and carboxymethyl cellulose | Carboxymethyl cellulose | [66] |
| Gelatin | The water-in-oil emulsion technique | Hydroxyethyl cellulose | [69] |
| Gelatin | Bacterial cellulose copolymerizes with gelatin | Bacterial cellulose | [70] |
4. Applications of Modified Cellulose Hydrogels in the Sensing Field
4.1. Applications in pH Sensors
4.2. Applications in Humidity Sensors
4.3. Applications in Pressure/Strain Sensors

4.4. Applications in Flexible Sensors
| Type of Application | Modified | Principle | Ref. |
|---|---|---|---|
| pH sensor | Polyelectrolyte CMC is deposited directly into the bacterial cellulose matrix | A universal pH indicator or glucose oxidase is added to act as a colorimetric pH or glucose sensor, respectively | [72] |
| pH Sensor | Lignin-based nanoparticles and cellulose nanofibril | The introduced lignin-based nanoparticles determine the pH response of heat-responsive hydrogels | [64] |
| pH sensor | Biopolymerized chitin and nanofibril | The addition of green citric acid crosslinkers enables rapid surface charge conversion, consequent expansion and selective and efficient removal of ionic dyes, depending on pH conditions | [73] |
| pH sensor | Repeated freeze–thaw method in aqueous NaOH/urea solution | GO-enhanced regenerated cellulose/PVA ternary hydrogel has pH sensitivity | [75] |
| Humidity sensor | Lignin modification | A fiber optic relative humidity sensor is prepared by using cellulose hydrogel as a water-sensitive material | [78] |
| Humidity sensor | Both graphene oxide and citral are loaded into acrylic/bagasse cellulose and have strong hydrogen bonding with hydrogels | The added graphene oxide is humidity-sensitive | [79] |
| Pressure sensor | By Ca2+/Zn2+ ion exchange at room temperature, three states of fluid, brittle and rigid cellulose hydrogels are designed and prepared | The Ca2+ ion forms good compressive strength through a coordination crosslinking network, while the Zn2+ ion transforms cellulose (Sol-L-Zn2+) into a fluid state by eliminating the connections between cellulose molecules | [83] |
| Pressure sensors | Lignin modification | The free-ion-directed motion induced by external mechanical stimulation and the synergistic effect of negative LS (−) particles and positive QHEC (+) particles give the hydrogel good self-energy sensing ability | [84] |
| Pressure sensor | Lignin modification | Lignin is used as a conductive filler | [85] |
| Flexible sensor | Acrylonitrile and acrylamide copolymers are grafted onto the cellulose chain in the presence of zinc chloride, with zinc nitrate as an initiator | The cellulose hydrogels prepared have superstretchability, excellent tensile strength, high elasticity, good toughness and electrical conductivity, as well as fatigue resistance due to the presence of multiple hydrogen bond interactions in both dipole–dipole interactions and the hydrogel networks. | [90] |
| Flexible sensor | Lignin modification | Through a new dynamic REDOX system composed of sodium lignesulfonate/Fe3+, the introduction of Fe3+ can dynamically double-crosslink polymer chains, giving hydrogels good mechanical properties, better ionic conductivity, good sensing sensitivity and electrical self-repair. | [92] |
| Flexible Sensor | Introduction of polypyrrole-modified cellulose nanofibers | As a crosslinking agent, 4-formylphenylboric acid is used to crosslink polyvinyl alcohol and polyethylenimine, and a hydrogel network with borate ester bonds and imine bonds is constructed | [93] |
| Flexible sensor | Lignin graft modification | Lignin-grafted polyacrylamide/hydroxypropyl cellulose hydrogels have special skin adhesion and tensile properties | [61] |
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angewandate Chem. Int. Ed. 2005, 44, 3358. [Google Scholar]
- Eichhorn, S.J.; Young, R.J.; Davies, G.R. Modeling crystal and molecular deformation in regenerated cellulose fibers. Biomacromolecules 2005, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Schurz, J. “Trend in polymer science” a bright future for cellulose. Prog. Polym. Sci. 1999, 24, 481. [Google Scholar] [CrossRef]
- Chen, L.; Cao, S.; Huang, L.; Wu, H.; Hu, H.; Liu, K.; Lin, S. Progress in preparation of bamboo cellulose and its functional materials. J. For. Eng. 2021, 6, 1–13. [Google Scholar]
- Wang, L.; Lou, Y.; Tong, Z.; Meng, J.; Shi, X.; Cao, K.; Xia, Q.; Yu, H. Molecular dynamics mechanism of cellulose dissolved at room temperature by hydrated metal salt eutectic solvent. J. For. Eng. 2022, 7, 64–71. [Google Scholar]
- Yu, Y.; Li, Y.; Lou, Y.; Liu, Y.; Yu, H. Effect of lignin condensation on cellulolytic hydrolysis of wood fiber dissociated by low eutectic solvent. J. For. Eng. 2019, 6, 101–108. [Google Scholar]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of anisotropic hydrogels and their applications. Angewandate Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Tobias, K.; Etienne, C.; John, K.B.; Jana, S.S.; Peter, B.; Ingo, B. Smart Hierarchical Bio-Based Materials by Formation of Stimuli-Responsive Hydrogels inside the Microporous Structure of Wood. Adv. Mater. Interfaces 2016, 3, 1600233. [Google Scholar]
- Mondal, M.I.H. Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2009, 10, 9. [Google Scholar] [CrossRef]
- Ha, E.J.; Kim, Y.J.; An, S.S.A.; Kim, Y.K.; Lee, J.O.; Lee, S.G. Purification of his-tagged protein using Ni2+-poly(2-acetamidoacrylic acid) hydrogel. J. Chromatogr. B 2008, 876, 8. [Google Scholar] [CrossRef]
- Lee, Y.J.; Braun, P.V. Tunable inverse opal hydrogel pH sensors. Adv. Mater. 2003, 15, 563. [Google Scholar] [CrossRef]
- Sorber, J.; Steiner, G.; Schulz, V.; Guenther, M.; Gerlach, G.; Salzer, R. Hydrogel-based piezoresistive pH sensors: Investigations using FTIR attenuated total reflection spectroscopic imaging. Anal. Chem. 2008, 80, 2957. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymer in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088. [Google Scholar] [CrossRef]
- Wu, D.; Wang, T.; Lu, B.; Xu, X.; Cheng, S.; Jiang, X. Fabrication of supramolecular hydrogels for drug delivery and stem cell encapsulation. Langmuir 2008, 24, 10306. [Google Scholar] [CrossRef]
- Thomas, R.; Antje, P.; Johannes, H. Cellulose Science and Technology: Chemistry, Analysis, and Applications; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Feng, X.; Wang, C.; Zhang, H.; Song, Z.; Shang, S. Research progress of biomass-modified cellulose hydrogels. Mod. Chem. Ind. 2023, 43, 24–29. [Google Scholar]
- Csisza, R.E.; Fekete, E. Microstructure and surface properties of fibrous and ground cellulosic substrates. Langmuir ACS J. Surf. Colloids 2011, 27, 8444. [Google Scholar]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Dornyi, B.; Csiszar, E.; Somlai, C.; Sajo, I. Effect of liquid ammonia on the fine structure of linen fabrics. Text. Res. J. 2016, 76, 629–636. [Google Scholar] [CrossRef]
- Lewin, M.; Rau, R.O.; Sello, S.B. The Role of Liquid Ammonia in Functional Textile Finishes. Text. Res. J. 1974, 44, 680–686. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.Y.; Zhang, J.C.; Tang, Z.W. Effect of Different Ways of Ammonia Removal on the Fine Structure and Properties of Hemp Fibers. Adv. Mater. Res. 2011, 236, 91–97. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, X.; Ren, X.; Zhou, X. Structure and properties of cotton fiber before and after liquid ammonia treatment. J. Print. Dye. 2003, 29, 3. [Google Scholar]
- Zhang, H.; Feng, J.; Li, J. Effect of liquid ammonia treatment on structure and properties of ramie fabric. J. Print. Dye. 2008, 34, 4. [Google Scholar]
- Cai, Y.; Su, S.; Navik, R.; Wen, S.; Peng, X.; Pervez, M.N.; Lin, L. Cationic modification of ramie fibers in liquid ammonia. Cellulose 2018, 25, 4463–4475. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; Wang, Z.; Xu, X.; Li, H. Application status and development prospect of steam blasting technology. Food Ferment. Ind. 2021, 47, 322–328. [Google Scholar]
- Chen, H.; Li, Z. Study on steam blasting treatment of wheatgrass-ii. Analysis of mechanism of steam blasting treatment of wheatgrass. Cellul. Sci. Technol. 1999, 7, 9. [Google Scholar]
- Wu, Y.; Wu, Z.H.; Zhang, J.L. Preparation of cellulose micro/nano fibrils by sonochemical method and its morphological characterization. Key Eng. Mater. 2013, 562, 864–868. [Google Scholar] [CrossRef]
- Lu, H.; Wen, H.; Liu, X. Study on preparation of cellulose from potato residue by ultrasonic assisted acid method. China J. Cereals Oils 2012, 27, 5. [Google Scholar]
- Huang, L.J.; Xu, T.; Wang, H.T.; Wang, S.F. Preparation of Super Absorbent Polymer by Carboxymethyl Cellulose Grafting Acrylic Acid Using Low-Temperature Plasma Treatment. Adv. Mater. Res. 2011, 239, 2578–2583. [Google Scholar] [CrossRef]
- Mendhe, P.; Arolkar, G.; Shukla, S.; Deshmukh, R. Low-temperature plasma processing for the enhancement of surface properties and dyeability of wool fabric. J. Appl. Polym. Sci. 2016, 133, 43097. [Google Scholar] [CrossRef]
- Kol Ov, K.; Vosmansk, V.; Rimpelov, S. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Xu, W.; Wu, H.; Shao, Y.; Han, X.; Zhou, M.; Gu, P.; Li, Z. Preparation methods of cellulose nanocrystal and its application in treatment of environmental pollution: A mini-review. Colloid Interface Sci. Commun. 2023, 53, 100707. [Google Scholar] [CrossRef]
- Yang, C.; Lin, H.; Ling, X. Research progress on modification of cellulose. Appl. Chem. Ind. 2022, 51, 1408–1412. [Google Scholar]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, L.; Zhai, X.; Li, Z.; Wang, A.; Wu, T.; Hou, H. Etherification modification of microcrystalline cellulose and its application in starch membranes. Food Sci. 2021, 42, 65–71. [Google Scholar]
- Oyewo, O.A.; Mutesse, B.; Leswifi, T.Y.; Onyango, M.S. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 2019, 7, 103251. [Google Scholar] [CrossRef]
- Joshi, G.; Rana, V.; Naithani, S.; Varshney, V.K.; Sharma, A.; Rawat, J.S. Chemical modification of waste paper: An optimization towards hydroxypropyl cellulose synthesis. Carbohydr. Polym. 2019, 223, 115082. [Google Scholar] [CrossRef]
- Camy, S.; Montanari, S.; Rattaz, A.; Vignon, M.; Condoret, J.S. Oxidation of cellulose in pressurized carbon dioxide. J. Supercrit. Fluids 2009, 51, 188–196. [Google Scholar] [CrossRef][Green Version]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Harabagiu, V. Oxidized cellulose-Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef]
- Dias, G.J.; Peplow, P.V.; Teixeira, F. Osseous regeneration in the presence of oxidized cellulose and collagen. J. Mater. Sci. Mater. Med. 2003, 14, 739–745. [Google Scholar] [CrossRef]
- Wiseman, D.M.; Saferstein, L.; Wolf, S. Bioresorbable Oxidized Cellulose Composite Material for Prevention of Postsurgical Adhesions. U.S. Patent 6,500,777, 31 December 2002. [Google Scholar]
- Li, Q.; Wang, A.; Long, K.; He, Z.; Cha, R. Modified Fenton Oxidation of Cellulose Fibers for Cellulose Nanofibrils Preparation. ACS Sustain. Chem. Eng. 2018, 7, 1129–1136. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 219–225. [Google Scholar] [CrossRef]
- Azzam, F.; Galliot, M.; Putaux, J.L.; Heux, L.; Jean, B. Surface peeling of cellulose nanocrystals resulting from periodate oxidation and reductive amination with water-soluble polymers. Cellulose 2015, 22, 3701–3714. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A.; Vakutin, A.G. Oat-hull cellulose nitrates for explosive compositions. Dokl. Chem. 2019, 487, 221–225. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Fortunati, E.; Kenny, J.M.; Torre, L.; Foresti, M.L. Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 1358–1364. [Google Scholar] [CrossRef]
- Jiang, X.; Lou, C.; Hua, F.; Deng, H.; Tian, X. Cellulose nanocrystalsbased flocculants for high-speed and high-efficiency decolorization of colored effluents. J. Clean. Prod. 2020, 251, 119749. [Google Scholar] [CrossRef]
- Arredondo, J.; Woodcock, N.M.; Garclavaldez, O. Surface modification of cellulose nanocrystals via raft polymerization of CO2-responsive monomer-tuning hydrophobicity. Langmuir 2020, 36, 13989–13997. [Google Scholar] [CrossRef]
- Xing, X.; Lu, D.; Wang, X.; Liu, Z. Preparation and antibacterial function of quaternary ammonium salts grafted cellulose fiber initiated by Fe2+-H2O2Redox. J. Macromol. Sci. Part A 2009, 46, 560–565. [Google Scholar] [CrossRef]
- Yoon, S.J.; Hyun, H.; Lee, D.W.; Yang, D.H. Visible Light-Cured Glycol Chitosan Hydrogel Containing a Beta-Cyclodextrin-Curcumin Inclusion Complex Improves Wound Healing In Vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, T.; Cai, S.; Gao, X.; Tang, X.; Peng, L.; Chen, Z.; Hu, Q.; Li, J.; Zhang, H. Novel ROS-scavenging hydrogel produced in situ crosslinking between cyclodextrin and cellulose for promoting diabetic wound healing. Chem. Eng. J. 2024, 486, 150373. [Google Scholar] [CrossRef]
- Liu, S.; Luo, W.; Huang, H. Characterization and behavior of composite hydrogel prepared from bamboo shoot cellulose and β-cyclodextrin. Int. J. Biol. Macromol. 2016, 89, 527–534. [Google Scholar] [CrossRef]
- Xia, N.; Wan, W.; Zhu, S.; Qiang, L.A. Preparation of crystalline nanocellulose/hydroxypropyl β-cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wei, L.; Sun, R.; Zhang, Y.; Zhang, Z. Progress of alginate fiber in the biomedical field. Cotton Text. Technol. 2021, 49, 74–79. [Google Scholar]
- Silva, K.M.N.; de Carvalho, D.; Valente, V.M.; Rubio, J.C.; Faria, P.E.; Silva-Caldeira, P.P. Concomitant and controlled release of furazolidone and bismuth(III) incorporated in a cross-linked sodium alginate-carboxymethyl cellulose hydrogel. Int. J. Biol. Macromol. 2019, 126, 359–366. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Bhattacharya, S.S.; Chattopadhyay, P. Trivalent ion cross-linked pH sensitive alginate-methyl cellulose blend hydrogel beads from aqueous template. Int. J. Biol. Macromol. 2013, 57, 297–307. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Ran, H.; Zeng, Y.; Luo, C.; Wu, B.; Lin, L.; Xu, S. Research Progress in PLA/lignin Composites. Chem. Eng. Equip. 2021, 197–199+175. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.-H.; Kim, H.; Kim, Y.H.; Ha, S.H.; Lee, S.H. Application of cellulose/lignin hydrogel beads as novel supports for immobilizing lipase. J. Mol. Catal. B Enzym. 2015, 25, 33–39. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, X.; Wang, Y.; Shi, J.; Luo, S.; Fan, J.; Sun, B.; Liu, Y.; Fan, Q. Skin-adhesive lignin-grafted-polyacrylamide/hydroxypropyl cellulose hydrogel sensor for real-time cervical spine bending monitoring in human-machine Interface. Int. J. Biol. Macromol. Struct. Funct. Interact. 2023, 247, 125833. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Rosenkranz, A.; Wang, B.; Yu, J.; Srinivasan, S.; Rajabzadeh, A.R. MXene/0D nanocomposite architectures: Design, properties and emerging applications. Mater. Today Nano 2023, 24, 100428. [Google Scholar] [CrossRef]
- Danica, Z.; Dragica, S.; Irina, O.; Natalia, K.; Marina, S.; Jasmina, G.; Svetlana, D.; Branko, M.; Nikola, T.; Vuk, M. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar]
- Lu, J.; Zhu, W.; Dai, L.; Si, C.; Ni, Y. Fabrication of thermo- and pH-sensitive cellulose nanofibrils-reinforced hydrogel with biomass nanoparticles. Carbohydr. Polym. 2019, 215, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Shao, Z.; Chen, X. Electrical Behavior of a Natural Polyelectrolyte Hydrogel: Chitosan/Carboxymethylcellulose HydrogeL. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.F.; Qian, F.; Zhu, Q.S. Interpolymer complex polyampholytic hydrogel of chitosan and carboxymethyl cellulose (CMC): Synthesis and ion effect. Polym. Int. 2001, 50, 1370–1374. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Q.; Liu, Z.; Liu, J.; Tang, K. Modified nano microfibrillated cellulose/carboxymethyl chitosan composite hydrogel with giant network structure and quick gelation formability. Int. J. Biol. Macromol. 2019, 135, 561–568. [Google Scholar] [CrossRef]
- Lv, F.; Wang, C.; Zhu, P.; Zhang, C. Characterization of chitosan microparticles reinforced cellulose biocomposite sponges regenerated from ionic liquid. Cellulose 2014, 21, 4405–4418. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Sun, Y.; Zhou, D. Progress in aquatic protein-derived gelatin. Food Res. Dev. 2020, 41, 207–213. [Google Scholar]
- Kajjari, P.B.; Manjeshwar, L.S.; Aminabhavi, T.M. Semi-Interpenetrating Polymer Network Hydrogel Blend Microspheres of Gelatin and Hydroxyethyl Cellulose for Controlled Release of Theophylline. Ind. Eng. Chem. Res. 2011, 50, 7833–7840. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Somchob, B.; Rodthongkum, N.; Hoven, V.P. Bacterial cellulose-based re-swellable hydrogel: Facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr. Polym. 2021, 256, 117506. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.; Bang, J. pH-sensitive cellulose/chitin nanofibrillar hydrogel for dye pollutant removal. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2023, 317, 121090. [Google Scholar] [CrossRef]
- Saygili, E.; Devamoglu, U.; Bayir, E.; Yesil-Celiktas, O. An optical pH-sensor integrated microfluidic platform multilayered with bacterial cellulose and gelatin methacrylate to mimic drug-induced lung injury. J. Ind. Eng. Chem. 2023, 121, 190–199. [Google Scholar] [CrossRef]
- Rui-Hong, X.; Peng-Gang, R.; Jian, H.; Fang, R.; Sun, Z.F. Preparation and properties of graphene oxide-regenerated cellulose/polyvinyl alcohol hydrogel with pH-sensitive behavior. Carbohydr. Polym. 2015, 138, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.L.; Sun, T.; Grattan, K.T.V. Fibre-optic sensor technologies for humidity and moisture measurement. Sens. Actuator A Phys. 2008, 144, 280–295. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hassan, S.H.B.; Yahya, M.; Lee, H.V. Novel Superabsorbent Cellulose-Based Hydrogels: Present Status, Synthesis, Characterization, and Application Prospects. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composites: A Reference Series; Springer: New York, NY, USA, 2018; pp. 1–41. [Google Scholar]
- Shi, X.; Zhang, Z.; Yang, M.; Ji, H.; Ji, X.; Tian, Z.; Chen, J. Cellulose Fibers Extraction from Cotton Stalk via One-Step Acid Bleachable Pretreatment for Fiber-Optic Humidity Sensor Fabrication. Waste Biomass Valorization 2023, 14, 823–832. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, H.; Cheng, J.H. Constructing a novel humidity sensor using acrylic acid/bagasse cellulose porous hydrogel combining graphene oxide and citral for antibacterial and intelligent fruit preservation. Carbohydr. Polym. 2024, 326, 121639. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Sun, H.; Hong, D.; Li, L.; Yang, Y.; Yong, X.; Zhang, C.; Cui, J. An optical fiber relative humidity sensor based on hollow-core fiber and hydroxypropyl methylcellulose hydrogel film. Optik 2019, 195, 163172. [Google Scholar] [CrossRef]
- Xiao, T.; Ji, H.; Liu, X.H.; Zhu, W. Lignin-containing fibers extraction and hydrogel preparation for fiber-optic relative humidity sensor fabrication. Ind. Crops Prod. 2021, 173, 114112. [Google Scholar]
- Ma, J.; Zhong, J.; Sun, F.; Liu, B.; Peng, Z.; Lian, J.; Wu, X.; Li, L.; Hao, M.; Zhang, T. Hydrogel sensors for biomedical electronics. Chem. Eng. J. 2024, 481, 148317. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, K.; Bukhvalov, D.; Zhang, X.F.; He, M. Cellulose Hydrogels by Reversible Ion-Exchange as Flexible Pressure Sensors. Adv. Mater. Technol. 2020, 5, 2000358. [Google Scholar] [CrossRef]
- Wang, Q.H.; Pan, X.F.; Guo, J.J.; Huang, L.; Ni, Y. Lignin and cellulose derivatives-induced hydrogel with asymmetrical adhesion, strength, and electriferous properties for wearable bioelectrodes and self-powered sensors. Chem. Eng. J. 2021, 414, 128903. [Google Scholar] [CrossRef]
- Li, M.F.; Tu, Q.Y.; Long, X.; Zhang, Q.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Flexible conductive hydrogel fabricated with polyvinyl alcohol, carboxymethyl chitosan, cellulose nanofibrils, and lignin-based carbon applied as strain and pressure sensor. Int. J. Biol. Macromol. 2021, 166, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.C.; Wang, B.; Li, J.P.; Xu, J.; Li, J.; Zeng, J.; Gaoa, W.; Chena, K. A self-healing, recyclable and conductive gelatin/nanofibrillated cellulose/Fe3+ hydrogel based on multi-dynamic interactions for a multifunctional strain sensor. Mater. Horiz. 2022, 9, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, K.; An, L.; Ding, D.; Chen, S.; Liu, Z.; Liu, Y.; Xu, F. Multi-physics coupling reinforced polyvinyl alcohol/cellulose nanofibrils based multifunctional hydrogel sensor for human motion monitoring. Int. J. Biol. Macromol. 2023, 235, 123841. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Ao, X.; Li, C.; Yang, L. Preparation and Properties of Cellulose Nanofiber-Reinforced Ionic Conductive Hydrogels Sensor. Polym. Sci. Ser. A 2022, 64, 765–774. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; Luo, Y.; Wu, Z.; Yu, J.; Chen, H.; Zhou, Y.; Zhang, H.; Tao, K.; Chen, X.; et al. High-Performance Hydrogel Sensors Enabled Multimodal and Accurate Human–Machine Interaction System for Active Rehabilitation. Adv. Mater. 2024, 36, 2309868. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, Tough, Antifreezing, and Conductive Cellulose Hydrogel for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256. [Google Scholar] [CrossRef]
- Jing, X.; Li, H.; Mi, H.Y.; Liu, Y.; Feng, P.Y.; Tan, Y.M.; Turng, L.S. Highly Transparent, Stretchable, and Rapid Self-Healing Polyvinyl Alcohol/Cellulose Nanofibril Hydrogel Sensors for Sensitive Pressure Sensing and Human Motion Detection. Sens. Actuators B Chem. 2019, 295, 159–167. [Google Scholar] [CrossRef]
- Song, Y.; Niu, L.; Ma, P.; Li, X.; Jacko, F.; Liu, Z. Rapid preparation of carboxymethyl cellulose conductive hydrogel at room temperature and its application in flexible strain sensors. Polym. Mater. Sci. Eng. 2023, 39, 11. [Google Scholar]
- Qiu, Y.; Lin, J.; Qin, J.; Wu, J.; Lin, F.; Lu, B.; Tang, L.; Huang, B. Dual dynamic covalent bond cross-linked nanocellulose-conductive hydrogels and their flexible sensors. Chem. Ind. Prog. 2022, 41, 4406–4416. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, S.; Maimaitiyiming, X. High tensile poly(vinyl alcohol)/Carboxymethyl cellulose sodium/Polyacrylamide/Borax dual network hydrogel for lifting heavy weight and multi-functional sensors. Cellulose 2023, 30, 11721–11736. [Google Scholar] [CrossRef]
- Hang, T.; Chen, Y.; Yin, Z.Z.J. Highly stretchable polyvinyl alcohol composite conductive hydrogel sensors reinforced by cellulose nanofibrils and liquid metal for information transmission. Int. J. Biol. Macromol. Struct. Funct. Interact. 2024, 258, 128855. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Liu, W.; Liu, J. Highly Sensitive and Robust Polysaccharide-Based Composite Hydrogel Sensor Integrated with Underwater Repeatable Self-Adhesion and Rapid Self-Healing for Human Motion Detection. ACS Appl. Mater. Interfaces 2022, 14, 24741–24754. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, L.; Fu, Q. Low-Temperature tolerance and conformal adhesion zwitterionic hydrogels as electronic skin for strain and temperature responsiveness. Chem. Eng. J. 2022, 431, 133782. [Google Scholar] [CrossRef]
- Qin, Y.; Wei, E.; Cui, C.; Xie, J. High Tensile, Antibacterial, and Conductive Hydrogel Sensor with Multiple Cross-Linked Networks Based on PVA/Sodium Alginate/Zinc Oxide. ACS Omega 2024, 9, 16851–16859. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yuan, Q.-Y.; Lou, C.-W.; Li, T.-T.; Lin, J.-H. Modified Nanocellulose Hydrogels and Applications in Sensing Fields. Gels 2025, 11, 140. https://doi.org/10.3390/gels11020140
Yang L, Yuan Q-Y, Lou C-W, Li T-T, Lin J-H. Modified Nanocellulose Hydrogels and Applications in Sensing Fields. Gels. 2025; 11(2):140. https://doi.org/10.3390/gels11020140
Chicago/Turabian StyleYang, Lan, Qian-Yu Yuan, Ching-Wen Lou, Ting-Ting Li, and Jia-Horng Lin. 2025. "Modified Nanocellulose Hydrogels and Applications in Sensing Fields" Gels 11, no. 2: 140. https://doi.org/10.3390/gels11020140
APA StyleYang, L., Yuan, Q.-Y., Lou, C.-W., Li, T.-T., & Lin, J.-H. (2025). Modified Nanocellulose Hydrogels and Applications in Sensing Fields. Gels, 11(2), 140. https://doi.org/10.3390/gels11020140













