Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration
Abstract
:1. Introduction

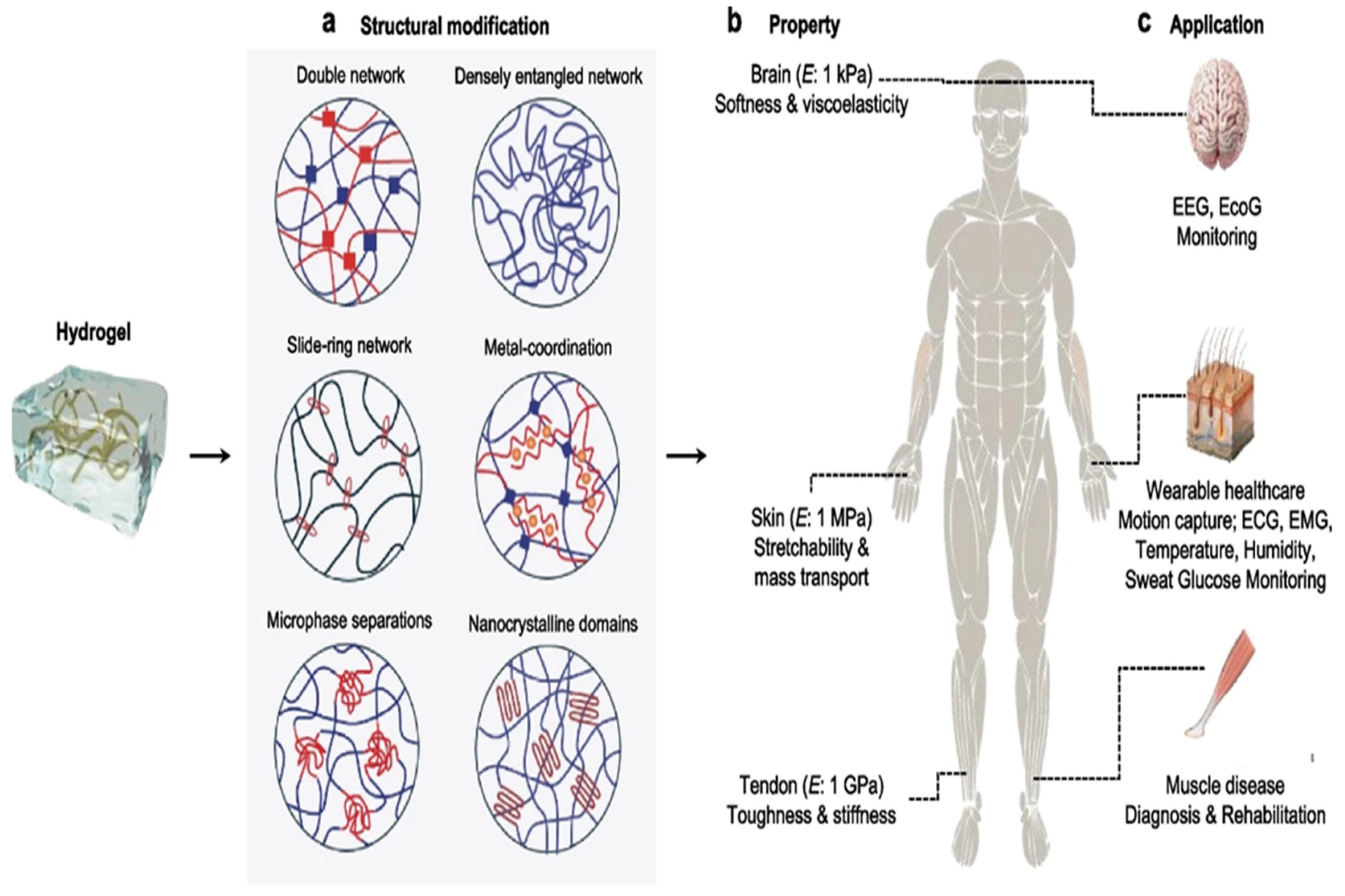
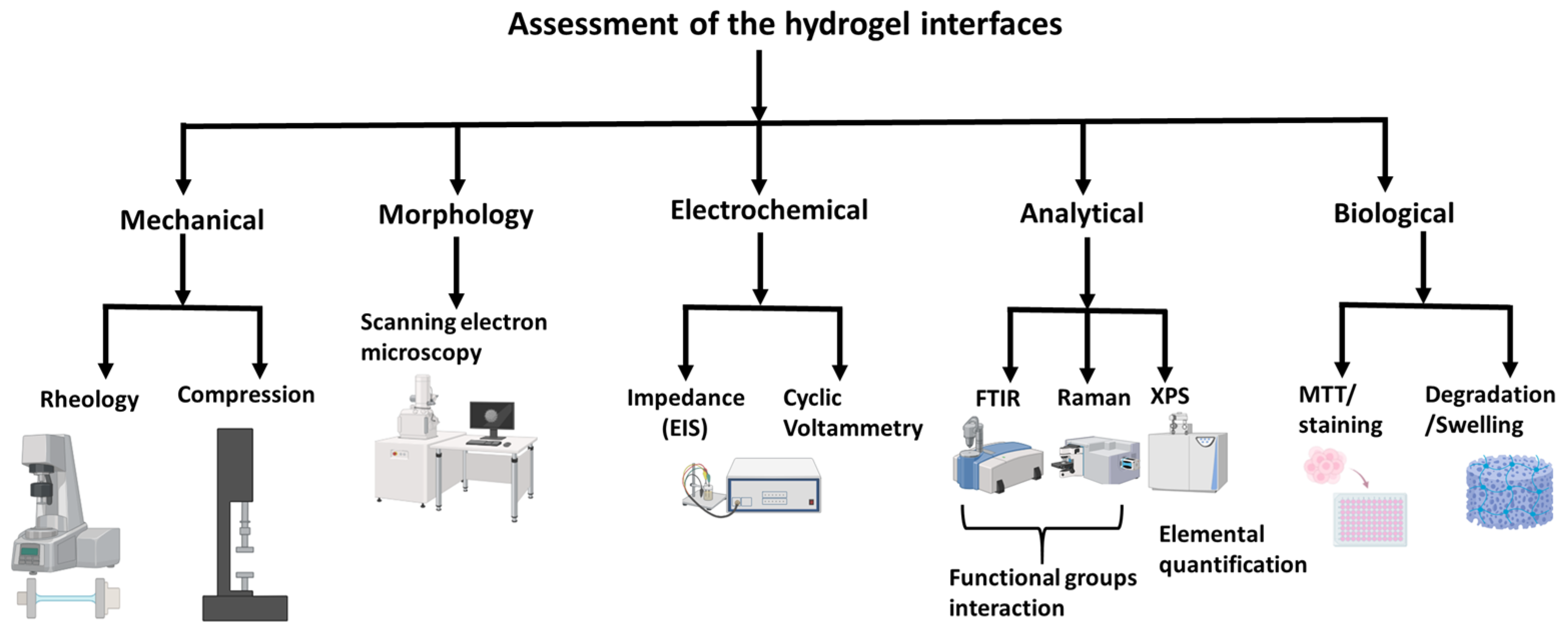
2. Critical Attributes of Hydrogels for HMI Integration
2.1. Biocompatibility
2.2. Electrical Conductivity
2.3. Optical Clarity
2.4. Intrinsic Healing Behavior
2.5. Adhesion
3. Recent Innovations in Hydrogel Design
3.1. Conductive Hydrogels

3.2. Hybrid and Composite Hydrogels
3.3. Fabrication Techniques
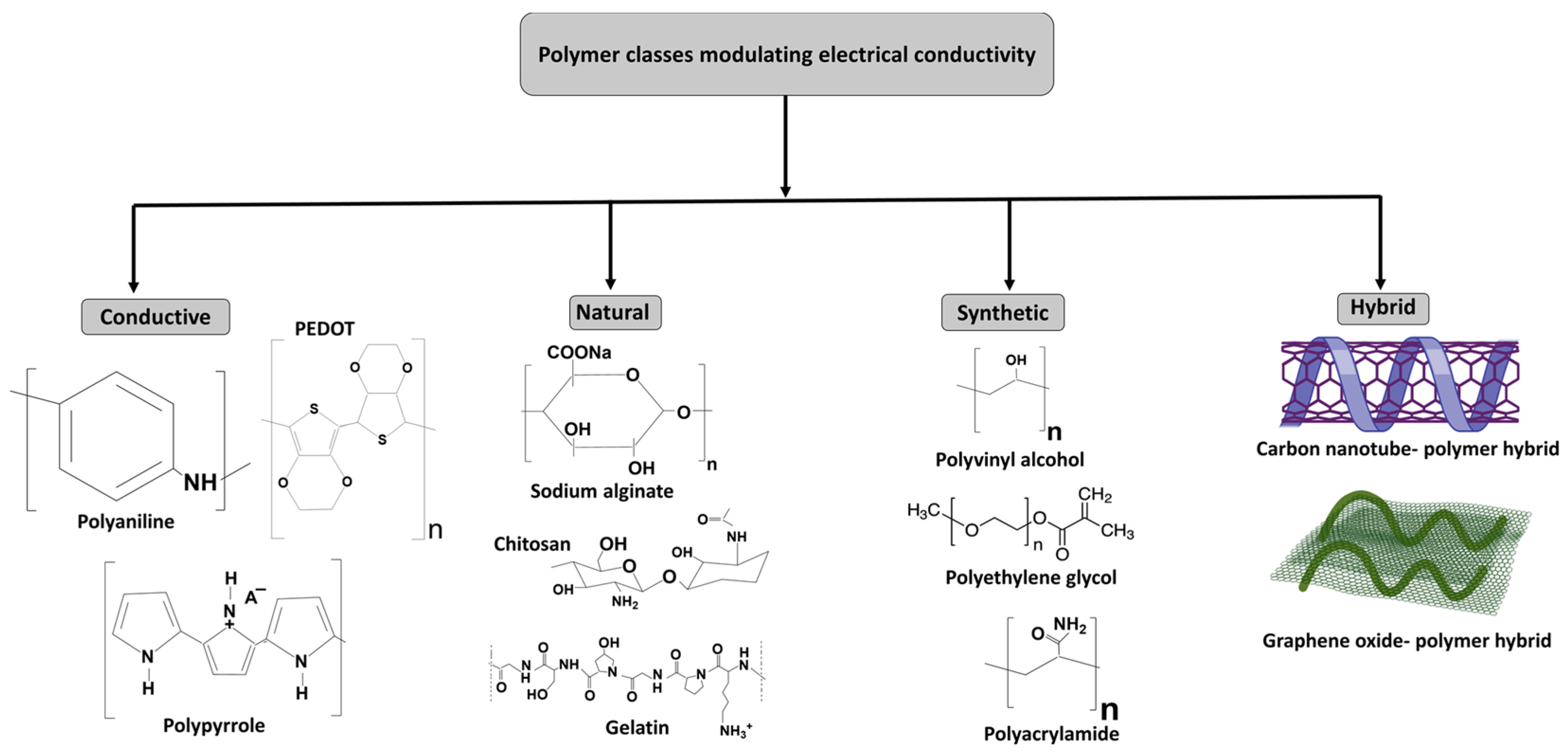
4. Polymer Classes Modulating Electrical Conductivity in Hydrogels
- i.
- Conductive polymers
- ii.
- Natural polymers
- iii.
- Synthetic polymers
- iv.
- Hybrid polymers for conductive hydrogels
5. Spectrum of the Mechanisms of Hydrogel–Machine Interfacing
6. Hydrogel Interfaces for Seamless Human–Machine Integration
| Category | Application | Hydrogel Used | Level of Invasiveness | References |
|---|---|---|---|---|
| Epidermal and wearable | Ultrasound probe | Couplant for ultrasonic imaging | [138,142,143] | |
| Epidermal electrode | Skin contact applications | |||
| Wound dressing | Wound healing and moisture retention | Noninvasive communication | ||
| Sweat sensor | Monitoring sweat biomarkers | |||
| Contact lens | Soft contact lenses for comfort and biocompatibility | |||
| Implantable | Implantable electrode | Deep-tissue or neural interfaces | [144] | |
| Tissue adhesive | Bonding tissues in surgical applications | [145] | ||
| Drug delivery carrier | Controlled drug release within the body | Fully invasive (long-term contact with internal organs and tissues) | [146] | |
| Anti-FBR coating | Reduced foreign body response (FBR) to implants | [147] | ||
| Optical waveguide | Light-based medical sensors | [148] | ||
| Ingestible | Ingestible device | Gastrointestinal monitoring and drug delivery | [149] | |
| Catheter | Reduce friction and enhanced biocompatibility | [150] | ||
| Guidewire | Minimally invasive procedures | Minimally invasive in body cavities (thoracic and abdominal cavities) and tubular organs | [138] | |
| Stent | Blood vessel support and drug delivery | [138,151] |
6.1. Epidermal and Wearable Applications
| Product | Application | Hydrogel Use | Refs. |
|---|---|---|---|
| Neuralink | Implantable brain–computer interface (BCI) | Biocompatible hydrogel coating for electrode stability and decreased immune response | [176] |
| Medtronic Deep Brain Stimulation (DBS) Systems | Neuromodulation, Parkinson’s disease, and epilepsy treatment | Soft electrode coatings with potential hydrogel applications for improved conductivity and tissue integration | [122] |
| Cortec Neuroprosthetic Electrodes | High-resolution neural recording and stimulation | Hydrogel-modified electrodes being researched for stable neural signal transmission | [177] |
| BrainCo-BMI Headset | Noninvasive brain–computer interface | Hydrogel-based EEG electrodes for improved signal detection and comfort | [94] |
| Synchron Stentrode | Implantable neural interface for paralyzed patients | Exploring hydrogel coatings for electrode longevity and reduced inflammation | [122] |
| Bio-Signal Technologies EEG and EMG Electrodes | Neural signal acquisition for rehabilitation | Soft hydrogel interfaces for high- fidelity signal recording | [65,122] |
6.2. Implantable Applications
6.3. Minimally Invasive Applications
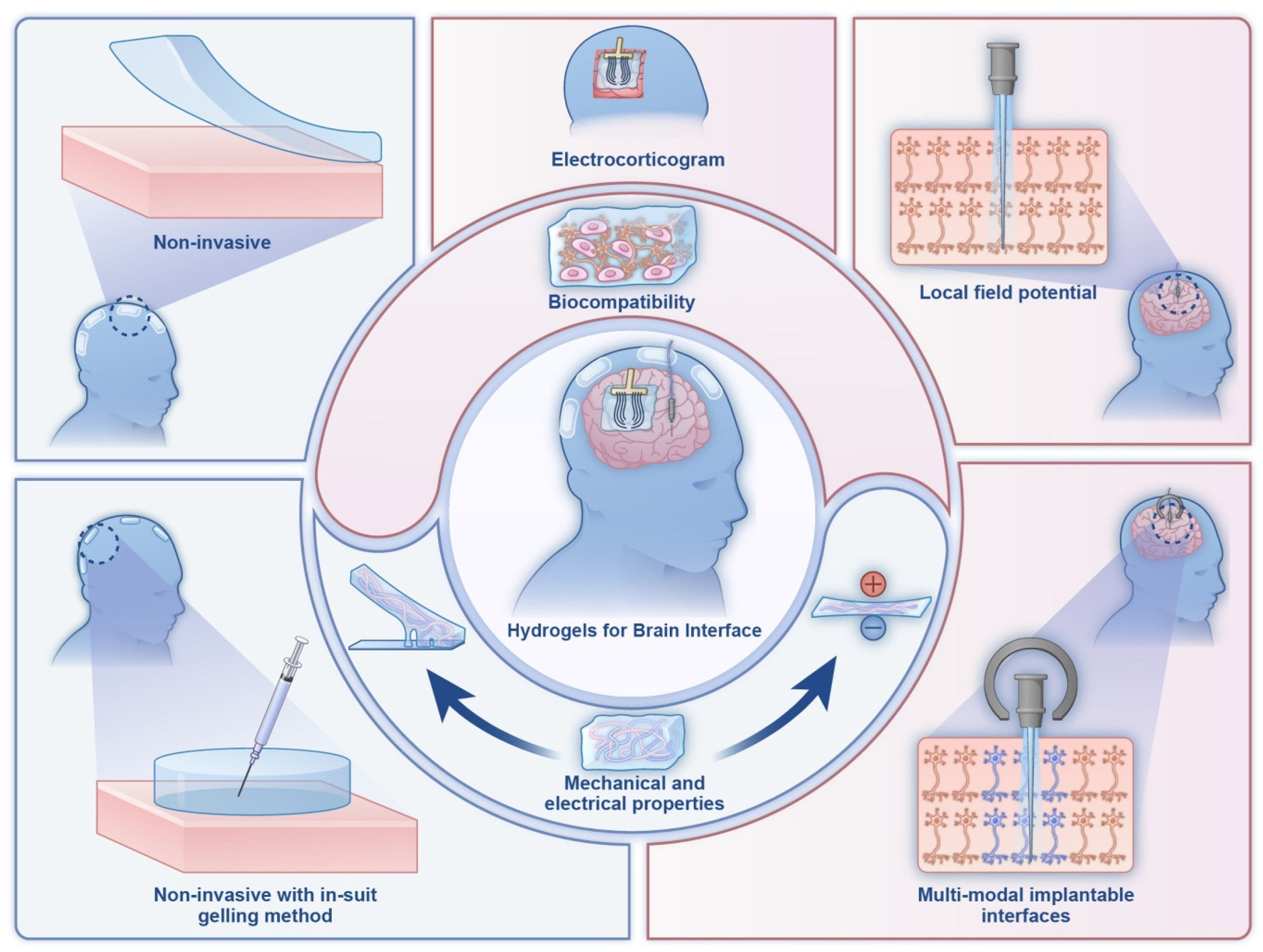
6.4. Neural and Bioelectronic Interfaces
6.5. Soft Robotics and Actuators
6.6. Haptics and Sensory Systems
7. Regulatory Requirements and the Patent Landscape of Hydrogel Biointerfaces
8. Challenges and Limitations of Hydrogel Interfaces in HMI
9. Future Perspectives
10. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HMI | Human–machine interfacing |
| BMI | Brain–machine interfaces |
| CMC | Carboxymethyl cellulose |
| PVA | Polyvinyl alcohol |
| PAA | Polyacrylic acid |
| PAAm | Polyacrylamide |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSS | Poly(styrene sulfonate) |
| PEDOT:SL-PAA | Poly(3,4-ethylenedioxythiophene): sulfonated lignin on poly (acrylic acid) |
| P(SBMA-co-AAm) | Poly(2-(methacryloyloxy) ethyl)dimethyl-(3-sulfopropyl)ammonium hydrox-ide-co-acrylamide) |
| LED | Light-emitting diode |
| ECM | Extracellular matrix |
| ECG | Electrocardiogram |
| EMG | Electromyogram |
| ECoG | Electrocorticography |
| AFP | Antifreeze proteins |
| FBR | Foreign body response |
| CNT | Carbon nanotube |
| LM | Liquid metal |
| LFP | Local field potential |
| GelMA | Gelatin methacrylate |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| PEG | Polyethylene glycol |
| IOP | Intraocular pressure |
| PAc | Polyactylene |
| PTh | Polythiophene |
References
- Vermeulen, N.; Parker, J.N.; Penders, B. Understanding life together: A brief history of collaboration in biology. Endeavour 2013, 37, 162–171. [Google Scholar] [CrossRef]
- Licardo, J.T.; Domjan, M.; Orehovački, T. Intelligent robotics—A systematic review of emerging technologies and trends. Electronics 2024, 13, 542. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Ge, J.; Wang, Y. Advancements in brain-machine interfaces for application in the metaverse. Front. Neurosci. 2024, 18, 1383319. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Antonini, M.-J.; Anikeeva, P. Next-generation interfaces for studying neural function. Nat. Biotechnol. 2019, 37, 1013–1023. [Google Scholar] [CrossRef]
- Lotti, F.; Ranieri, F.; Vadalà, G.; Zollo, L.; Di Pino, G. Invasive intraneural interfaces: Foreign body reaction issues. Front. Neurosci. 2017, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Fleming, G.A.; Petrie, J.R.; Holl, R.W.; Bergenstal, R.M.; Peters, A.L. Insulin pump risks and benefits: A clinical appraisal of pump safety standards, adverse event reporting, and research needs: A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015, 38, 716–722. [Google Scholar]
- Farra, R.; Sheppard, N.F., Jr.; McCabe, L.; Neer, R.M.; Anderson, J.M.; Santini, J.T., Jr.; Cima, M.J.; Langer, R. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl. Med. 2012, 4, 122ra121. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Lieber, C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 330–345. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Yeo, W.-H.; Akhtar, A.; Norton, J.J.; Kwack, Y.-J.; Li, S.; Jung, S.-Y.; Su, Y.; Lee, W.; Xia, J. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- de la Oliva, N. Biological Response to Implanted Intraneural Electrodes. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2018. [Google Scholar]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Green, R.A.; Hassarati, R.T.; Goding, J.A.; Baek, S.; Lovell, N.H.; Martens, P.J.; Poole-Warren, L.A. Conductive hydrogels: Mechanically robust hybrids for use as biomaterials. Macromol. Biosci. 2012, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I. Transcutaneous Electrical Nerve Stimulation (TENS): Research to Support Clinical Practice; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Green, R.A.; Baek, S.; Poole-Warren, L.A.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11, 014107. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Yin, R.; Xu, Z.; Mei, M.; Chen, Z.; Wang, K.; Liu, Y.; Tang, T.; Priydarshi, M.K.; Meng, X.; Zhao, S. Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram. Nat. Commun. 2018, 9, 2334. [Google Scholar] [CrossRef] [PubMed]
- Blacklow, S.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Ji, T.; Xue, Y.; Zhao, L.; Zhao, K.; Jia, B.; Wang, B.; Wang, J.; Zhang, S. Self-healing hydrogel bioelectronics. Adv. Mater. 2024, 36, 2306350. [Google Scholar] [CrossRef] [PubMed]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2024, 35, 2416390. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Liao, S.; Liu, H.; Qiao, Y.; Zhou, J. Multifunctional conductive hydrogel interface for bioelectronic recording and stimulation. Adv. Healthc. Mater. 2024, 13, 2400562. [Google Scholar] [CrossRef]
- Jia, L.; Li, Y.; Ren, A.; Xiang, T.; Zhou, S. Degradable and Recyclable Hydrogels for Sustainable Bioelectronics. ACS Appl. Mater. Interfaces 2024, 16, 32887–32905. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Z.; Rogers, J.A. Functional hydrogel interface materials for advanced bioelectronic devices. Acc. Mater. Res. 2021, 2, 1010–1023. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, J.; Zhang, L.; Guana, H.; Lei, Z.; Zhang, X.; Yang, C.; Zhua, Y.; Sun, Q.; Xua, L. Biocompatible wearable touch panel based on ionically conductive organic hydrogels with anti-freezing, anti-dehydration, self-healing, and underwater adhesion properties. arXiv 2023, arXiv:2308.14006. [Google Scholar]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT: Sulfonated lignin as conductive materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, J.; Zhou, M.; Yang, Y.; Wang, X.; Wang, J.; Cao, Y.; Wang, W.; Wu, D. A multi-model, large range and anti-freezing sensor based on a multi-crosslinked poly (vinyl alcohol) hydrogel for human-motion monitoring. J. Mater. Chem. B 2020, 8, 11010–11020. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jing, R.; Ren, X.; Gao, G. Fish-inspired anti-icing hydrogel sensors with low-temperature adhesion and toughness. J. Mater. Chem. A 2020, 8, 9373–9381. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Xiang, P.; Dai, Y.; Gao, Y.; Xu, H.; Yu, J.; Gao, G.; Chen, K. Protein-assisted freeze-tolerant hydrogel with switchable performance toward customizable flexible sensor. Chem. Eng. J. 2022, 428, 131171. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic salts induce thermally reversible and anti-freezing cellulose hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Wu, S.; Wang, T.-W.; Du, Y.; Yao, B.; Duan, S.; Yan, Y.; Hua, M.; Alsaid, Y.; Zhu, X.; He, X. Tough, anti-freezing and conductive ionic hydrogels. NPG Asia Mater. 2022, 14, 65. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly (vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ji, C.; Meng, Q.; Mi, H.; Yang, Q.; Li, Z.; Yang, N.; Qiu, J. Freeze-tolerant hydrogel electrolyte with high strength for stable operation of flexible zinc-ion hybrid supercapacitors. Small 2022, 18, 2200055. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V. Strategies in the preparation of conductive polyvinyl alcohol hydrogels for applications in flexible strain sensors, flexible supercapacitors, and triboelectric nanogenerator sensors: An overview. Adv. Compos. Hybrid Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Dai, X.; Long, Y.; Jiang, B.; Guo, W.; Sha, W.; Wang, J.; Cong, Z.; Chen, J.; Wang, B.; Hu, W. Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res. 2022, 15, 5461–5468. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Moon, S.E.; Kim, J.H.; Kang, S.M. Ultrasensitive and highly stretchable multiple-crosslinked ionic hydrogel sensors with long-term stability. Nano-Micro Lett. 2023, 15, 51. [Google Scholar] [CrossRef]
- Azizullah; Haider, A.; Kortz, U.; Joshi, S.A.; Iqbal, J. Polyethyleneimine-Polyoxometalate-Based Supramolecular Self-assembled pH-Responsive Hydrogels: Formulation and in vitro Evaluation. ChemistrySelect 2017, 2, 5905–5912. [Google Scholar] [CrossRef]
- Wang, C.; Wiener, C.G.; Sepulveda-Medina, P.I.; Ye, C.; Simmons, D.S.; Li, R.; Fukuto, M.; Weiss, R.; Vogt, B.D. Antifreeze hydrogels from amphiphilic statistical copolymers. Chem. Mater. 2018, 31, 135–145. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Ren, Y.; Jin, G.; Zhang, C.; Chen, W.; Yan, F. Poly (ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 2020, 7, 919–927. [Google Scholar] [CrossRef]
- Gong, X.; Zhao, C.; Wang, Y.; Luo, Y.; Zhang, C. Antifreezing, ionically conductive, transparent, and antidrying carboxymethyl chitosan self-healing hydrogels as multifunctional sensors. ACS Biomater. Sci. Eng. 2022, 8, 3633–3643. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene nanocomposite organohydrogel for flexible, healable, low-temperature tolerant strain sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Zhou, X.; Liu, Y.; Wang, N.; Gao, C. Highly sensitive zwitterionic hydrogel sensor for motion and pulse detection with water retention, adhesive, antifreezing, and self-healing properties. ACS Appl. Mater. Interfaces 2022, 14, 47100–47112. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Q.; Da, G.; Yao, J.; Shao, Z.; Chen, X. A highly stretchable and anti-freezing silk-based conductive hydrogel for application as a self-adhesive and transparent ionotronic skin. J. Mater. Chem. C 2021, 9, 8955–8965. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A review of advanced hydrogel applications for tissue engineering and drug delivery systems as biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Xu, Z.; Li, Z.; Zhang, L.; Wan, P. Flexible conformally bioadhesive MXene hydrogel electronics for machine learning-facilitated human-interactive sensing. Adv. Mater. 2024, 36, 2401035. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ao, Q.; Jiang, T.; Tong, X.; Ding, R.; Li, X.; Tang, J. Sustainable and high performance MXene hydrogel with interlocked structure for machine learning-facilitated human-interactive sensing. Chem. Eng. J. 2024, 499, 156432. [Google Scholar] [CrossRef]
- Chen, K.; Liu, B.; Hu, N.; Fan, Q.; Zhan, F.; Zhang, Z.; Ni, Z.; Li, X.; Hu, T. A biodegradable, highly sensitive and multifunctional mechanical sensor based on rGO-silk fibroin hydrogel for human motion detection and gesture recognition. J. Mater. Chem. A 2024, 12, 3283–3293. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Y.; Das, P.; Ma, J.; Wang, S.; Zhu, G.; Wu, Z.-S. Advancing MXene-based integrated microsystems with micro-supercapacitors and/or sensors: Rational design, key progress, and challenging perspectives. J. Mater. 2023, 9, 1242–1262. [Google Scholar] [CrossRef]
- Zhang, C.W.; Chen, C.; Duan, S.; Yan, Y.; He, P.; He, X. Hydrogel-based soft bioelectronics for personalized healthcare. Med-X 2024, 2, 20. [Google Scholar] [CrossRef]
- Han, F.; Chen, S.; Wang, F.; Liu, M.; Li, J.; Liu, H.; Yang, Y.; Zhang, H.; Liu, D.; He, R. High-Conductivity, Self-Healing, and Adhesive Ionic Hydrogels for Health Monitoring and Human-Machine Interactions Under Extreme Cold Conditions. Adv. Sci. 2025, 2412726. [Google Scholar] [CrossRef]
- Villa, J.; Cury, J.; Kessler, L.; Tan, X.; Richter, C.-P. Enhancing biocompatibility of the brain-machine interface: A review. Bioact. Mater. 2024, 42, 531–549. [Google Scholar] [CrossRef]
- Wan, R.; Yu, J.; Quan, Z.; Ma, H.; Li, J.; Tian, F.; Wang, W.; Sun, Y.; Liu, J.; Gao, D. A reusable, healable, and biocompatible PEDOT: PSS hydrogel-based electrical bioadhesive interface for high-resolution electromyography monitoring and time–frequency analysis. Chem. Eng. J. 2024, 490, 151454. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, H.S.; Jeong, H.; Kim, D.-H. Recent Advances in Conductive Hydrogels for Soft Biointegrated Electronics: Materials, Properties, and Device Applications. Wearable Electron. 2024, 1, 255–280. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Pan, Y.; He, M.; Wu, J.; Qi, H.; Cheng, Y. One-step synthesis of MXene-functionalized PEDOT: PSS conductive polymer hydrogels for wearable and noninvasive monitoring of sweat glucose. Sens. Actuators B Chem. 2024, 401, 135055. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. High-performing conductive hydrogels for wearable applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Gong, X.; Chang, Y.; Zheng, J. Highly conductive and tough double-network hydrogels for smart electronics. SmartMat 2024, 5, e1160. [Google Scholar] [CrossRef]
- Kolodziej, L.; Iwasińska-Kowalska, O.; Wróblewski, G.; Giżewski, T.; Jakubowska, M.; Lekawa-Raus, A. Hydrogels and Carbon Nanotubes: Composite Electrode Materials for Long-Term Electrocardiography Monitoring. J. Funct. Biomater. 2024, 15, 113. [Google Scholar] [CrossRef]
- Chen, R.-S.; Gao, M.; Chu, D.; Cheng, W.; Lu, Y. Self-powered hydrogel wearable bioelectronics. Nano Energy 2024, 128, 109960. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Yun, G.; Gong, L.; Chen, Z.; Jin, S.; Du, H.; Jiang, Z.; Li, W. A laminated gravity-driven liquid metal-doped hydrogel of unparalleled toughness and conductivity. Adv. Funct. Mater. 2024, 34, 2308113. [Google Scholar] [CrossRef]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive ionic conductive alginate/gelatin organohydrogels with tunable functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar] [CrossRef]
- Fan, Z.; Ji, D.; Kim, J. Recent Progress in Mechanically Robust and Conductive-Hydrogel-Based Sensors. Adv. Intell. Syst. 2023, 5, 2300194. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Huang, S.; Geng, L.; Wu, J.; Mao, G.; Peng, X.; Cheng, Y. Self-assembly polysaccharide network regulated hydrogel sensors with toughness, anti-freezing, conductivity and wide working conditions. Chem. Eng. J. 2024, 497, 154409. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Wang, S.; Tian, G.; Yang, T.; Huang, L.; Ao, Y.; Lan, B.; Zhang, J.; Xu, T. Body temperature-triggered adhesive ionic conductive hydrogels for bioelectrical signal monitoring. Chem. Eng. J. 2024, 498, 155195. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Qian, R.; Yu, Z.; Ye, C. Interfacial engineering of liquid metal nanoparticles for the fabrication of conductive hydrogels: A review. Chem. Eng. J. 2024, 486, 150197. [Google Scholar] [CrossRef]
- He, Q.; Cheng, Y.; Deng, Y.; Wen, F.; Lai, Y.; Li, H. Conductive hydrogel for flexible bioelectronic device: Current progress and future perspective. Adv. Funct. Mater. 2024, 34, 2308974. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Zhao, X.; Yang, L.; Zhao, Y.; Wei, R.; Wu, J. Kirigami Design Smart Contact Lens for Highly Sensitive Eyelid Pressure Measurement. ACS Sens. 2024, 10, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, M.C.; Lee, J.Y. Nanomaterial-based electrically conductive hydrogels for cardiac tissue repair. Int. J. Nanomed. 2022, 17, 6181. [Google Scholar] [CrossRef]
- Zhao, R.; Luo, J.; Liu, J.; Ke, T.; Zhang, J.; Gaidau, C.; Zhou, J.; Gu, H. Ultra-flexible, anti-freezing, and adhesive collagen fiber-derived conductive organohydrogel e-skin for strain, humidity, temperature, and bioelectric sensing applications. Chem. Mater. 2024, 36, 8141–8158. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Lao, J.; Gao, H.; Yu, J. Hydrogels for flexible electronics. ACS Nano 2023, 17, 9681–9693. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Guo, J.; Zhu, K.; Zhang, L. Ultra-stretchable and force-sensitive hydrogels reinforced with chitosan microspheres embedded in polymer networks. Adv. Mater. 2016, 28, 8037–8044. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Ido, Y.; Miyake, T.; Nagamine, K.; Nishizawa, M. Conducting polymer electrodes printed on hydrogel. J. Am. Chem. Soc. 2010, 132, 13174–13175. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of conductive hydrogels for fabricating flexible strain sensors. Small 2022, 18, 2101518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef]
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Browning, M.B.; Russell, B.; Rivera, J.; Höök, M.; Cosgriff-Hernandez, E.M. Bioactive hydrogels with enhanced initial and sustained cell interactions. Biomacromolecules 2013, 14, 2225–2233. [Google Scholar] [CrossRef]
- López-Díaz, A.; Vázquez, A.S.; Vázquez, E. Hydrogels in soft robotics: Past, present, and future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef]
- Kn, H.P.; Meghana, C.; Raju, N.K.; Shilpa, S.; Yashaswini, M.; Manjunatha, C. Current developments in conductive nano-inks for flexible and wearable electronics. ECS Trans. 2022, 107, 11261. [Google Scholar] [CrossRef]
- Omidian, H.; Mfoafo, K. Three-dimensional printing strategies for enhanced hydrogel applications. Gels 2024, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hao, M.; Liu, B.; Chen, J.; Ren, G.; Zhao, Y.; Guo, J.; Zhuang, L.; Zhao, S.; Peng, Z. Recent progress of hydrogels in brain-machine interface. Soft Sci. 2024, 4, 39. [Google Scholar] [CrossRef]
- Wei, W.; Hao, M.; Zhou, K.; Wang, Y.; Lu, Q.; Zhang, H.; Wu, Y.; Zhang, T.; Liu, Y. In situ multimodal transparent electrophysiological hydrogel for in vivo miniature two-photon neuroimaging and electrocorticogram analysis. Acta Biomater. 2022, 152, 86–99. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xue, Y.; Lei, I.M.; Chen, X.; Zhang, P.; Cai, C.; Liang, X.; Lu, Y.; Liu, J. Engineering electrodes with robust conducting hydrogel coating for neural recording and modulation. Adv. Mater. 2023, 35, 2209324. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Guo, J.; Guan, F.; Sun, J.; Song, X.; He, J.; Yang, Q. Preparation of 3D printable polyvinyl alcohol based conductive hydrogels via incorporating k-carrageenan for flexible strain sensors. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132141. [Google Scholar] [CrossRef]
- Arsuffi, B.; Siqueira, G.; Nyström, G.; Titotto, S.; Magrini, T.; Daraio, C. Programmable Multi-Responsive Nanocellulose-Based Hydrogels With Embodied Logic. Adv. Funct. Mater. 2024, 34, 2409864. [Google Scholar] [CrossRef]
- Gao, C.; Song, S.; Lv, Y.; Huang, J.; Zhang, Z. Recent development of conductive hydrogels for tissue engineering: Review and perspective. Macromol. Biosci. 2022, 22, 2200051. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C.-H. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Fonner, J.M.; Forciniti, L.; Nguyen, H.; Byrne, J.D.; Kou, Y.-F.; Syeda-Nawaz, J.; Schmidt, C.E. Biocompatibility implications of polypyrrole synthesis techniques. Biomed. Mater. 2008, 3, 034124. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. Biosensing applications of polyaniline (PANI)-based nanocomposites: A review. Polym. Rev. 2021, 61, 553–597. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Z.; Zheng, L.; Ye, Z.; Yuan, Y.; Zhang, S.; Liang, B.; Li, T. Recent progress in the biomedical application of PEDOT: PSS hydrogels. Chin. Chem. Lett. 2024, 35, 109810. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Kaklamani, G.; Kazaryan, D.; Bowen, J.; Iacovella, F.; Anastasiadis, S.H.; Deligeorgis, G. On the electrical conductivity of alginate hydrogels. Regen. Biomater. 2018, 5, 293–301. [Google Scholar] [CrossRef]
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 164–174. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef]
- Spencer, A.R.; Primbetova, A.; Koppes, A.N.; Koppes, R.A.; Fenniri, H.; Annabi, N. Electroconductive gelatin methacryloyl-PEDOT: PSS composite hydrogels: Design, synthesis, and properties. ACS Biomater. Sci. Eng. 2018, 4, 1558–1567. [Google Scholar]
- Kumar, S.L.; Sureka, P.; Sowmitha, A.; Sentisenla, J.; Swathy, M. Recent advancements of hydroxyapatite and polyethylene glycol (PEG) composites for tissue engineering applications–A comprehensive review. Eur. Polym. J. 2024, 215, 113226. [Google Scholar]
- Milos, F.; del Campo, A. Polyacrylamide Hydrogels as Versatile Biomimetic Platforms to Study Cell-Materials Interactions. Adv. Mater. Interfaces 2024, 11, 2400404. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Sharma, S.S.A.; Bashir, S.; Kasi, R.; Subramaniam, R.T. The significance of graphene based composite hydrogels as smart materials: A review on the fabrication, properties, and its applications. FlatChem 2022, 33, 100352. [Google Scholar] [CrossRef]
- Lopes, P.A.; Vaz Gomes, D.; Green Marques, D.; Faia, P.; Góis, J.; Patrício, T.F.; Coelho, J.; Serra, A.; de Almeida, A.T.; Majidi, C. Soft bioelectronic stickers: Selection and evaluation of skin-interfacing electrodes. Adv. Healthc. Mater. 2019, 8, 1900234. [Google Scholar] [CrossRef]
- Davidson, M.D.; Burdick, J.A.; Wells, R.G. Engineered biomaterial platforms to study fibrosis. Adv. Healthc. Mater. 2020, 9, 1901682. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef]
- Harding, J.L.; Reynolds, M.M. Combating medical device fouling. Trends Biotechnol. 2014, 32, 140–146. [Google Scholar] [CrossRef]
- Yao, M.; Hsieh, J.-C.; Tang, K.W.K.; Wang, H. Hydrogels in wearable neural interfaces. Med-x 2024, 2, 23. [Google Scholar] [CrossRef]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, R.; Suo, Z. Topological adhesion of wet materials. Adv. Mater. 2018, 30, 1800671. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, G.; Guo, D.; Liu, J.; Zhang, X.; Lin, S.; Zhang, K. Soft elastic hydrogel couplants for ultrasonography. Mater. Sci. Eng. C 2021, 119, 111609. [Google Scholar] [CrossRef]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef]
- Lee, G.-H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC Trends Anal. Chem. 2019, 118, 488–501. [Google Scholar]
- Hendrickson, G.R.; Lyon, L.A. Bioresponsive hydrogels for sensing applications. Soft Matter 2009, 5, 29–35. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhou, J.; Chow, L.; Zhao, G.; Huang, Y.; Ma, Z.; Zhang, Q.; Yang, Y.; Yiu, C.K.; et al. A three-dimensional liquid diode for soft, integrated permeable electronics. Nature 2024, 628, 84–92. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv. Healthc. Mater. 2021, 10, 2100646. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef]
- Cai, P.; Hu, B.; Leow, W.R.; Wang, X.; Loh, X.J.; Wu, Y.L.; Chen, X. Biomechano-interactive materials and interfaces. Adv. Mater. 2018, 30, 1800572. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Liang, S.; Li, C.; Niu, M.; Zhu, P.; Pan, Z.; Mao, Y. Ionic hydrogels-based triboelectric nanogenerators for self-powered human–machine interfaces. J. Phys. Mater. 2023, 7, 012001. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, M.; Patel, R.; Thakkar, K.; Patel, D.; Balar, P.C.; Patel, P.; Desai, U. Advancements in HMI. In Human-Machine Interface Technology Advancements and Applications; CRC Press: Boca Raton, FL, USA, 2023; p. 111. [Google Scholar]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Smart contact lenses—A step towards non-invasive continuous eye health monitoring. Biosensors 2023, 13, 933. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, D.; Xu, C.; Zhang, T.; Gao, M.; Xie, C.; Zhang, D.; Lu, X. Polyphenol-Mediated Multifunctional Human–Machine Interface Hydrogel Electrodes in Bioelectronics. Small Sci. 2025, 5, 2400362. [Google Scholar] [CrossRef]
- Li, S.; Cong, Y.; Fu, J. Tissue adhesive hydrogel bioelectronics. J. Mater. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Ji, J.; Zhang, P. Materials Strategies to Overcome the Foreign Body Response. Adv. Healthc. Mater. 2024, 13, 2304478. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, X.; Ying, Y. Soft and stretchable optical waveguide: Light delivery and manipulation at complex biointerfaces creating unique windows for on-body sensing. ACS Sens. 2021, 6, 1446–1460. [Google Scholar] [CrossRef]
- Liu, X.; Steiger, C.; Lin, S.; Parada, G.A.; Liu, J.; Chan, H.F.; Yuk, H.; Phan, N.V.; Collins, J.; Tamang, S. Ingestible hydrogel device. Nat. Commun. 2019, 10, 493. [Google Scholar] [CrossRef]
- Mau, M.M.; Sarker, S.; Terry, B.S. Ingestible devices for long-term gastrointestinal residency: A review. Prog. Biomed. Eng. 2021, 3, 042001. [Google Scholar] [CrossRef]
- Liu, W.; Choi, S.J.; George, D.; Li, L.; Zhong, Z.; Zhang, R.; Choi, S.Y.; Selaru, F.M.; Gracias, D.H. Untethered shape-changing devices in the gastrointestinal tract. Expert Opin. Drug Deliv. 2023, 20, 1801–1822. [Google Scholar] [CrossRef]
- Hu, H.; Hu, C.; Guo, W.; Zhu, B.; Wang, S. Wearable ultrasound devices: An emerging era for biomedicine and clinical translation. Ultrasonics 2024, 142, 107401. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Kim, J.H.; Yu, C.C.; Dagdeviren, C. An emerging era: Conformable ultrasound electronics. Adv. Mater. 2024, 36, 2307664. [Google Scholar] [CrossRef]
- Huang, H.; Wu, R.S.; Lin, M.; Xu, S. Emerging wearable ultrasound technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 71, 713–729. [Google Scholar] [CrossRef]
- La, T.G.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2022, 7, 2100798. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, T.; Kim, H.; Jo, Y.; Kim, M.-G.; Kim, S.; Bae, H.-M.; Lee, H.J. Calcium-modified silk patch as a next-generation ultrasound coupling medium. ACS Appl. Mater. Interfaces 2021, 13, 55827–55839. [Google Scholar] [CrossRef] [PubMed]
- Vega-Chacón, J.; Arbeláez, M.I.A.; Jorge, J.H.; Marques, R.F.C.; Jafelicci, M., Jr. pH-responsive poly (aspartic acid) hydrogel-coated magnetite nanoparticles for biomedical applications. Mater. Sci. Eng. C 2017, 77, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.L.; Hanson, S.R.; King III, S.B.; Sahatjian, R.A.; Scott, N.A. Local delivery of a synthetic antithrombin with a hydrogel-coated angioplasty balloon catheter inhibits platelet-dependent thrombosis. J. Am. Coll. Cardiol. 1994, 23, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Cho, S.; Choe, A.; Yeom, J.; Ro, Y.G.; Kim, J.; Kang, D.-h.; Lee, S.; Ko, H. Soft Sensors and Actuators for Wearable Human–Machine Interfaces. Chem. Rev. 2024, 124, 1464–1534. [Google Scholar] [CrossRef]
- Niu, M.; Chen, K.; Li, W.; Hu, J.; Zhang, J.; Zhu, P.; Pan, Z.; Mao, Y. Ionic hydrogels-based electronic skins for electrophysiological monitoring. J. Mater. Res. 2024, 39, 188–211. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Shi, S.; Zheng, Y.; Ye, Z.; Liao, J.; Sun, Q.; Dang, B.; Shen, X. Myelin Sheath-Inspired Hydrogel Electrode for Artificial Skin and Physiological Monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Khademolqorani, S.; Jadhav, V.V.; Chamanehpour, E.; Mishra, Y.K.; Mostafavi, E.; Kaushik, A. Recent progress of bio-based smart wearable sensors for healthcare applications. Mater. Today Electron. 2023, 5, 100055. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive polymer-based hydrogels for wearable electrochemical biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Ma, X.; Wei, L.; Wang, J. Antibacterial peptide NZ2114-loaded hydrogel accelerates Staphylococcus aureus-infected wound healing. Appl. Microbiol. Biotechnol. 2022, 106, 3639–3656. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef]
- Ji, J.; Wu, S.; Su, H.; An, S.; Ruan, J.; Zeng, D. Research progress of PVA conductive hydrogel-based wearable biosensors in sweat detection. Chem. Eng. Sci. 2024, 300, 120620. [Google Scholar] [CrossRef]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and advances of hydrogel-based wearable electrochemical biosensors for real-time monitoring of biofluids: From lab to market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Li, H.; Wu, J.; Wan, Q.; Chen, T.; Liu, W.; Peng, H.; Zhang, H.; Luo, Y. Smart Hydrogel Sensors for Health Monitoring and Early Warning. Adv. Sens. Res. 2024, 3, 2400003. [Google Scholar] [CrossRef]
- Wu, K.Y.; Dave, A.; Carbonneau, M.; Tran, S.D. Smart Contact Lenses in Ophthalmology: Innovations, Applications, and Future Prospects. Micromachines 2024, 15, 856. [Google Scholar] [CrossRef]
- Kim, J.; Cha, E.; Park, J.U. Recent advances in smart contact lenses. Adv. Mater. Technol. 2020, 5, 1900728. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, R.M. Neuroengineering tools/applications for bidirectional interfaces, brain–computer interfaces, and neuroprosthetic implants–a review of recent progress. Front. Neuroeng. 2010, 3, 112. [Google Scholar] [CrossRef]
- Cheng, S.; Zhu, R.; Xu, X. Hydrogels for next generation neural interfaces. Commun. Mater. 2024, 5, 99. [Google Scholar] [CrossRef]
- Heng, W.; Solomon, S.; Gao, W. Flexible electronics and devices as human–machine interfaces for medical robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Zhou, Y.; Bai, Y.; Ju, Z.; Guo, J.; Gu, G.; Bai, K.; Ouyang, G.; Chen, S. Soft human–machine interfaces: Design, sensing and stimulation. Int. J. Intell. Robot. Appl. 2018, 2, 313–338. [Google Scholar] [CrossRef]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef]
- Chen, Q.; Du, S.; Guo, Q.; Zhou, Y.; Tian, Y.; Sun, R.; Yang, P.; Wu, D.; Zhou, Z.; Li, R. Hydrogel Strain Sensors Based on Ordered Iron Nanowires for Human-Machine Interaction. IEEE Sens. J. 2024, 24, 38835–38842. [Google Scholar] [CrossRef]
- Wicaksono, I.; Dagdeviren, C. Flexible and Stretchable Devices for Human-Machine Interfaces. In Handbook of Flexible and Stretchable Electronics; CRC Press: Boca Raton, FL, USA, 2019; pp. 415–466. [Google Scholar]
- Rao, Z.; Ershad, F.; Almasri, A.; Gonzalez, L.; Wu, X.; Yu, C. Soft electronics for the skin: From health monitors to human–machine interfaces. Adv. Mater. Technol. 2020, 5, 2000233. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Dong, Y.; Teng, L.; Li, D.; Han, H.; Zhu, S.; Sun, X.; Zeng, Z.; Zeng, X. A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator. Nanomaterials 2024, 14, 1365. [Google Scholar] [CrossRef]
- Esposito, D.; Centracchio, J.; Andreozzi, E.; Gargiulo, G.D.; Naik, G.R.; Bifulco, P. Biosignal-based human–machine interfaces for assistance and rehabilitation: A survey. Sensors 2021, 21, 6863. [Google Scholar] [CrossRef]
- Singh, H.P.; Kumar, P. Developments in the human machine interface technologies and their applications: A review. J. Med. Eng. Technol. 2021, 45, 552–573. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in biodegradable electronic skin: Material progress and recent applications in sensing, robotics, and human–machine interfaces. Adv. Mater. 2023, 35, 2203193. [Google Scholar] [CrossRef] [PubMed]
- Kutner, N.; Kunduru, K.R.; Rizik, L.; Farah, S. Recent advances for improving functionality, biocompatibility, and longevity of implantable medical devices and deliverable drug delivery systems. Adv. Funct. Mater. 2021, 31, 2010929. [Google Scholar] [CrossRef]
- Fang, K.; Wan, Y.; Wei, J.; Chen, T. Hydrogel-Based Sensors for Human–Machine Interaction. Langmuir 2023, 39, 16975–16985. [Google Scholar] [CrossRef]
- Huang, X.; Yang, N.; Sun, S.; Cheng, Y.; Cheng, L. Recent progress of hydrogel-based bioelectronics for mechanophysiological signal sensing. Mater. Sci. Eng. R Rep. 2025, 162, 100888. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J. Optical waveguides and integrated optical devices for medical diagnosis, health monitoring and light therapies. Sensors 2020, 20, 3981. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, R.; Pani, B.; Sarkar, A.; Tripathi, M. A Review on Engineering the Future with Hydrogels: Advancements in Energy Storage Devices and Biomedical Technologies. New J. Chem. 2024, 48, 10347–10369. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, J.; Qiu, X.; Yan, Y.; Zhang, Z.; Fang, K.; Yang, Y.; Zhang, X.; Huang, J. Manufacturing and post-engineering strategies of hydrogel actuators and sensors: From materials to interfaces. Adv. Colloid Interface Sci. 2022, 308, 102749. [Google Scholar] [CrossRef]
- Suzuki, S.; Ikada, Y. Biomaterials for Surgical Operation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Jia, B.; Huang, H.; Dong, Z.; Ren, X.; Lu, Y.; Wang, W.; Zhou, S.; Zhao, X.; Guo, B. Degradable biomedical elastomers: Paving the future of tissue repair and regenerative medicine. Chem. Soc. Rev. 2024, 53, 4086–4153. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef]
- Dai, S.; Gao, Y.; Duan, L. Recent advances in hydrogel coatings for urinary catheters. J. Appl. Polym. Sci. 2023, 140, e53701. [Google Scholar] [CrossRef]
- Mariello, M.; Eş, I.; Proctor, C.M. Soft and flexible bioelectronic micro-systems for electronically controlled drug delivery. Adv. Healthc. Mater. 2024, 13, 2302969. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.M.; Lye, T.; Redekop, G.; Brevner, A.; Hamilton, M.; Kozey, M.; Easton, D. Infection rates in standard vs. hydrogel coated ventricular catheters. Can. J. Neurol. Sci. 2004, 31, 506–510. [Google Scholar] [CrossRef]
- Ku, J.C. Preclinical Assessment of Endovascular Hydrogel Embolization for Neurovascular Conditions; University of Toronto: Toronto, ON, Canada, 2024. [Google Scholar]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel paint. Adv. Mater. 2019, 31, 1903062. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zeng, L.; Wu, Z.L.; Guo, H.; Hourdet, D. Stimuli-responsive toughening of hydrogels. Chem. Mater. 2021, 33, 7633–7656. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Dey Chowdhury, S. Hydrogel Composites for Multifunctional Biomedical Applications. J. Compos. Sci. 2024, 8, 154. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D.; Wilson, R.L. Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine. Gels 2024, 10, 238. [Google Scholar] [CrossRef]
- Bento, C.S.; Gaspar, M.C.; Coimbra, P.; de Sousa, H.C.; Braga, M.E. A review of conventional and emerging technologies for hydrogels sterilization. Int. J. Pharm. 2023, 634, 122671. [Google Scholar] [CrossRef]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef]
- Bai, H.; Li, S.; Shepherd, R.F. Elastomeric haptic devices for virtual and augmented reality. Adv. Funct. Mater. 2021, 31, 2009364. [Google Scholar] [CrossRef]
- Acqualagna, L.; Blankertz, B. Gaze-independent BCI-spelling using rapid serial visual presentation (RSVP). Clin. Neurophysiol. 2013, 124, 901. [Google Scholar] [CrossRef] [PubMed]
- Karikari, E.; Koshechkin, K.A. Review on brain-computer interface technologies in healthcare. Biophys. Rev. 2023, 15, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Baek, S.; Sluyter, R.; Konstantinov, K.; Kim, J.; Kim, S.; Kim, Y. Wearable and implantable bioelectronics as eco-friendly and patient-friendly integrated nanoarchitectonics for next-generation smart healthcare technology. EcoMat 2023, 5, e12356. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Mingxuan, Z.; Mingming, H.; Botao, L.; Jianping, C.; Guoqiang, R.; Yinchao, Z.; Jinxiu, G.; Liping, Z.; Shunying, Z.; Zhaoxiang, P.; et al. Recent progress of hydrogels in brain-machine interface. Soft Sci. 2024, 4, 39. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Wang, J.; Yang, L.; Ren, X.; Song, G.; Chen, S.; Yuan, Y.; Liu, R.; Pan, L.; et al. A chemically mediated artificial neuron. Nat. Electron. 2022, 5, 586–595. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Li, Y.; Wang, H.; Perz, M.; Tang, Q.; Wing, K.; Tang, K.; Pyatnitskiy, I.; Reyes, R.; Ding, H.; et al. Design of hydrogel-based wearable EEG electrodes for medical applications. J. Mater. Chem. B 2022, 10, 7260–7280. [Google Scholar] [CrossRef]
- Minev, I. Electronic tissue technologies for seamless biointerfaces. J. Polym. Sci. 2023, 61, 1707–1712. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, K.; Jia, S.; Huang, Y.; Zhao, G.; Zhang, B.; Liu, Y.; Yu, X. Advances in materials for haptic skin electronics. Matter 2024, 7, 2826–2845. [Google Scholar] [CrossRef]
- Ying, B. Hydrogel Artificial Ionic Skins for Wearable Electronics and Soft Robotics; McGill University: Montreal, QC, Canada, 2020. [Google Scholar]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Lee, P.S. Functional fibers and fabrics for soft robotics, wearables, and human–robot interface. Adv. Mater. 2021, 33, 2002640. [Google Scholar] [CrossRef] [PubMed]
- Cajić, M.; Lazarević, M.; Karličić, D.; Rosić, N.; Paunović, S.; Lin, J.; Fu, Z.; Sun, H.; Hong, Y. Hydrogel Ionotronics for Soft Robotic Applications. Serbia-China Bilateral Project (2024–2026). 2024. Available online: https://www.mi.sanu.ac.rs/novi_sajt/research/documents/Bilateral%20Project%20-Cajic.pdf (accessed on 10 March 2025).
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.; Sun, J.-Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D printing of hydrogels for stretchable ionotronic devices. Adv. Funct. Mater. 2021, 31, 2107437. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.; Zou, C.; Liu, L.; Chen, H. Voltage-induced wrinkle performance in a hydrogel by dielectric elastomer actuation. Polymers 2018, 10, 697. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Xu, C.; Zheng, J. A novel dielectric elastomer actuator based on compliant polyvinyl alcohol hydrogel electrodes. J. Mater. Sci. Mater. Electron. 2015, 26, 9213–9218. [Google Scholar] [CrossRef]
- Park, N.; Kim, J. Hydrogel-based artificial muscles: Overview and recent progress. Adv. Intell. Syst. 2020, 2, 1900135. [Google Scholar] [CrossRef]
- Ahmed, F.; Waqas, M.; Jawed, B.; Soomro, A.M.; Kumar, S.; Hina, A.; Khan, U.; Kim, K.H.; Choi, K.H. Decade of bio-inspired soft robots: A review. Smart Mater. Struct. 2022, 31, 073002. [Google Scholar] [CrossRef]
- Enyan, M.; Bing, Z.; Amu-Darko, J.N.O.; Issaka, E.; Otoo, S.L.; Agyemang, M.F. Advances in smart materials soft actuators on mechanisms, fabrication, materials, and multifaceted applications: A review. J. Thermoplast. Compos. Mater. 2025, 38, 302–370. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive hydrogel-based soft robot: A review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef] [PubMed]
- Bonardd, S.; Nandi, M.; Hernandez Garcia, J.I.; Maiti, B.; Abramov, A.; Diaz Diaz, D. Self-healing polymeric soft actuators. Chem. Rev. 2022, 123, 736–810. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Susanto, G.J.; Anwar Ali, H.P.; Tee, B.C. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-integrated hydrogels as multifunctional composite materials for biomedical applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Boateng, D.; Li, X.; Zhu, Y.; Zhang, H.; Wu, M.; Liu, J.; Kang, Y.; Zeng, H.; Han, L. Recent advances in flexible hydrogel sensors: Enhancing data processing and machine learning for intelligent perception. Biosens. Bioelectron. 2024, 261, 116499. [Google Scholar] [CrossRef]
- Patel, S.; Rao, Z.; Yang, M.; Yu, C. Wearable Haptic Feedback Interfaces for Augmenting Human Touch. Adv. Funct. Mater. 2025, 2417906. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, T.; Wang, J.; Hu, Y.; Gui, Z. Engineering robust and transparent dual-crosslinked hydrogels for multimodal sensing without conductive additives. J. Colloid Interface Sci. 2024, 675, 14–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, P.; Tan, M.; Niu, M.; Liang, S.; Mao, Y. Recent Advances in Hydrogel-Based Self-Powered Artificial Skins for Human–Machine Interfaces. Adv. Intell. Syst. 2023, 5, 2300162. [Google Scholar] [CrossRef]
- Sonar, H.A. Comprehensive Interactive Soft Interfaces for Wearable Tactile Feedback; EPFL: Lausanne, Switzerland, 2021. [Google Scholar]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.; Ponraj, G.; Kirthika, S.K.; Suman, M.V.; Lim, C.M.; Ren, H. Hydrogel-shielded soft tactile sensor for biocompatible drug delivery monitoring. J. Med. Devices 2019, 13, 044503. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, C.; Song, Z.; Huang, Y.; Yao, K.; Wong, T.; Zhou, J.; Zhao, L.; Huang, X.; Nejad, S.K. Electronic skin as wireless human-machine interfaces for robotic VR. Sci. Adv. 2022, 8, eabl6700. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible conductive hydrogels: Applications in the field of biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Zhang, Z.; Shi, Q.; He, T.; Liu, H.; Chen, T.; Lee, C. Haptic-feedback smart glove as a creative human-machine interface (HMI) for virtual/augmented reality applications. Sci. Adv. 2020, 6, eaaz8693. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Li, Z.; Xue, Y.; Wang, F.; Shan, L.; Wang, Y.; Shi, X.; Wu, K.; Liu, J. Conducting Hydrogel-Based Neural Biointerfacing Technologies. Adv. Funct. Mater. 2025, 2422869. [Google Scholar] [CrossRef]
- Jyothish, K.; Mishra, S. A Survey on Robotic Prosthetics: Neuroprosthetics, Soft Actuators, and Control Strategies. ACM Comput. Surv. 2024, 56, 195. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Hauser, A.W.; Hayward, R.C. Shape-morphing materials from stimuli-responsive hydrogel hybrids. Acc. Chem. Res. 2017, 50, 161–169. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; Luo, Y.; Wu, Z.; Yu, J.; Chen, H.; Zhou, Y.; Zhang, H.; Tao, K.; Chen, X. High-Performance Hydrogel Sensors Enabled Multimodal and Accurate Human–Machine Interaction System for Active Rehabilitation. Adv. Mater. 2024, 36, 2309868. [Google Scholar] [CrossRef]
- Kweon, O.Y.; Samanta, S.K.; Won, Y.; Yoo, J.H.; Oh, J.H. Stretchable and self-healable conductive hydrogels for wearable multimodal touch sensors with thermoresponsive behavior. ACS Appl. Mater. Interfaces 2019, 11, 26134–26143. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart materials for flexible electronics and devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Hu, J.; Wang, B.; Xia, X.; Cheng, Y.; Wang, C.-H.; Lu, Y. Biomimetic Wearable Sensors: Emerging Combination of Intelligence and Electronics. Adv. Sci. 2023, 11, 2303264. [Google Scholar] [CrossRef]
- Fu, M.; Sun, Z.; Liu, X.; Huang, Z.; Luan, G.; Chen, Y.; Peng, J.; Yue, K. Highly Stretchable, Resilient, Adhesive, and Self-Healing Ionic Hydrogels for Thermoelectric Application. Adv. Funct. Mater. 2023, 33, 2306086. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Chen, B.; Li, H.; Mao, Y. Recent Progress of Bioinspired Triboelectric Nanogenerators for Electronic Skins and Human–Machine Interaction. Nanoenergy Adv. 2024, 4, 45–69. [Google Scholar] [CrossRef]
- Biswas, S.; Visell, Y. Haptic Perception, Mechanics, and Material Technologies for Virtual Reality. Adv. Funct. Mater. 2021, 31, 2008186. [Google Scholar] [CrossRef]
- Woerly, S.; Pinet, E.; de Robertis, L.; Van Diep, D.; Bousmina, M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel™). Biomaterials 2001, 22, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical application, patent repository, clinical trial and regulatory updates on hydrogel: An extensive review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar]
- Rules of Procedure. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/docs/rules_procedure_2016_en.pdf (accessed on 10 March 2025).
- Asher, S.A.; Holtz, J.H. Polymerized Crystalline Colloidal Array Sensor Methods. U.S. Patent 5,854,078, 29 December 1998. [Google Scholar]
- Stewart, R.F. Phase Change Sensor. 2010. Available online: https://patents.google.com/patent/US20070249059A1/en (accessed on 10 March 2025).
- Penterman, R.; Van Lierop, S.; Immink, A.H.J.; Broer, D.J. Temperature Sensor and Biosensor Using the Same. U.S. Patent Application 12/743,827, 28 October 2010. [Google Scholar]
- Parker, K.K.; O’grady, M. Porous Electroactive Hydrogels and Uses Thereof. U.S. Patent 8,999,378, 7 April 2015. [Google Scholar]
- Han, I.S.; Lew, S.; Han, M.H. Photometric Glucose Measurement System Using Glucose-Sensitive Hydrogel. U.S. Patent 6,835,553, 28 December 2004. [Google Scholar]
- Lewis, J.A.; Gladman, A.S. Method of 4d Printing a Hydrogel Composite Structure. U.S. Patent Application 14/954,228, 1 June 2017. [Google Scholar]
- Daunert, S.; Peteu, S.F.; Bachas, L.G.; Madou, M.J.; Moschou, E. Artificial Muscle Hydrogel Blends Reversibly Electroactuated Near Neutral pH, Implantable Actuating Devices, and Methods Using the Same. U.S. Patent 7,482,381, 27 June 2009. [Google Scholar]
- Richardson-Burns, S.; Hendricks, J.L.; Martin, D.C.; Sereno, A.; King, Z.; Jan, E. Co-Electrodeposited Hydrogel-Conducting Polymer Electrodes for Biomedical Applications. U.S. Patent 9,084,546, 21 July 2015. [Google Scholar]
- Edwards, L.; McCrimmon, P.; Watson, R.T. Tactile-Feedback Touch Screen. U.S. Patent 8,427,433 B2, 23 April 2013. [Google Scholar]
- Chen, Z.J.; Shen, T.Y.; Xiao, X.; He, X.C.; Luo, Y.L.; Jin, Z.; Li, C.H. An Ultrahigh-Modulus Hydrogel Electrolyte for Dendrite-Free Zinc Ion Batteries. Adv. Mater. 2024, 36, 2413268. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y. A flexible triboelectric nanogenerator based on PVA/PTT/LiCl conductive hydrogel for gait monitoring in basketball. AIP Adv. 2023, 13, 075303. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Lampe, K.J.; Bjugstad, K.B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012, 72, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, N.; Erickson, B.; Murphy, B.B.; Richardson, A.G.; Robbins, G.; Apollo, N.V.; Mentzelopoulos, G.; Mathis, T.; Hantanasirisakul, K.; Bagga, P. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Transl. Med. 2021, 13, eabf8629. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Z.; Hu, H.; Chen, B.; Wang, Y.; Mao, Y.; Li, H.; Zhang, B. Recent Progress in Self-Healing Triboelectric Nanogenerators for Artificial Skins. Biosensors 2025, 15, 37. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef]
- Heinemann, C.; Buchner, F.; Lee, P.S.; Bernhardt, A.; Kruppke, B.; Wiesmann, H.-P.; Hintze, V. Effects of Gamma Irradiation and Supercritical Carbon Dioxide Sterilization on Methacrylated Gelatin/Hyaluronan Hydrogels. J. Funct. Biomater. 2023, 14, 317. [Google Scholar] [CrossRef]
- Kronenfeld, J.M.; Rother, L.; Saccone, M.A.; Dulay, M.T.; DeSimone, J.M. Roll-to-roll, high-resolution 3D printing of shape-specific particles. Nature 2024, 627, 306–312. [Google Scholar] [CrossRef]
- Hu, X.; Lei, B.; Li, S.S.; Chen, L.J.; Li, Q. Living cell-laden hydrogels: Unleashing the future of responsive biohybrid systems. Responsive Mater. 2023, 1, e20230009. [Google Scholar] [CrossRef]
| Product | Application | Hydrogel Interface Used | Reference |
|---|---|---|---|
| HapticGel VR Glove | Virtual reality (VR) haptic feedback | Conductive ionic hydrogel | [254] |
| Skinfeel e-skin | Wearable electronic skin (e-skin) for tactile sensing | Hydrogel-based electrode for neural signal transmission | [243] |
| Softsense prosthetic interface | Prosthetic limb sensory restoration | Biocompatible conductive hydrogel | [238] |
| Neurogel neural interface | Neural interfacing for sensory restoration | Hydrogel-based electrode for neural signal transmission | [255] |
| S. No. | Patent No. and Country | Title | Intended Use | Details | Reference |
|---|---|---|---|---|---|
| 1 | US5854078A (USA) | Polymerized crystalline colloidal array sensor methods | Sensor devices | Hydrogels capable of shrinking and swelling in response to stimuli, changing light diffraction to detect concentration changes | [259] |
| 2 | US7794657B2 (USA) | Phase change sensor | Biosensors | Sensor material undergoes volume change upon target molecule binding, enabling signal detection | [260] |
| 3 | US20100272608A1 (USA) | Temperature sensor and biosensor using the same | Temperature sensors and biosensors | Evanescent wave excitation-based temperature and biomolecule detection | [261] |
| 4 | US8999378B2 (USA) | Porous electroactive hydrogels and uses thereof | Actuators and biomedical applications | Electroactive hydrogels with tunable deformation angle | [262] |
| 5 | US6835553B2 (USA) | Photometric glucose measurement system using a glucose-sensitive hydrogel | Glucose biosensor | Implantable biosensor using a hydrogel filament to detect glucose concentration photometrically | [263] |
| 6 | US20170151733A1 (USA) | Method of 4D printing a hydrogel composite structure | 4D printing | Hydrogel composite structures with swelling-induced 3D shape transformation | [264] |
| 7 | US7482381B2 (USA) | Artificial muscle hydrogel blends reversibly electroactuated near neutral pH, implantable actuating devices, and methods using the same | Artificial muscles and drug delivery | Electroactuated hydrogel materials for fluid release and implantable actuators | [265] |
| 8 | US9084546B2 (USA) | Co-electrodeposited hydrogel-conducting polymer electrodes for biomedical applications | Biomedical electrodes | Bioelectrodes with enhanced biocompatibility for electronic signal detection | [266] |
| 9 | US8427433B2 (USA) | Tactile-feedback touchscreen | Tactile displays | Gel layer-based system providing tactile feedback for touchscreen interfaces | [267] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels 2025, 11, 232. https://doi.org/10.3390/gels11040232
Ullah A, Kim DY, Lim SI, Lim H-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels. 2025; 11(4):232. https://doi.org/10.3390/gels11040232
Chicago/Turabian StyleUllah, Aziz, Do Youn Kim, Sung In Lim, and Hyo-Ryoung Lim. 2025. "Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration" Gels 11, no. 4: 232. https://doi.org/10.3390/gels11040232
APA StyleUllah, A., Kim, D. Y., Lim, S. I., & Lim, H.-R. (2025). Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human–Machine Integration. Gels, 11(4), 232. https://doi.org/10.3390/gels11040232







