Influence of Honey Bee Brood Protein on the Hydrophilic, Mechanical, and Thermal Properties of Polysaccharide Gel Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Amino Acid Composition of BBP
2.2. Carboxymethyl Starch Characterization
2.3. Composite Film Characterization
2.3.1. Color and Optical Properties
2.3.2. Mechanical Properties
2.3.3. Water Susceptibility
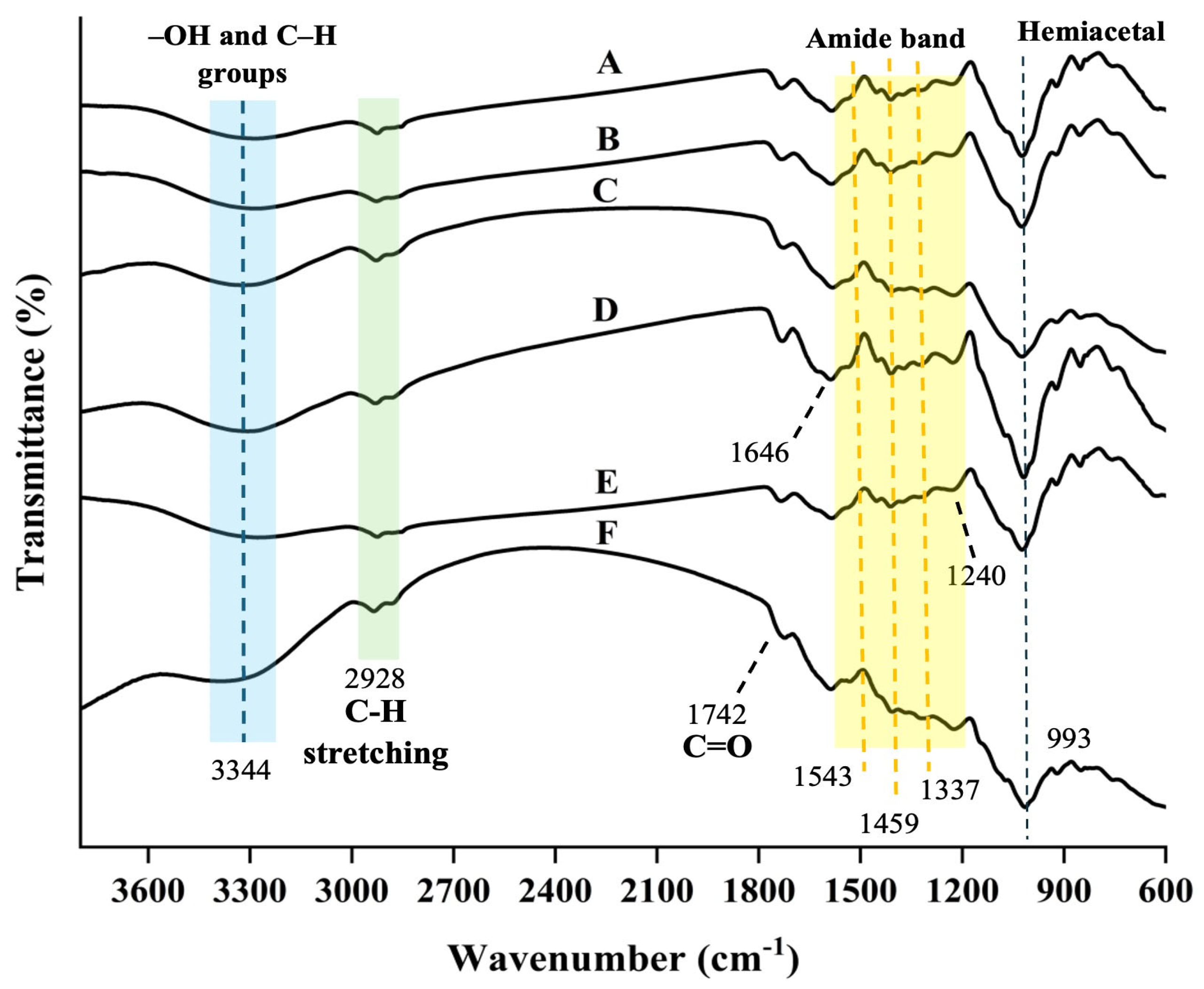
2.3.4. FTIR Analysis
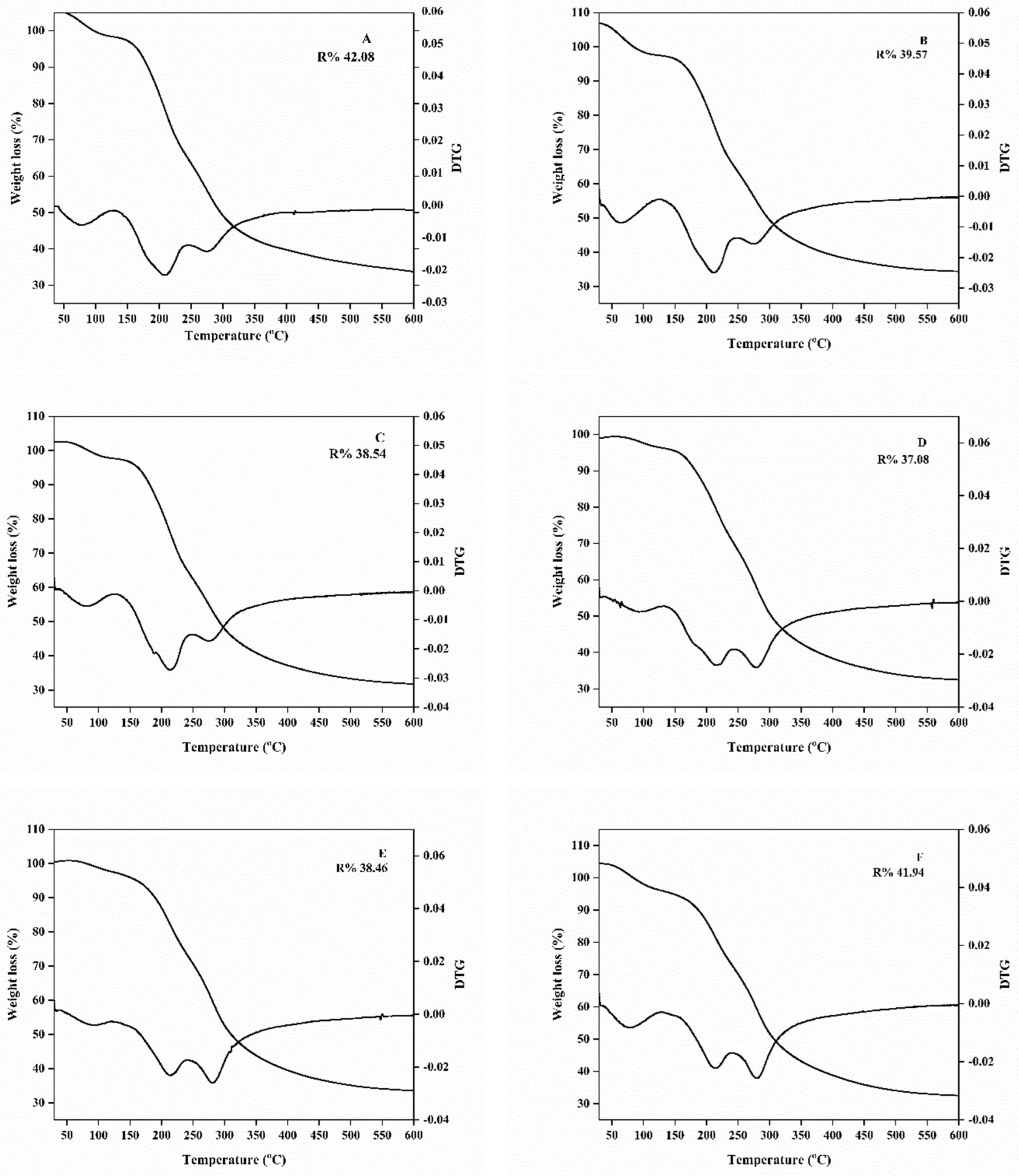
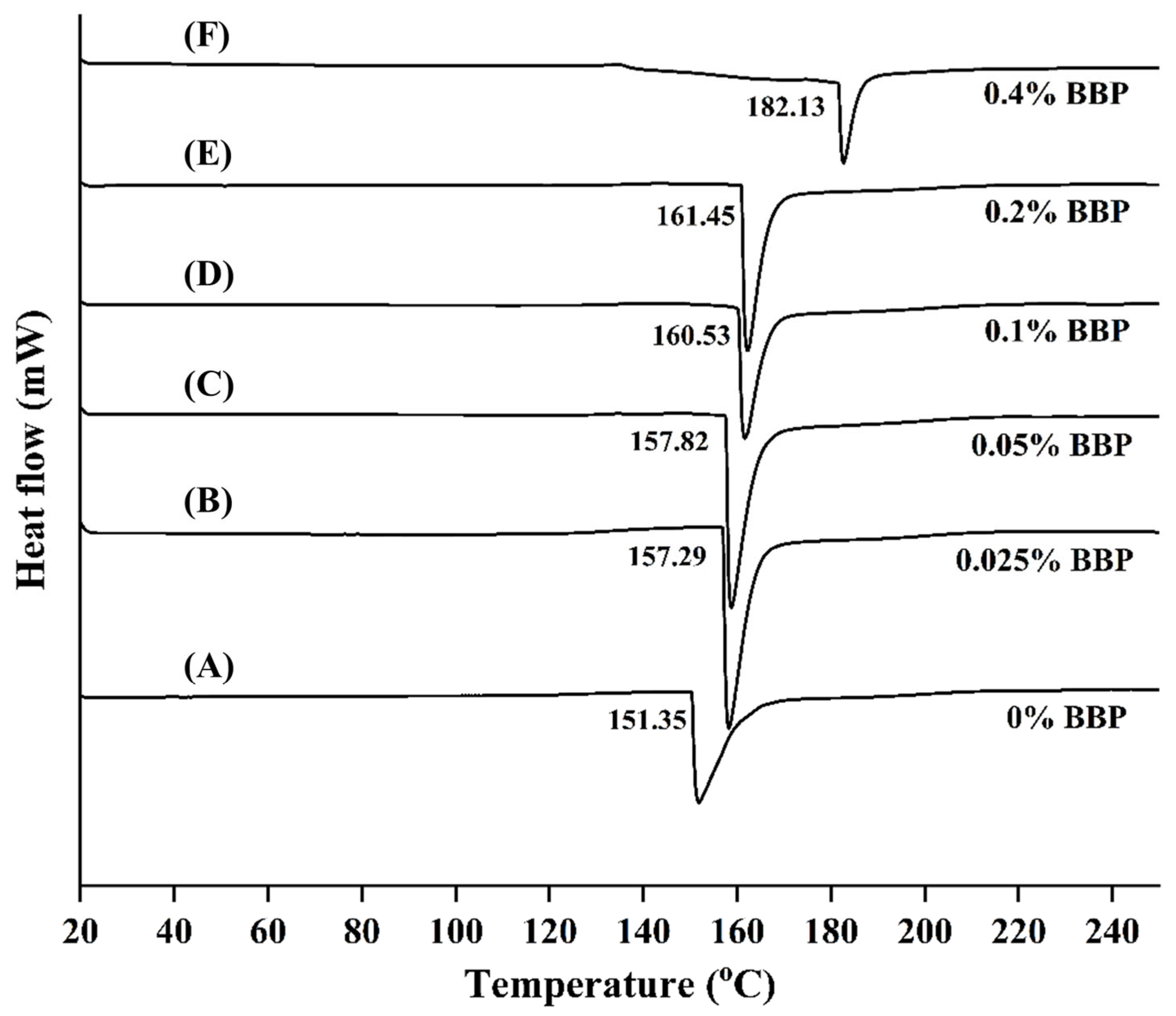
2.3.5. Thermal Properties
2.3.6. Scanning Electron Microscopy (SEM)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Carboxymethyl Starch (CMS) Synthesis
4.3. Honey Bee Brood Protein Extraction (BBP)
4.4. Polysaccharide Gel Film Preparation
4.5. Polysaccharide Gel Film Physical Properties
4.5.1. Film Thickness
4.5.2. Surface Color Measurements
4.5.3. Opacity
4.5.4. Moisture Content of the Film
4.5.5. Water Absorption and Thickness Swelling
4.5.6. Water Contact Angle
4.6. Water Vapor Permeability (WVP)
4.7. Polysaccharide Gel Film Mechanical Properties
4.8. Fourier Transform Infrared (FT-IR) Spectroscopy
4.9. Polysaccharide Gel Film Thermal Properties
4.10. Scanning Electron Microscope (SEM)
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramesh, M.; Muthukrishnan, M. 25-Biodegradable Polymer Blends and Composites for Food-Packaging Applications. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing Series in Composites Science and Engineering; Rangappa, S.M., Parameswaranpillai, J., Ramesh, M., Siengchin, S., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 693–716. [Google Scholar]
- Sun, Y.; Bai, Y.; Yang, W.; Bu, K.; Tanveer, S.K.; Hai, J. Global Trends in Natural Biopolymers in the 21st Century: A Scientometric Review. Front. Chem. 2022, 10, 915648. [Google Scholar] [CrossRef]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; Lopez, G.F.G.; Belloso, O.M.; Gonzalez, C.R. Design and Characterization of Corn Starch Edible Films Including Beeswax and Natural Antimicrobials. Food Bioprocess Technol. 2017, 10, 103–114. [Google Scholar]
- Chaichi, M.; Badii, F.; Mohammadi, A. Novel Bioactive Composite Films Based on Pectin-Nanocellulose-Synergistic Triple Essential Oils: Development and Characterization. Food Bioprocess Technol. 2023, 16, 1794–1805. [Google Scholar]
- Bizymis, A.-P.; Giannou, V.; Tzia, C. Contribution of Hydroxypropyl Methylcellulose to the Composite Edible Films and Coatings Properties. Food Bioprocess Technol. 2023, 16, 1488–1501. [Google Scholar] [CrossRef]
- Yin, W.; Qiu, C.; Ji, H.; Li, X.; Sang, S.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Recent advances in biomolecule-based films and coatings for active and smart food packaging applications. Food Biosci. 2023, 52, 102378. [Google Scholar]
- Souza, M.A.d.; Vilas-Boas, I.T.; Leite-da-Silva, J.M.; Abrahão, P.d.N.; Teixeira-Costa, B.E.; Veiga-Junior, V.F. Polysaccharides in Agro-Industrial Biomass Residues. Polysaccharides 2022, 3, 95–120. [Google Scholar] [CrossRef]
- Qiao, S.; Zhu, J.; Yang, Y.; Dai, H.; Fu, Y.; Chen, H.; Ma, L.; Zhang, Y.; Wang, H. Effect of electrostatic repulsion on barrier properties and thermal performance of gelatin films by carboxymethyl starch, and application in food cooking. Int. J. Biol. Macromol. 2024, 261, 129380. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Hong, P.; Zhou, C. Di-aldehyde starch crystal: A novel bio-crosslinker for strengthening the structure and physio-chemical properties of gelatin-based films. Food Biosci. 2021, 43, 10138. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar]
- Hernández-Muñoz, P.; Villalobos, R.; Chiralt, A. Effect of cross-linking using aldehydes on properties of glutenin-rich films. Food Hydrocoll. 2004, 18, 403–411. [Google Scholar]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar]
- Wang, K.; Li, W.; Wu, L.; Li, Y.; Li, H. Preparation and characterization of chitosan/dialdehyde carboxymethyl cellulose composite film loaded with cinnamaldehyde@zein nanoparticles for active food packaging. Int. J. Biol. Macromol. 2024, 261, 129586. [Google Scholar]
- Li, M.; Qu, H.; Li, Q.; Lu, S.; Wu, Y.; Tang, Z.; Liu, X.; Yuan, Z.; Huang, L.; Chen, L.; et al. A carboxymethyl cellulose/chitosan-based hydrogel harvests robust adhesive, on-demand detachment and self-healing performances for deep burn healing. Chem. Eng. J. 2024, 498, 155552. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Correia, P.M.R.; Anjos, O.; Coelho, C.; Costa, C.A. Honey Bee (Apis mellifera L.) Broods: Composition, Technology and Gastronomic Applicability. Foods 2022, 11, 2750. [Google Scholar] [CrossRef] [PubMed]
- Rutka, I.; Galoburda, R.; Galins, J.; Galins, A. Bee drone brood homogenate chemical composition, Stabilization and application: A review. Livest. Res. Rural Dev. 2021, 36, 93–103. [Google Scholar]
- Finke, M.D. Nutrient Composition of Bee Brood and its Potential as Human Food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar]
- WHO; FAO; UNU. Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007.
- Mishyna, M.; Martinez, J.-J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar]
- Kchaou, H.; Chaabouni, S.E.; Hamdi, M. Influence of natural additives on the optical, mechanical, and barrier properties of biodegradable films. J. Food Sci. 2019, 84, 331–340. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Kontominas, M.G.; McHugh, T.H. Edible films and coatings from lipids and resins. Innov. Food Sci. Emerg. Technol. 2010, 11, 519–525. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Plutino, M.; Pignatti, G.; Karabagias, I.K.; Martinelli, E.; Souto, E.B.; Santini, A.; Lucini, L. Antioxidant Properties of Bee Products Derived from Medicinal Plants as Beekeeping Sources. Agriculture 2021, 11, 1136. [Google Scholar] [CrossRef]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates in the diet of the honey bee (Apis mellifera). Sci. Rep. 2020, 10, 6572. [Google Scholar]
- Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Honey bees and their brood: A potentially valuable resource of food, worthy of greater appreciation and scientific attention. J. Ecol. Environ. 2021, 45, 31. [Google Scholar] [CrossRef]
- Li, D.; Liu, R.; Tao, Y.; Shi, Y.; Wang, P.; Han, Y. Enhancement of the carboxymethylation of corn starch via induced electric field. Carbohydr. Polym. 2023, 319, 121137. [Google Scholar] [CrossRef] [PubMed]
- Fatimah Zuhra, C.; Ginting, M.; Masyita, A.; Az-zahra, W. Carboxymethyl Starch Synthesis from Breadfruit Starch (Artocarpus Communis) through Esterification Reaction with Monochloro Acetate. In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), Niagara, Indonesia, 9–11 October 2019; pp. 143–148. [Google Scholar]
- Jainan, A.; Deenu, A.; Naruenartwongsakul, S.; Raviyan, P.; Sungsuwan, J.; Kamthai, S. Preliminary study of alkaline pretreatment effect on carboxymethyl flour (CMF) from Chiang Mai University (CMU) purple rice properties. Chiang Mai J. Sci. 2017, 44, 1624–1632. [Google Scholar]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.O.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar]
- Kchaou, H.; Abbe, N.B.; Chargui, R.; Hamdi, M. Biodegradable active packaging materials for shelf-life extension of food products. Environ. Sci. Pollut. Res. 2020, 27, 9181–9191. [Google Scholar] [CrossRef]
- Perdana, J.; Bernaert, N.; Akhtar, M.; De Meulenaer, B. Biopolymer-based edible packaging to prevent lipid oxidation in food products: A review. Trends Food Sci. Technol. 2020, 106, 229–240. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Ng, P.K. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings: A review. Innov. Food Sci. Emerg. Technol. 2014, 11, 586–597. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT—Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Asad, R.; Lagnika, C.; Luo, H.; Nie, M.; Dai, Z.; Liu, C.; Abdin, M.; Hashim, M.M.; Li, D.; Song, J. Effect of Chinese chives (Allium tuberosum) addition to carboxymethyl T cellulose based food packaging films. Carbohydr. Polym. 2020, 235, 1159. [Google Scholar]
- Clara, P.; Sanchez-Gonzalez, L.; Chiralt, A.; Chafer, M.; Gonzalez-Martinez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocoll. 2012, 27, 22–29. [Google Scholar]
- Extabide, A.; Vairo, C.; Santos-Vizcaino, E.; Guerrero, P.; Pedraz, J.L.; Igartua, M.; Caba, K. de la.; Hernandez, R.M. Ultrathin hydro-films based on lactose-crosslinked fish gelatin for wound healing applications. Int. J. Pharm. 2017, 530, 455–467. [Google Scholar]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and characterization of edible dialdehyde carboxymethyl cellulose crosslinked feather keratin films for food packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef]
- Bharti, K.; Kshtriya, V.; Singh, R.; Walia, S.; Bhatia, D.; Ballabh, K.; Gour, N. Unusual aggregates formed by the self-assembly of proline, hydroxyproline, and lysine. ACS Chem. Neurosci. 2021, 12, 3237–3249. [Google Scholar] [CrossRef]
- Huang, X.; Ian, G. Apparent contact angle around the periphery of a liquid drop on roughened surfaces. Sci. Rep. 2020, 10, 8220. [Google Scholar]
- Mahdiyar, S.; Ahamdi, S.J.; Seif, A.; Rajabzadeh, G. Carboxymethyl cellulose film modification through surface photocrosslinking and chemical crosslinking for food packaging applications. Food Hydrocoll. 2016, 61, 378–389. [Google Scholar]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar]
- Cheng, J.; Wang, J.; Li, Z.; Cui, L. Improving the mechanical and water-resistance properties of pea protein-based edible film via wet-heating Maillard reaction: Insights into the simultaneous effect of heating and Maillard reaction. Food Packag. Shelf Life 2023, 35, 101024. [Google Scholar]
- Martucci, J.F.; Accareddu, A.E.M.; Ruseckaite, R.A. Preparation and characterization of plasticized gel films cross-linked with low concentrations of glutaraldehyde. J. Mater. Sci. 2012, 47, 3282–3292. [Google Scholar]
- Xie, Y.F.; Ding, J.; Li, Y.; Wei, P.; Liu, S.; Yang, R. The formation of protein-chitosan complexes: Their interaction, applications, and challenges. Foods 2024, 13, 3572. [Google Scholar] [CrossRef]
- Saberi, B.; Chokchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar]
- Sun, F.F.; Shan, P.; Liu, B.; Li, Y.; Wang, K.; Zhuang, Y.; Ning, D.; Li, H. Gelatin-based multifunctional composite films integrated with dialdehyde carboxymethyl cellulose and coffee leaf extract for active food packaging. Int. J. Biol. Macromol. 2024, 263, 130302. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Huang, G.; Yan, L. Predicting Protein Surface Property with its Surface Hydrophobicity. Protein Pept. Lett. 2021, 28, 938–944. [Google Scholar]
- Kong, J.; Shaoning, Y. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Horvatinec, J.; Svečnjak, L. Infrared (FTIR) spectral features of honey bee (Apis mellifera L.) hemolymph. J. Cent. Eur. Agric. 2020, 21, 37–41. [Google Scholar] [CrossRef]
- Li, S.; Kong, L.; Ziegler, G.R. Electrospinning of Octenylsuccinylated Starch-Pullulan Nanofibers from Aqueous Dispersions. Carbohydr. Polym. 2021, 258, 116933. [Google Scholar]
- Liang, Q.; Gao, Q. Effect of amylose content on the preparation for carboxymethyl starch/pullulan electrospun nanofibers and their properties as encapsulants of thymol. Food Hydrocoll. 2023, 136, 108250. [Google Scholar]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent Sericin-Chitosan Scaffold: Physical Properties and Low Cytotoxicity Effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Sun, R.; Wang, W.; Xia, Q. Preparation, characterization, and evaluation of tamarind seed polysaccharide-carboxymethylcellulose buccal films loaded with soybean peptides-chitosan nanoparticles. Food Hydrocoll. 2023, 141, 108684. [Google Scholar]
- Chen, P.S. Amino acid and protein metabolism. In Comprehensive Insect Physiology Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 10, pp. 177–218. [Google Scholar]
- Wellner, N.; Belton, P.S.; Tatham, A.S. Fourier transform IR spectroscopic study of hydration-induced structure changes in the solid state of ω-gliadins. Biochem. J. 1996, 319, 741–747. [Google Scholar] [CrossRef]
- Bai, W.; Vidal, N.P.; Roman, L.; Portillo-Perez, G.; Martinez, M.M. Preparation and characterization of self-standing biofilms from compatible pectin/starch blends: Effect of pectin structure. Int. J. Biol. Macromol. 2023, 251, 126383. [Google Scholar]
- Sucheta; Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Zarandona, I.; Correia, D.M.; Moreira, J.; Costa, C.M.; Lanceros-Mendez, S.; Guerrero, P.; Caba, K. de la. Magnetically responsive chitosan-pectin films incorporating Fe3O4 nanoparticles with enhanced antimicrobial activity. Int. J. Biol. Macromol. 2023, 227, 1070–1077. [Google Scholar]
- Wang, B.; Yang, X.; Qiao, C.; Li, Y.; Li, T.; Xu, C. Effects of chitosan quaternary ammonium salt on the physicochemical properties of sodium carboxymethyl cellulose-based films. Carbohydr. Polym. 2018, 184, 37–46. [Google Scholar]
- Tavares, K.M.; de Campos, A.; Luchesi, B.R.; Resende, A.A.; de Oliveira, J.E.; Marconcini, J.M. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr. Polym. 2020, 246, 116521. [Google Scholar]
- Terzioglu, P.; Guney, F.; Parin, F.N.; Sen, I.; Tuna, S. Biowaste orange peel incorporated chitosan/polyvinyl alcohol composite films for food packaging applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar]
- Yang, L.; Xie, M.; Fang, J.; Zhang, T.; Wang, X.; Chen, L. Effect of additives on properties of cross-linked carboxymethyl starch/polyvinyl alcohol composite films. J. Appl. Polym. Sci. 2021, 139, 51546. [Google Scholar]
- Guo, J.; Li, X.; Mu, C.; Zhang, H.; Qin, P.; Li, D. Freezing–thawing effects on the properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-MMT composite films. Food Hydrocoll. 2013, 33, 273–279. [Google Scholar]
- Juikar, S.K.; Warkar, S.G. Biopolymers for packaging applications: An overview. Packag. Technol. Sci. J. 2023, 36, 229–251. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Oz, F.; Khan, H.M.; Roy, S.; Esatbeyogly, T.; Pratap-Singh, A. Thermal Properties of Biopolymer Films: Insights for Sustainable Food Packaging Applications. Food Eng. Rev. 2024, 16, 497–512. [Google Scholar] [CrossRef]
- Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P.; Kanthakat, G.; Wongwatcharayothin, W.; Somjai, C.; Danmek, K.; Chuttong, B. Application of Carboxymethyl Cellulose and Glycerol Monostearate as Binder Agents for Protein Powder Production from Honey Bee Brood Using Foam-Mat Drying Technique. Foods 2024, 13, 2265. [Google Scholar] [CrossRef] [PubMed]
- Tachai, K.; Deenu, A.; Kamthai, S. Short Synthesis Time and Characterization of Dialdehyde Carboxymethyl Cellulose (DCMC) from High Bagasse Carboxymethyl Cellulose (CMCB) Concentration. J. Nat. Fibers 2024, 21, 70. [Google Scholar]
- ASTM E313-20; Standard Practice for Calculating Yellowness and Whiteness Indices from Instrumentally Measured Color Coordinates. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D882-83; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 1983.
- Jafari, Z.; Goli, M.; Toghyani, M. The Effects of Phosphorylation and Microwave Treatment on the Functional Characteristics of Freeze-Dried Egg White Powder. Foods 2022, 11, 2711. [Google Scholar] [CrossRef]



| Amino Acid | Content (mg/g Dry Basis) |
|---|---|
| Essential amino acid | |
| Threonine | 0.08 ± 0.01 |
| Valine | 0.39 ± 0.01 |
| Isoleucine | 0.08 ± 0.01 |
| Leucine | 0.30 ± 0.02 |
| Methionine | 0.06 ± 0.01 |
| Phenylalanine | 0.37 ± 0.01 |
| Lysine | 1.01 ± 0.06 |
| Histidine | 0.58 ± 0.02 |
| Nonessential amino acid | |
| Aspartic acid | 0.05 ± 0.02 |
| Serine | 0.02 ± 0.01 |
| Glutamic acid | 0.72 ± 0.04 |
| Proline | 1.51 ± 0.16 |
| Glycine | 0.22 ± 0.01 |
| Alanine | 1.48 ± 0.07 |
| Tyrosine | ND * |
| Arginine | ND * |
| Film | Surface Color | Opacity (mm−1) | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | YI | ||
| CMS/CS/PT | 78.19 ± 0.11 c | 2.85 ± 0.57 a | 27.28 ± 0.85 a | 34.44 ± 0.93 ab | 49.91 ± 5.74 ab | 3.66 ± 0.16 c |
| CMS/CS/PT/0.025 BBP | 77.83 ± 0.10 d | 3.13 ± 0.50 b | 27.92 ± 0.09 a | 35.19 ± 0.10 a | 51.30 ± 4.39 ab | 3.63 ± 0.27 c |
| CMS/CS/PT/0.05 BBP | 79.02 ± 0.35 b | 2.81 ± 0.50 b | 27.04 ± 0.34 a | 33.80 ± 0.54 b | 48.97 ± 6.76 ab | 4.00 ± 0.07 b |
| CMS/CS/PT/0.1 BBP | 79.57 ± 0.82 ab | 2.23 ± 0.32 c | 25.40 ± 0.81 b | 32.07 ± 0.95 c | 45.63 ± 3.65 b | 3.24 ± 0.08 d |
| CMS/CS/PT/0.2 BBP | 80.05 ± 0.05 a | 2.13 ± 0.31 d | 22.47 ± 0.30 d | 29.36 ± 0.24 d | 40.18 ± 6.33 b | 3.53 ± 0.03 c |
| CMS/CS/PT/0.4 BBP | 79.99 ± 0.30 a | 2.56 ± 0.52 d | 23.67 ± 0.78 c | 30.42 ± 0.84 d | 42.36 ± 7.28 b | 4.22 ± 0.10 a |
| Film | Thickness (mm) NS | Tensile Strength (MPa) | Tensile Strength After 24 h Water Absorption (MPa) | Elongation at Break (%) | Elongation at Break After 24 h Water Absorption (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|
| CMS/CS/PT | 0.11 ± 0.01 | 7.95 ± 0.19 ab | 0.66 ± 0.07 c | 34.90 ± 0.33 a | 12.81 ± 0.53 c | 65.70 ± 1.28 e |
| CMS/CS/PT/0.025 BBP | 0.11 ± 0.01 | 6.72 ± 0.26 c | 0.33 ± 0.02 d | 25.55 ± 0.21 d | 7.68 ± 0.50 d | 105.54 ± 1.70 a |
| CMS/CS/PT/0.05 BBP | 0.11 ± 0.01 | 6.74 ± 0.19 c | 0.34 ± 0.01 d | 29.19 ± 0.18 c | 8.38 ± 0.93 d | 47.55 ± 1.31 f |
| CMS/CS/PT/0.1 BBP | 0.11 ± 0.01 | 7.73 ± 0.09 b | 0.35 ± 0.01 d | 32.23 ± 0.15 b | 15.45 ± 0.87 a | 97.84 ± 1.02 b |
| CMS/CS/PT/0.2 BBP | 0.11 ± 0.01 | 5.64 ± 0.22 de | 1.02 ± 0.03 b | 20.37 ± 0.13 e | 14.85 ± 0.24 b | 83.75 ± 1.16 d |
| CMS/CS/PT/0.4 BBP | 0.11 ± 0.01 | 5.16 ± 0.27 e | 2.52 ± 0.01 a | 17.96 ± 0.09 f | 14.25 ± 0.32 b | 88.46 ± 1.12 c |
| Film | Moisture Content (%) NS | Contact Angle (°) | Water Absorption (%) | Thickness Swelling (%) | Water Solubility (%) | WVP × 10−6 (g m−1 d−1 Pa−1) |
|---|---|---|---|---|---|---|
| CMS/CS/PT (Control) | 7.75 ± 0.13 | 76.18 ± 0.50 b | 288.11 ± 2.70 d | 117.52 ± 1.38 d | 35.63 ± 0.83 d | 4.50 ± 0.14 a |
| CMS/CS/PT/0.025 BBP | 7.38 ± 0.57 | 65.86 ± 0.23 d | 327.74 ± 9.50 b | 243.32 ± 1.81 b | 58.54 ± 0.56 a | 4.33 ± 0.28 ab |
| CMS/CS/PT/0.05 BBP | 7.46 ± 0.39 | 73.68 ± 0.45 c | 317.14 ± 3.46 c | 227.12 ± 1.36 c | 42.86 ± 0.43 b | 4.22 ± 0.29 ab |
| CMS/CS/PT/0.1 BBP | 6.92 ± 0.60 | 83.68 ± 0.48 a | 369.01 ± 4.43 a | 248.75 ± 1.65 a | 29.16 ± 0.11 e | 3.93 ± 0.15 b |
| CMS/CS/PT/0.2 BBP | 6.93 ± 0.42 | 55.76 ± 0.52 e | 87.47 ± 4.77 ef | 34.33 ± 1.33 e | 40.53 ± 0.33 c | 2.20 ± 0.36 c |
| CMS/CS/PT/0.4 BBP | 6.96 ± 0.60 | 51.86 ± 0.48 f | 81.39 ± 1.73 f | 21.06 ± 1.87 f | 36.20 ± 0.58 d | 2.11 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamthai, S.; Wiriyacharee, P.; Naruenartwongsakul, S.; Khaw-on, P.; Deenu, A.; Chaipoot, S.; Phongphisutthinant, R.; Tachai, K.; Orpool, S. Influence of Honey Bee Brood Protein on the Hydrophilic, Mechanical, and Thermal Properties of Polysaccharide Gel Films. Gels 2025, 11, 236. https://doi.org/10.3390/gels11040236
Kamthai S, Wiriyacharee P, Naruenartwongsakul S, Khaw-on P, Deenu A, Chaipoot S, Phongphisutthinant R, Tachai K, Orpool S. Influence of Honey Bee Brood Protein on the Hydrophilic, Mechanical, and Thermal Properties of Polysaccharide Gel Films. Gels. 2025; 11(4):236. https://doi.org/10.3390/gels11040236
Chicago/Turabian StyleKamthai, Suthaphat, Pairote Wiriyacharee, Srisuwan Naruenartwongsakul, Patompong Khaw-on, Aree Deenu, Supakit Chaipoot, Rewat Phongphisutthinant, Kamonwan Tachai, and Sawichaya Orpool. 2025. "Influence of Honey Bee Brood Protein on the Hydrophilic, Mechanical, and Thermal Properties of Polysaccharide Gel Films" Gels 11, no. 4: 236. https://doi.org/10.3390/gels11040236
APA StyleKamthai, S., Wiriyacharee, P., Naruenartwongsakul, S., Khaw-on, P., Deenu, A., Chaipoot, S., Phongphisutthinant, R., Tachai, K., & Orpool, S. (2025). Influence of Honey Bee Brood Protein on the Hydrophilic, Mechanical, and Thermal Properties of Polysaccharide Gel Films. Gels, 11(4), 236. https://doi.org/10.3390/gels11040236











