Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogel Patches Recover Wound Incision Rate
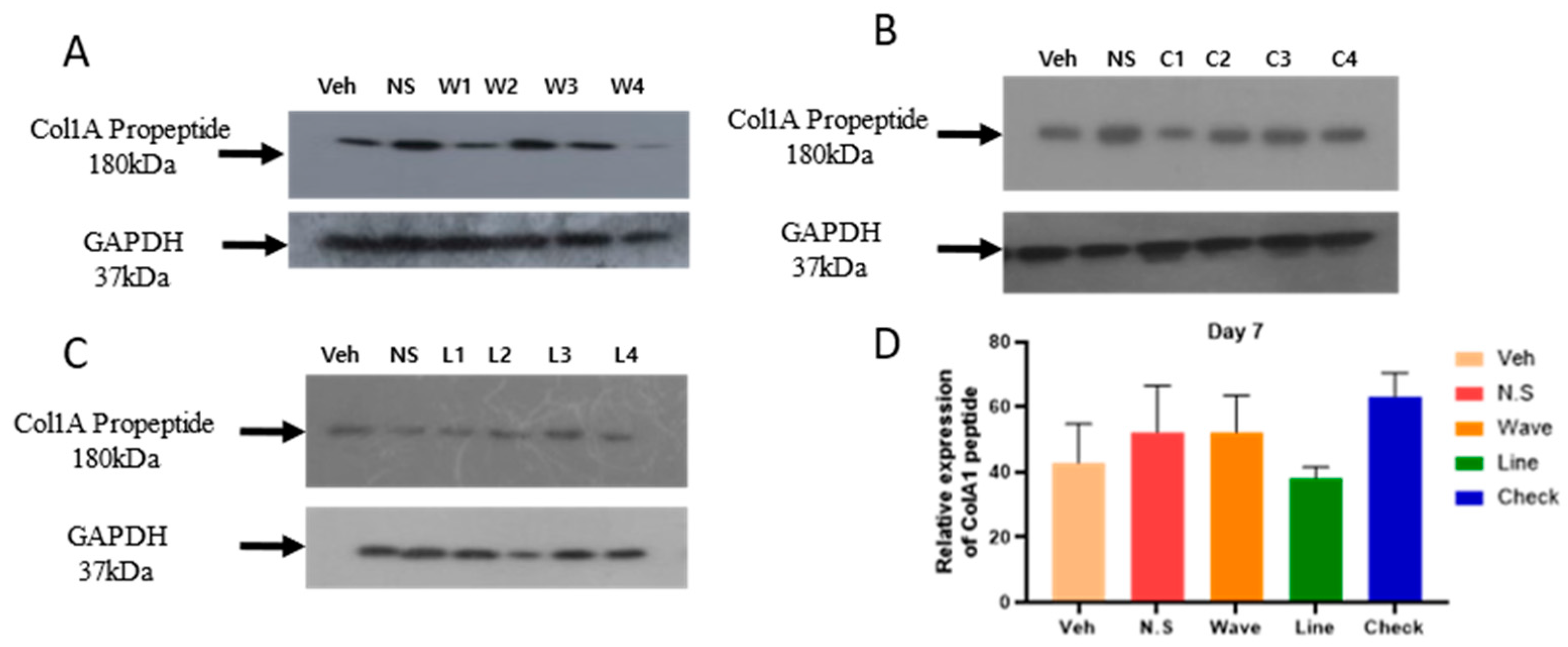
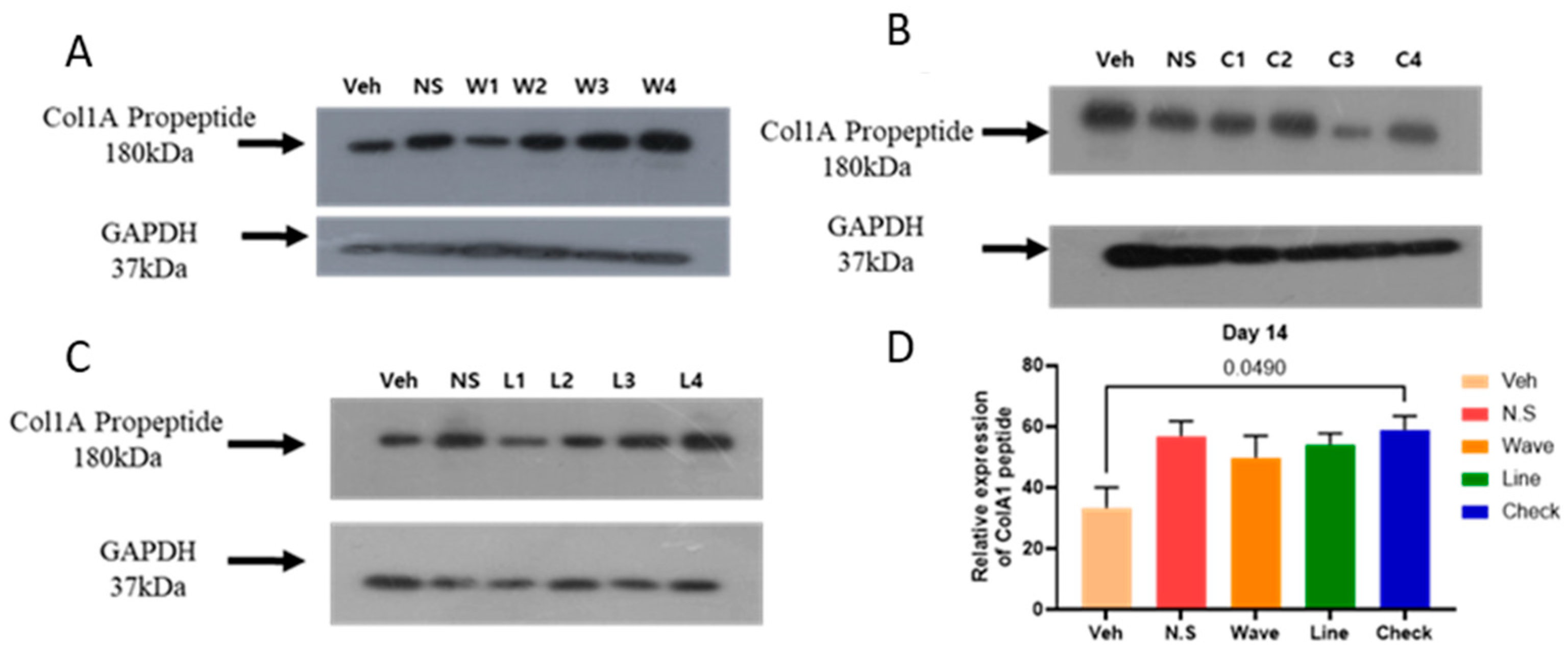
2.2. Hydrogel Improves the Expression of COl1A1 Protein in the Skin
2.3. Discussion
3. Conclusions
4. Materials and Methods
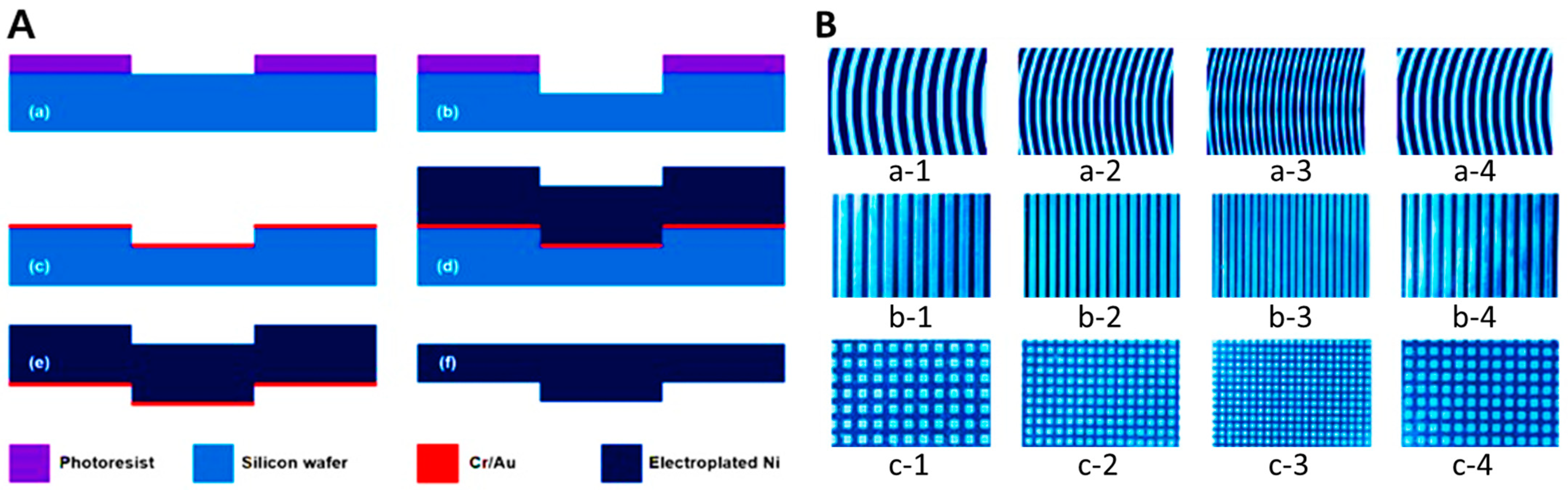
4.1. Nickel (Ni) Stamp
4.1.1. Structure
4.1.2. Manufacturing Process
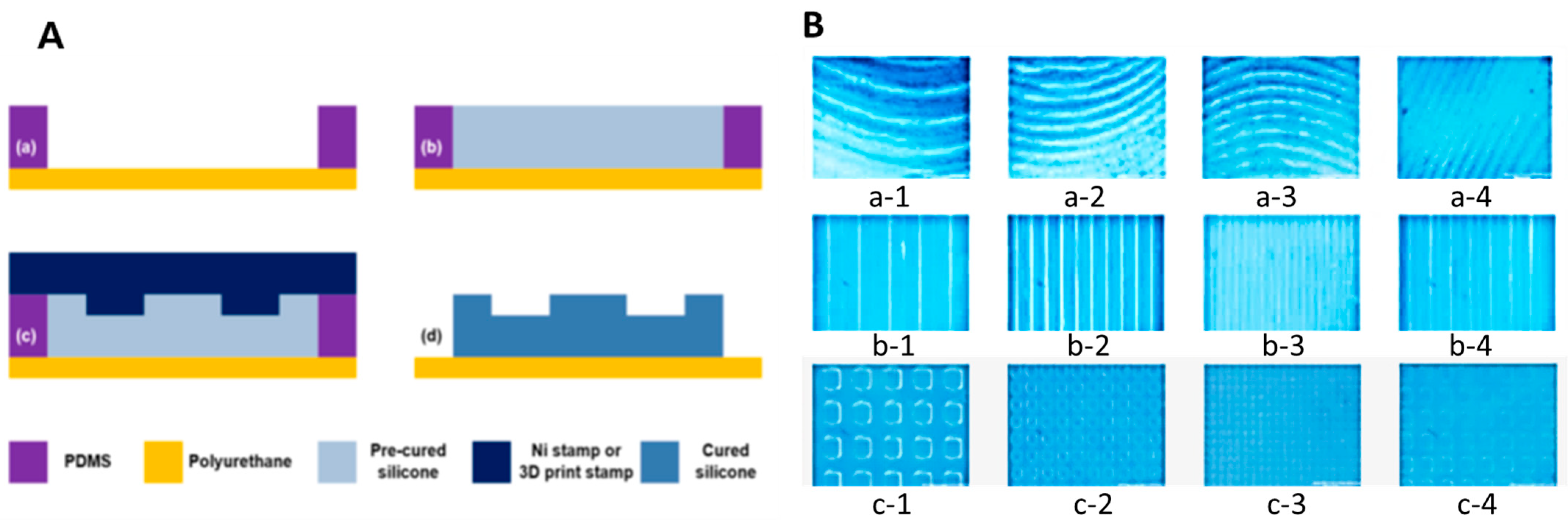
4.2. Wrinkle Shape Replication Process Using a Hot-Embossing Process
4.2.1. Polymer Preparation
4.2.2. Manufacturing Process
4.2.3. Production Result Analysis
4.3. Animal Study
4.3.1. Experimental Animals
4.3.2. Production of Secondary Union Skin Damage (Wound) Animal Model
4.3.3. Experimental Group and Micro-Patterned Hydrogel Patch Application
4.3.4. Wound Closure Rate
4.3.5. Western Blot
4.3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, J.; Parker, C.N.; Bui, U.; MacAndrew, M.; Mitchell, J.; Finlayson, K.J. What is the evidence on skin care for maintaining skin integrity and prevention of wounds?: An integrative review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2024, 32, 25–33. [Google Scholar]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [PubMed]
- Chen, Y.; Tian, L.; Yang, F.; Tong, W.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Liang, X.; et al. Tannic acid accelerates cutaneous wound healing in rats via activation of the ERK 1/2 signaling pathways. Adv. Wound Care 2019, 8, 341–354. [Google Scholar]
- Su, X.; Liu, X.; Wang, S.; Li, B.; Pan, T.; Liu, D.; Wang, F.; Diao, Y.; Li, K.J.B. Wound-healing promoting effect of total tannins from Entada phaseoloides (L.) Merr. in rats. Burns 2017, 43, 830–838. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E.J.B. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar]
- Kamoun, E.A.; Kenawy ER, S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Tian, Y.; Pang, L.; Zhang, R.; Xu, T.; Wang, S.; Yu, B.; Gao, L.; Cong, H.; Shen, Y. Poly-tetrahydropyrimidine antibacterial hydrogel with injectability and self-healing ability for curing the purulent subcutaneous infection. Acs Appl. Mater. Interfaces 2020, 12, 50236–50247. [Google Scholar] [PubMed]
- Han, Y.; Xu, Y.; Tian, L.; Zhou, J.; Zhou, X. Research Progress of Polyvinyl Alcohol (PVA) Based on Hydrogel Dressings. Zhongguo Yi Liao Qi Xie Za Zhi = Chin. J. Med. Instrum. 2018, 42, 437–439. [Google Scholar]
- Chen, S.L.; Fu, R.H.; Liao, S.F.; Liu, S.P.; Lin, S.Z.; Wang, Y.C. A PEG-based hydrogel for effective wound care management. Cell Transplant. 2018, 27, 275–284. [Google Scholar]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar]
- Hartatiek, H.; Yudyanto, Y.; Utomo, J.; Nurhuda, M.; Santjojo, D.J.; Masruroh, M. Morphology, Porosity, and Biodegradation of PVA/CS/PEG/HAp Nanofiber Composites as Scaffold in Bone Tissue Engineering; AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2020. [Google Scholar]
- Sengwa, R.J.; Choudhary, S. Structural characterization of hydrophilic polymer blends/montmorillonite clay nanocomposites. J. Appl. Polym. Sci. 2014, 131, 16. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.; Meijer, E.W. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar]
- Arbab, S.; Ullah, H.; Muhammad, N.; Wang, W.; Zhang, J. Latest advance anti-inflammatory hydrogel wound dressings and traditional Lignosus rhinoceros used for wound healing agents. Front. Bioeng. Biotechnol. 2024, 12, 1488748. [Google Scholar]
- Nifontova, G.; Safaryan, S.; Khristidis, Y.; Smirnova, O.; Vosough, M.; Shpichka, A.; Timashev, P. Advancing wound healing by hydrogel-based dressings loaded with cell-conditioned medium: A systematic review. Stem Cell Res. Ther. 2024, 15, 371. [Google Scholar]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-based dressings designed to facilitate wound healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar]
- Pinthong, T.; Yooyod, M.; Daengmankhong, J.; Tuancharoensri, N.; Mahasaranon, S.; Viyoch, J.; Jongjitwimol, J.; Ross, S.; Ross, G.M. Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings. Gels 2023, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci. Rep. 2021, 11, 23094. [Google Scholar]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; de Santiago, G.T. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar]
- D’Urso, M.; Kurniawan, N.A. Mechanical and physical regulation of fibroblast–myofibroblast transition: From cellular mechanoresponse to tissue pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A.J. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [PubMed]
- Smith, J.; Rai, V. Novel factors regulating proliferation, migration, and differentiation of fibroblasts, keratinocytes, and vascular smooth muscle cells during wound healing. Biomedicines 2024, 12, 1939. [Google Scholar] [CrossRef]
- Smithmyer, M.E.; Sawicki, L.A.; Kloxin, A.M. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater. Sci. 2014, 2, 634–650. [Google Scholar]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.-M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024, 15, 6820. [Google Scholar]
- Dunn, G.A.; Brown, A.F. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J. Cell Sci. 1986, 83, 313–340. [Google Scholar] [CrossRef]
- van der Putten, C.; D’Urso, M.; Bril, M.; Woud, T.E.; Bouten, C.V.; Kurniawan, N.A. Generation of multicue cellular microenvironments by UV-photopatterning of three-dimensional cell culture substrates. J. Vis. Exp. 2022, 184, e63988. [Google Scholar]
- Thanikachalam, T.; Selvaraj, T.K.R.; Ayyappan, M.; Arumugam, G. Gap closure of different shape wounds: In vitro and in vivo experimental models in the presence of engineered protein adhesive hydrogel. J. Tissue Eng. Regen. Med. 2019, 13, 174–178. [Google Scholar]
- Mohite, P.; Asane, G.; Rebello, N.; Munde, S.; Ade, N.; Boban, T.; Damiri, F.; Singh, S. Polymeric Hydrogel Sponges for Wound Healing Applications: A Comprehensive Review. Regen. Eng. Transl. Med. 2024, 10, 416–437. [Google Scholar] [CrossRef]
- Hao, R.; Cui, Z.; Zhang, X.; Tian, M.; Zhang, L.; Rao, F.; Xue, J. Rational design and preparation of functional hydrogels for skin wound healing. Front. Chem. 2022, 9, 839055. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Sun, M.; Wang, P.; Okubo, T.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J.; Li, Y. Possible contribution of fibrocytes to increased type I collagen synthesis during the early stage of dermal wound repair in human skin. J. Investig. Dermatol. 2018, 138, 240–242. [Google Scholar] [CrossRef]
- Yeh ChungJu, Y.C.; Chen ChinChuan, C.C.; Leu YannLii, L.Y.; Lin MingWei, L.M.; Chiu MeiMiao, C.M.; Wang ShuHuei, W.S. The effects of artocarpin on wound healing: In vitro and in vivo studies. Sci. Rep. 2017, 7, 15599. [Google Scholar]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive assessment of Nile tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, J.; Wu, H.; Lin, X.; Cai, L. Stimuli-responsive hydrogel dressing for wound healing. APL Mater. 2025, 13, 010601. [Google Scholar]


| Hydrogel Microstructure | Average of Time Constant (Day) |
|---|---|
| Veh 1 (Control) | 6.7 |
| N.S. 2 (Flat) | 5.8 |
| Waves | 4.0 |
| Lines | 3.8 |
| Checks | 2.7 |
| Pattern | Flat | Wave | Wave | Wave | Wave | Line | Line | Line | Line | Check | Check | Check | Check |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | N.S. | a-1 | a-2 | a-3 | a-4 | b-1 | b-2 | b-3 | b-4 | c-1 | c-2 | c-3 | c-4 |
| Interval | - | 500 μm | 200 μm | 100 μm | 250 μm | 500 μm | 200 μm | 100 μm | 250 μm | 500 μm | 200 μm | 100 μm | 250 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.J.; Khan, Z.A.; Ansari, A.; Choi, J.; Kim, E.J.; Han, S.-H.; Song, H.-J.; Jeong, O.C.; Hong, Y. Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model. Gels 2025, 11, 239. https://doi.org/10.3390/gels11040239
Choi HJ, Khan ZA, Ansari A, Choi J, Kim EJ, Han S-H, Song H-J, Jeong OC, Hong Y. Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model. Gels. 2025; 11(4):239. https://doi.org/10.3390/gels11040239
Chicago/Turabian StyleChoi, Hong Jin, Zeeshan Ahmad Khan, AbuZar Ansari, Jeonghyun Choi, Eun Jin Kim, Seo-Hee Han, Ho-Jun Song, Ok Chan Jeong, and Yonggeun Hong. 2025. "Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model" Gels 11, no. 4: 239. https://doi.org/10.3390/gels11040239
APA StyleChoi, H. J., Khan, Z. A., Ansari, A., Choi, J., Kim, E. J., Han, S.-H., Song, H.-J., Jeong, O. C., & Hong, Y. (2025). Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model. Gels, 11(4), 239. https://doi.org/10.3390/gels11040239








