Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance
Abstract
:1. Introduction
2. Results and Discussion
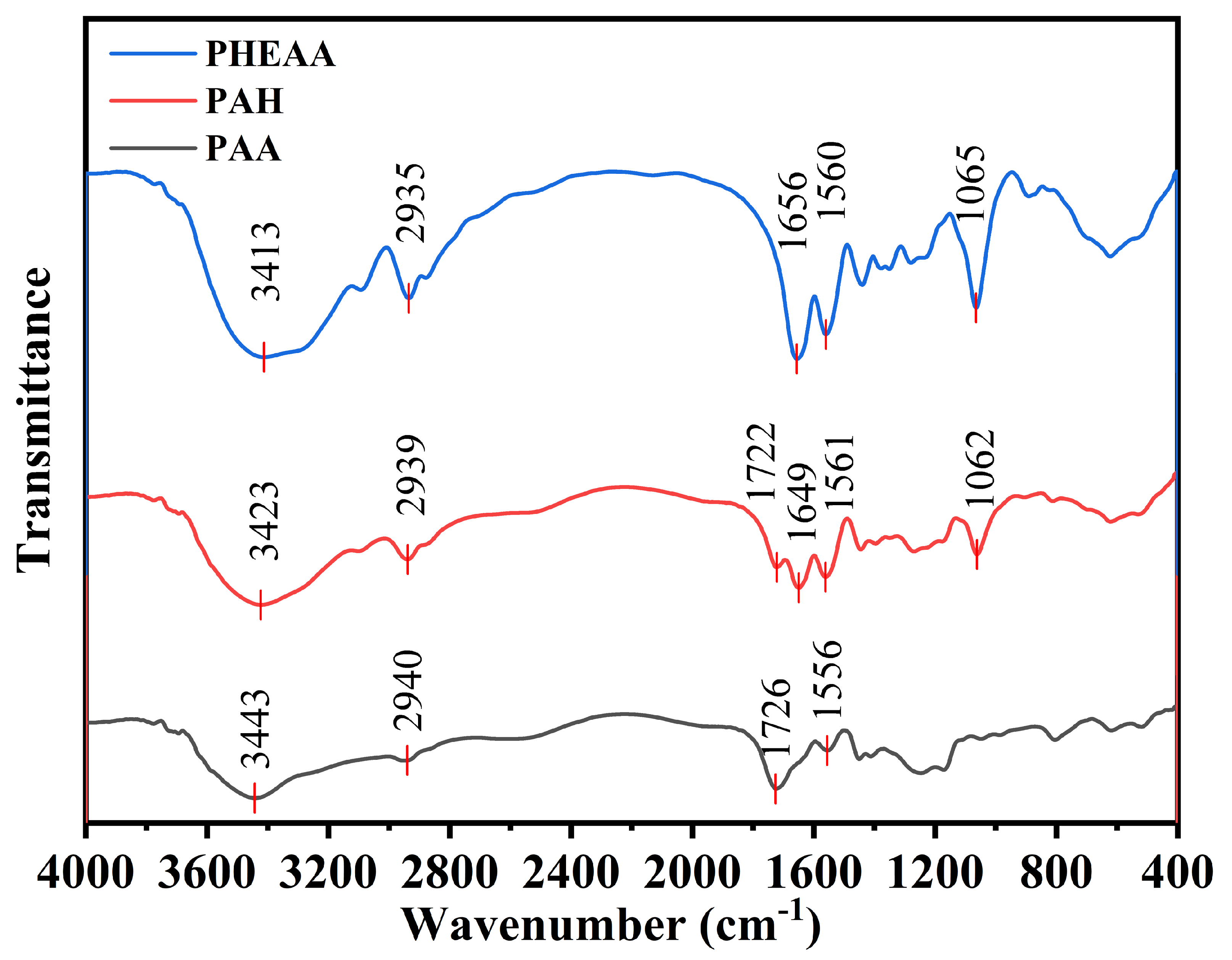
2.1. Infrared Characterization of PHA Hydrogels
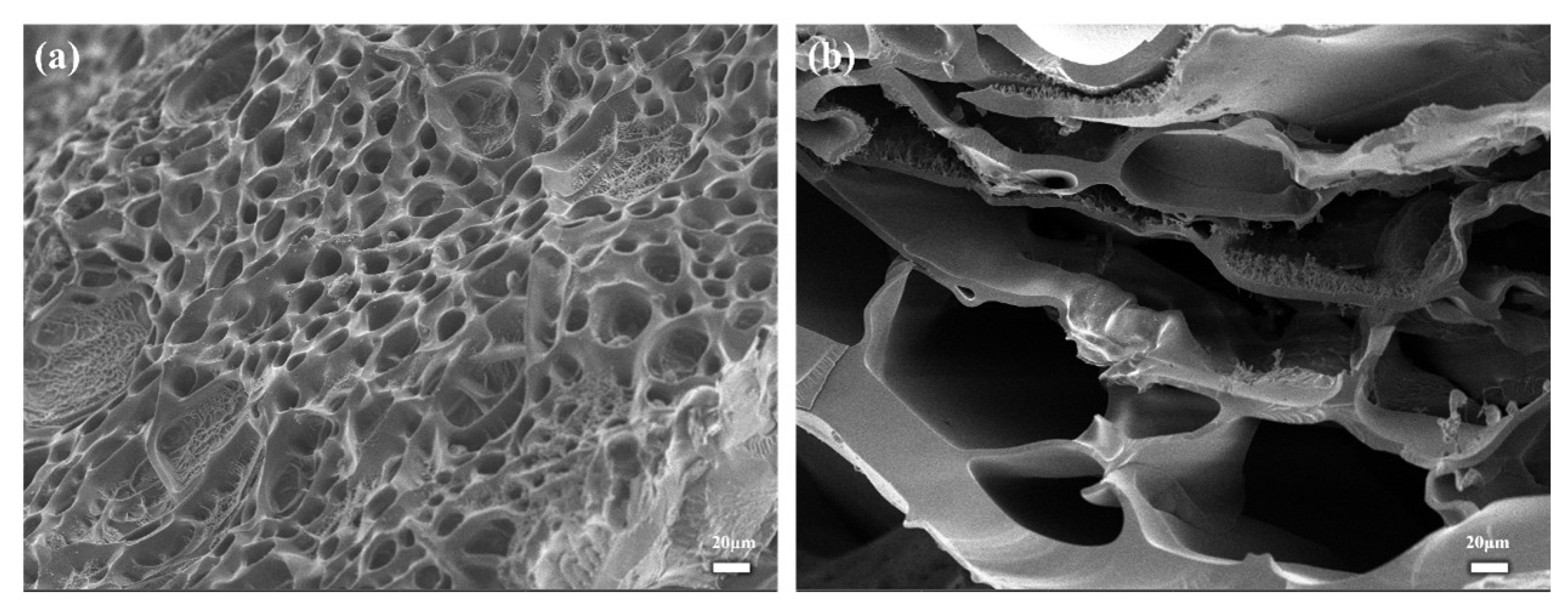
2.2. Morphological Characterization of PHA Hydrogels
2.3. pH-Responsive Behavior of Hydrogels
2.3.1. pH Response of Hydrogels with Different Monomer Ratios
2.3.2. Effect of pH on the Swelling Behavior of Hydrogels
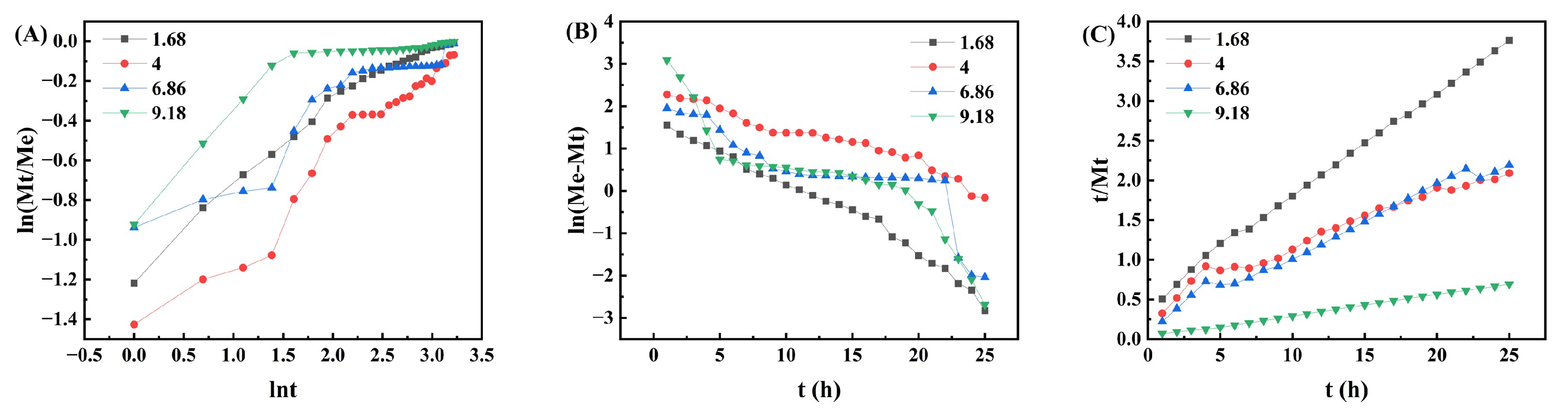
2.4. Swelling Kinetics
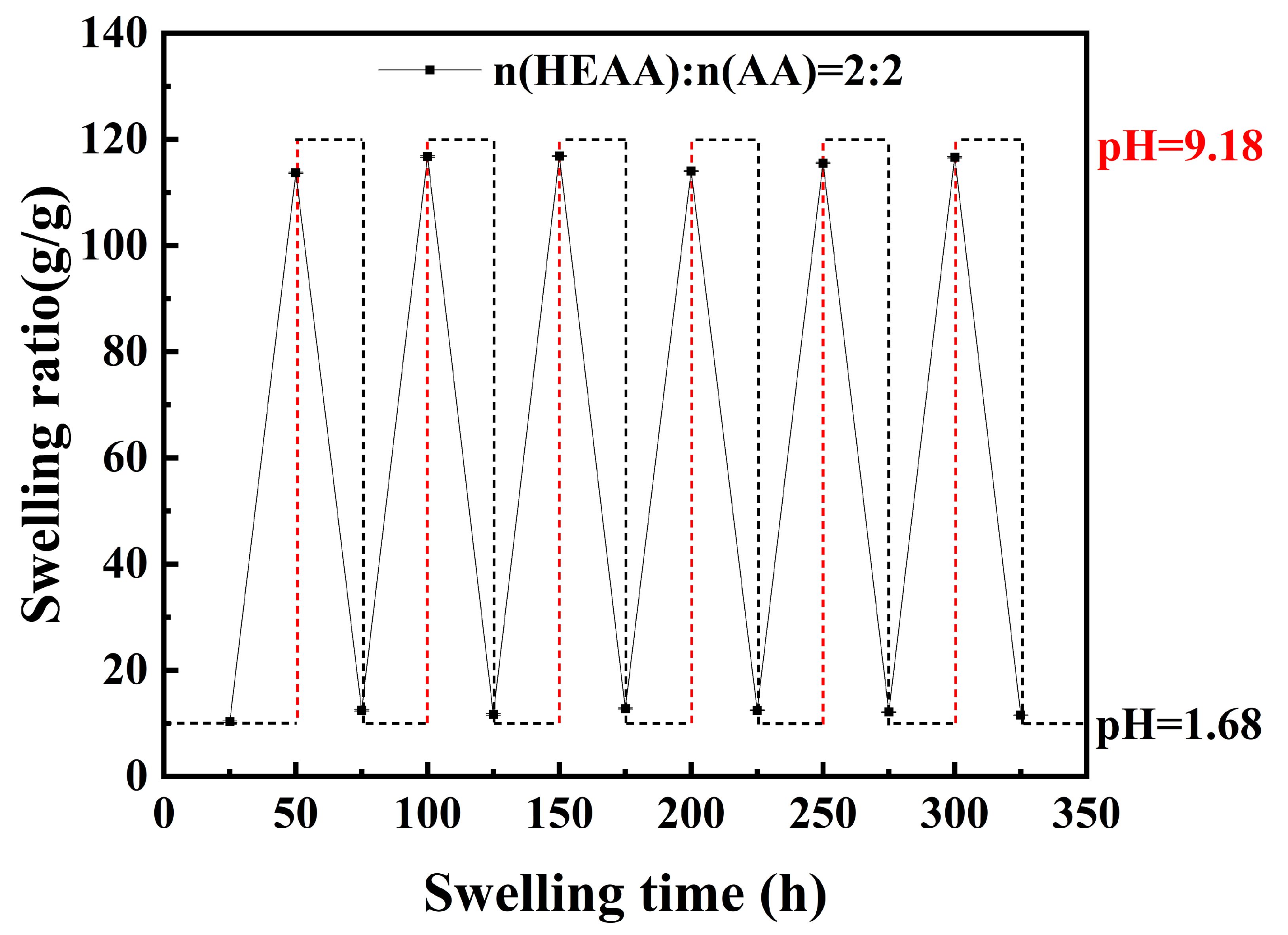
2.5. Reversible pH Swelling of Hydrogels
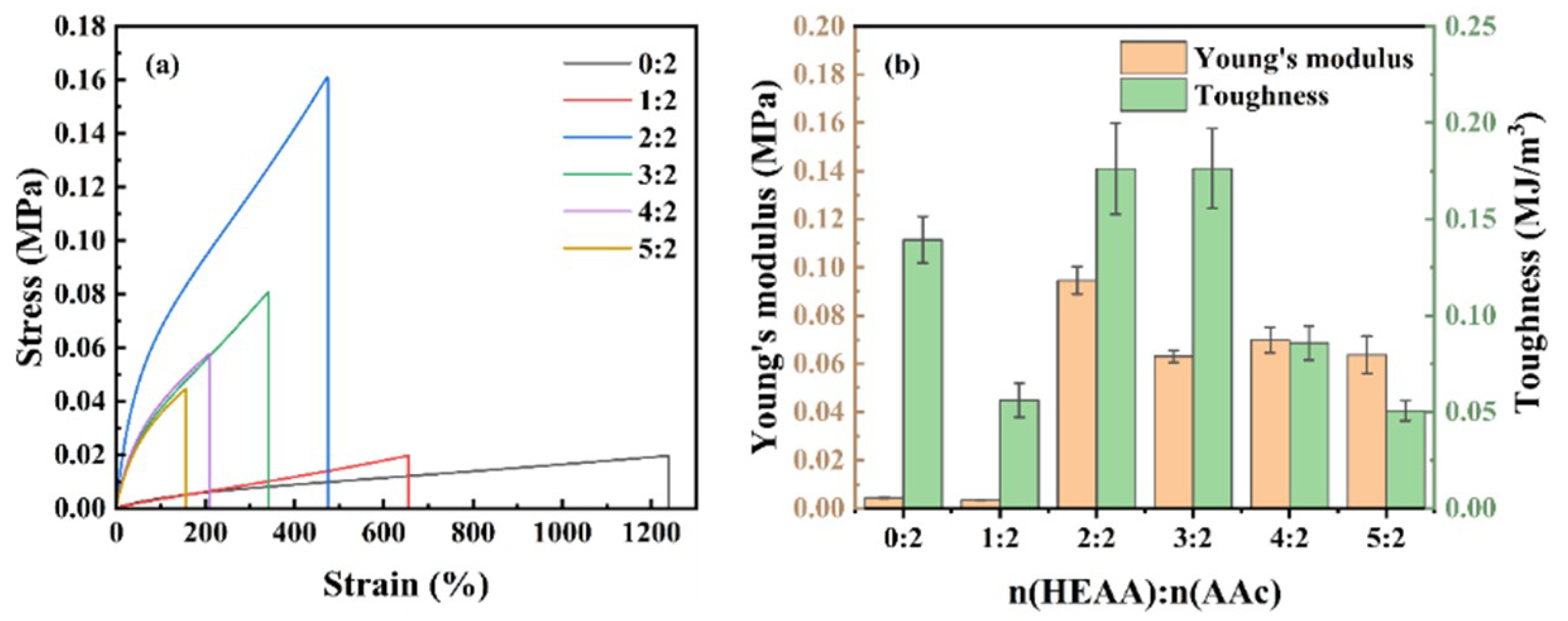
2.6. Mechanical Properties of Hydrogels
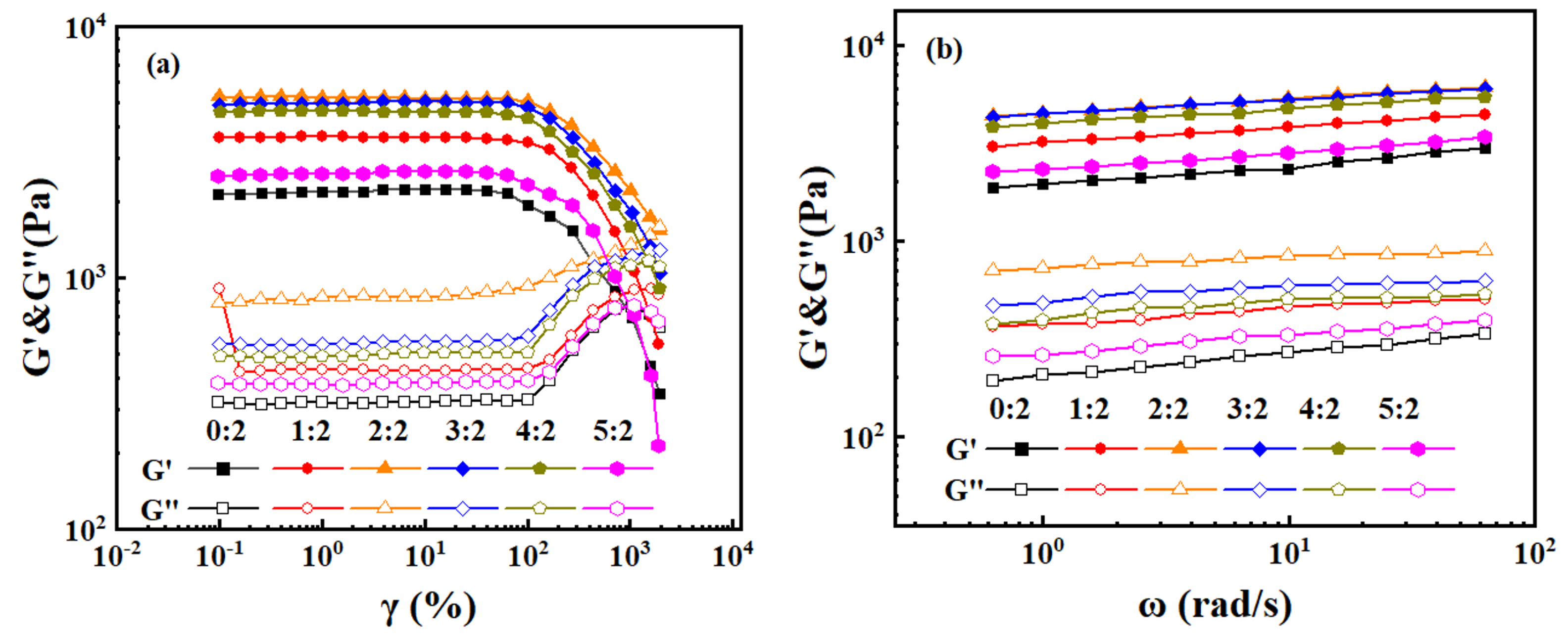
2.7. Rheological Properties of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Poly (N-(2-Hydroxyethyl) Acrylamide-acrylic acid) (PHA) Hydrogel
4.3. Characterization of PHA Hydrogels
4.4. Testing of Hydrogel Swelling Properties
4.4.1. Determination of Swelling Degree of Hydrogels
4.4.2. pH Sensitivity Testing of Hydrogels
4.5. Testing of Mechanical Properties of Hydrogels
4.6. Rheological Testing of Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Du, Y.; Yan, Y.; Song, S.; Qi, J.; Xia, X.; Hu, X.; Chen, Q.; Liu, J.; Zeng, X.; et al. pH-responsive dual-functional hydrogel integrating localized delivery and anti-cancer activities for highly effective therapy in PDX of OSCC. Mater. Today 2023, 62, 71–97. [Google Scholar]
- Zixuan, C.; Bin, Z.; Liyang, J.; Yunyi, L.; Guohe, X.; Jingjun, M. Intelligent-Responsive Hydrogels-Based Controlled Drug Release Systems and Its Applications. Prog. Chem. 2019, 31, 1653. [Google Scholar]
- Zhang, J.; Xiao, S.; Shen, M.; Sun, L.; Chen, F.; Fan, P.; Zhong, M.; Yang, J. Aqueous lubrication of poly (N-hydroxyethyl acrylamide) brushes: A strategy for their enhanced load bearing capacity and wear resistance. RSC Adv. 2016, 6, 21961–21968. [Google Scholar]
- Wang, S.; Wang, F.; Shi, K.; Yuan, J.; Sun, W.; Yang, J.; Chen, Y.; Zhang, D.; Che, L. Osteichthyes skin-inspired tough and sticky composite hydrogels for dynamic adhesive dressings. Compos. Part B Eng. 2022, 241, 110010. [Google Scholar]
- De Breuck, J.; Streiber, M.; Ringleb, M.; Schröder, D.; Herzog, N.; Schubert, U.S.; Zechel, S.; Traeger, A.; Leiske, M.N. Amino-Acid-Derived Anionic Polyacrylamides with Tailored Hydrophobicity–Physicochemical Properties and Cellular Interactions. ACS Polym. Au 2024, 4, 222–234. [Google Scholar]
- Zhang, J.; Qu, D.; Wang, S.; Qi, S.; Zuo, H. Structure, Property Optimization, and Adsorption Properties of N, N′-methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions. Polymers 2024, 16, 1990. [Google Scholar] [CrossRef]
- Bal, A.; Özkahraman, B.; Özbaş, Z. Preparation and characterization of p H responsive poly (methacrylic acid-acrylamide-N-hydroxyethyl acrylamide) hydrogels for drug delivery systems. J. Appl. Polym. Sci. 2016, 133, 43226. [Google Scholar]
- Awasthi, S.; Singhal, R. Mathematical modeling for the prediction of the overall swelling profile from poly (AM-co-AA-co-HEA) hydrogels: Effect of glycidyl methacrylate and ammonium per sulphate. Int. J. Plast. Technol. 2015, 19, 241–262. [Google Scholar]
- Zagni, C.; Dattilo, S.; Mecca, T.; Gugliuzzo, C.; Scamporrino, A.A.; Privitera, V.; Puglisi, R.; Carroccio, S.C. Single and dual polymeric sponges for emerging pollutants removal. Eur. Polym. J. 2022, 179, 111556. [Google Scholar]
- Didehban, K.; Hayasi, M.; Kermajani, F. Removal of anionic dyes from aqueous solutions using polyacrylamide and polyacrylic acid hydrogels. Korean J. Chem. Eng. 2017, 34, 1177–1186. [Google Scholar]
- Barbani, N.; Bertoni, F.; Ciardelli, G.; Cristallini, C.; Silvestri, D.; Coluccio, M.; Giusti, P. Bioartificial materials based on blends of dextran and poly (vinyl alcohol-co-acrylic acid). Eur. Polym. J. 2005, 41, 3004–3010. [Google Scholar]
- Ma, M.; Li, Y.; Wang, D.; Li, J.; Ye, H.; Du, D.; Chi, R.; Han, Q. Highly efficient and sustainable polyacrylic acid-polyacrylamide double-network hydrogels prepared by cross-linking of waste residues for heavy metals ions removal. J. Environ. Chem. Eng. 2024, 12, 114294. [Google Scholar]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar]
- Wang, X.; Wang, Y.; He, S.; Hou, H.; Hao, C. Ultrasonic-assisted synthesis of superabsorbent hydrogels based on sodium lignosulfonate and their adsorption properties for Ni2+. Ultrason. Sonochem. 2018, 40, 221–229. [Google Scholar]
- Rodríguez-Loredo, N.A.; Ovando-Medina, V.M.; Pérez, E.; Corona-Rivera, M.A.; Cervantes-González, E.; Antonio-Carmona, I.D.; Ramos-Torres, C.J. Preparation of poly (acrylic acid)/linseed mucilage/chitosan hydrogel for ketorolac release. J. Vinyl Addit. Technol. 2023, 29, 890–900. [Google Scholar]
- Markovic, M.D.; Panic, V.V.; Seslija, S.I.; Spasojevic, P.M.; Ugrinovic, V.D.; Boskovic-Vragolovic, N.M.; Pjanovic, R.V. Modification of hydrophilic polymer network to design a carrier for a poorly water-soluble substance. Polym. Eng. Sci. 2020, 60, 2496–2510. [Google Scholar]
- Wang, Y.; He, G.; Li, Z.; Hua, J.; Wu, M.; Gong, J.; Zhang, J.; Ban, L.t.; Huang, L. Novel biological hydrogel: Swelling behaviors study in salt solutions with different ionic valence number. Polymers 2018, 10, 112. [Google Scholar] [CrossRef]
- Corona Rivera, M.A.; Cisneros Covarrubias, C.A.; Fernández Escamilla, V.V.A.; Mendizábal Mijares, E.; Pérez López, J.E. Synthesis and characterization of pH-responsive water-dispersed nanohydrogels of cross-linked polyacrylamide-co-polyacrylic acid. Polym. Eng. Sci. 2022, 62, 1797–1810. [Google Scholar]
- Thakur, S.; Arotiba, O.A. Synthesis, swelling and adsorption studies of a pH-responsive sodium alginate–poly (acrylic acid) superabsorbent hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar]
- Li, Q.; Ma, W.; Ma, H.; Liu, H.; Shang, H.; Qiao, N.; Sun, X. pH-responsive biomass-based hydrogels for 5-fu delivery: Investigating the influential role of itaconic acid content on structural characteristic, swelling behavior, and drug loading capacity. Mater. Chem. Phys. 2024, 322, 129604. [Google Scholar]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly (sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar]
- Jing, Z.; Xu, A.; Liang, Y.Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable poly (acrylic acid-co-acrylamide)/poly (vinyl alcohol) double network hydrogels with tunable mechanics and high self-healing performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, S.; Liu, X.; Chen, Y.; Qi, P.; Liu, X. Molybdenum disulfide enhanced polyacrylamide-acrylic acid-Fe3+ ionic conductive hydrogel with high mechanical properties and anti-fatigue abilities as strain sensors. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610, 125692. [Google Scholar]
- Jing, Z.; Zhang, Q.; Liang, Y.Q.; Zhang, Z.; Hong, P.; Li, Y. Synthesis of poly (acrylic acid)–Fe3+/gelatin/poly (vinyl alcohol) triple-network supramolecular hydrogels with high toughness, high strength and self-healing properties. Polym. Int. 2019, 68, 1710–1721. [Google Scholar]
- Hu, X.; Feng, L.; Wei, W.; Xie, A.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel semi-IPN hydrogel based on Salecan and poly (N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Carbohydr. Polym. 2014, 105, 135–144. [Google Scholar]
- Shin, J.; Choi, S.; Kim, J.H.; Cho, J.H.; Jin, Y.; Kim, S.; Min, S.; Kim, S.K.; Choi, D.; Cho, S.W. Tissue tapes—phenolic hyaluronic acid hydrogel patches for off-the-shelf therapy. Adv. Funct. Mater. 2019, 29, 1903863. [Google Scholar]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, Y.; Wang, N.; Lei, Z. Facile synthesis of an acrylic acid-co-2-acrylamide-2-methylpropanesulfonic acid copolymer/polyvinylpyrrolidone semi-IPN hydrogel with excellent swelling and anti-leakage properties. Mater. Today Commun. 2024, 39, 109190. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhanitabar, A. A promising porous polymer-nanoclay hydrogel nanocomposite as water reservoir material: Synthesis and kinetic study. J. Porous Mater. 2018, 25, 665–675. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Chen, C.; Zhao, D.; Su, Z.; Ma, G.; Yu, R. A rapid, non-invasive and non-destructive method for studying swelling behavior and microstructure variations of hydrogels. Carbohydr. Polym. 2016, 151, 1251–1260. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Dong, C.; An, Y.; Liu, J.; Li, J.; Li, X.; Si, C.; Zhang, M. Synthesis and characterization of temperature/pH dual sensitive hemicellulose-based hydrogels from eucalyptus APMP waste liquor. Carbohydr. Polym. 2020, 247, 116717. [Google Scholar] [CrossRef]
- Ramazani-Harandi, M.; Zohuriaan-Mehr, M.; Yousefi, A.; Ershad-Langroudi, A.; Kabiri, K. Effects of structural variables on AUL and rheological behavior of SAP gels. J. Appl. Polym. Sci. 2009, 113, 3676–3686. [Google Scholar] [CrossRef]
- Chen, M.; Shen, Y.; Xu, L.; Xiang, G.; Ni, Z. Synthesis of a super-absorbent nanocomposite hydrogel based on vinyl hybrid silica nanospheres and its properties. RSC Adv. 2020, 10, 41022–41031. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Zhao, J.; Qiu, X.; Zhang, W.; Ma, G.; Lei, Z. Preparation and anti-leakage properties of sesbania gum-grafted copolymers. J. Appl. Polym. Sci. 2021, 138, 50103. [Google Scholar] [CrossRef]


| Peppas Model | First-Order Model | Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| pH | R2 | n | K1 | R2 | K2 | R2 | K3 |
| 1.68 | 0.9641 | 0.355 | 0.343 | 0.9802 | 0.166 | 0.9986 | 0.035 |
| 4 | 0.9595 | 0.45 | 0.225 | 0.954 | 0.092 | 0.9744 | 0.01 |
| 6.86 | 0.8883 | 0.299 | 0.388 | 0.748 | 0.123 | 0.9859 | 0.028 |
| 9.18 | 0.6899 | 0.203 | 0.563 | 0.8167 | 0.161 | 0.9987 | 0.028 |
| HEAA: AA Molar Ratio | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Toughness (MJ/m3) |
|---|---|---|---|---|
| 0:2 | 0.019 | 1238.19 | 0.004 | 0.139 |
| 1:2 | 0.020 | 655.73 | 0.003 | 0.056 |
| 2:2 | 0.160 | 474.26 | 0.095 | 0.176 |
| 3:2 | 0.081 | 341.76 | 0.063 | 0.176 |
| 4:2 | 0.058 | 209.19 | 0.070 | 0.086 |
| 5:2 | 0.045 | 156.60 | 0.064 | 0.051 |
| HEAA/AA Ratios | G′ (Pa) | G″ (Pa) |
|---|---|---|
| 0:2 | 346∼2248 | 315∼768 |
| 1:2 | 544∼3637 | 424∼904 |
| 2:2 | 1738∼5257 | 791∼1584 |
| 3:2 | 1043∼5021 | 541∼1285 |
| 4:2 | 905∼4617 | 480∼1162 |
| 5:2 | 214∼2645 | 378∼776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xi, G.; Wu, T.; Li, P.; Zhan, P.; Liu, N.; Wu, Z. Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance. Gels 2025, 11, 241. https://doi.org/10.3390/gels11040241
Liu Q, Xi G, Wu T, Li P, Zhan P, Liu N, Wu Z. Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance. Gels. 2025; 11(4):241. https://doi.org/10.3390/gels11040241
Chicago/Turabian StyleLiu, Qiang, Ge Xi, Tao Wu, Peining Li, Peng Zhan, Na Liu, and Zhiping Wu. 2025. "Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance" Gels 11, no. 4: 241. https://doi.org/10.3390/gels11040241
APA StyleLiu, Q., Xi, G., Wu, T., Li, P., Zhan, P., Liu, N., & Wu, Z. (2025). Preparation of pH-Sensitive Poly (N-(2-Hydroxyethyl) Acrylamide-co-acrylic Acid) Hydrogels and Their Performance. Gels, 11(4), 241. https://doi.org/10.3390/gels11040241





