Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications
Abstract
1. Introduction
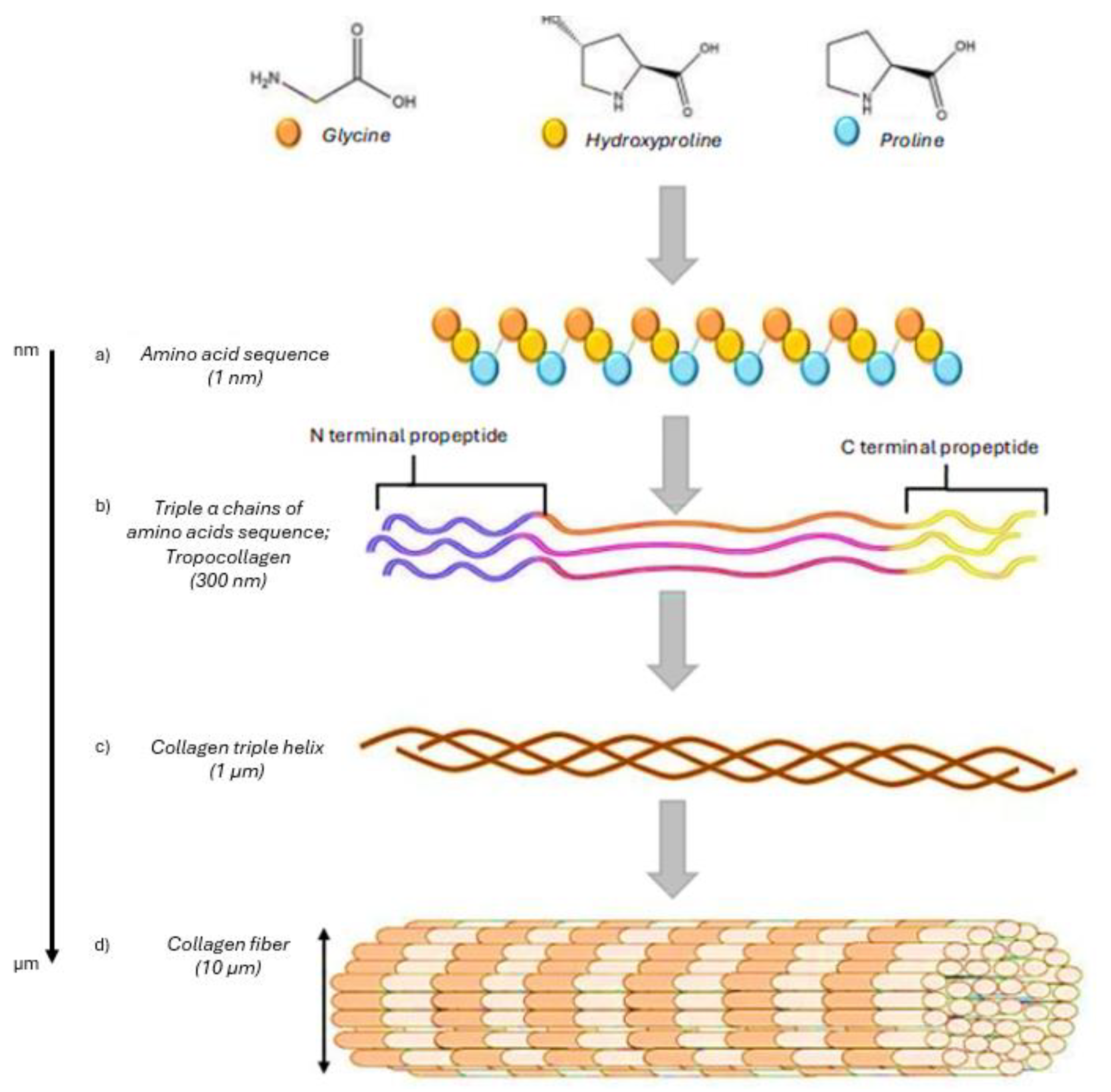
2. Composition, Types, and Sources of Collagen
3. Properties of Collagen—Suitability for Wound Dressings
4. Biological Functions of Collagen in Wound Healing
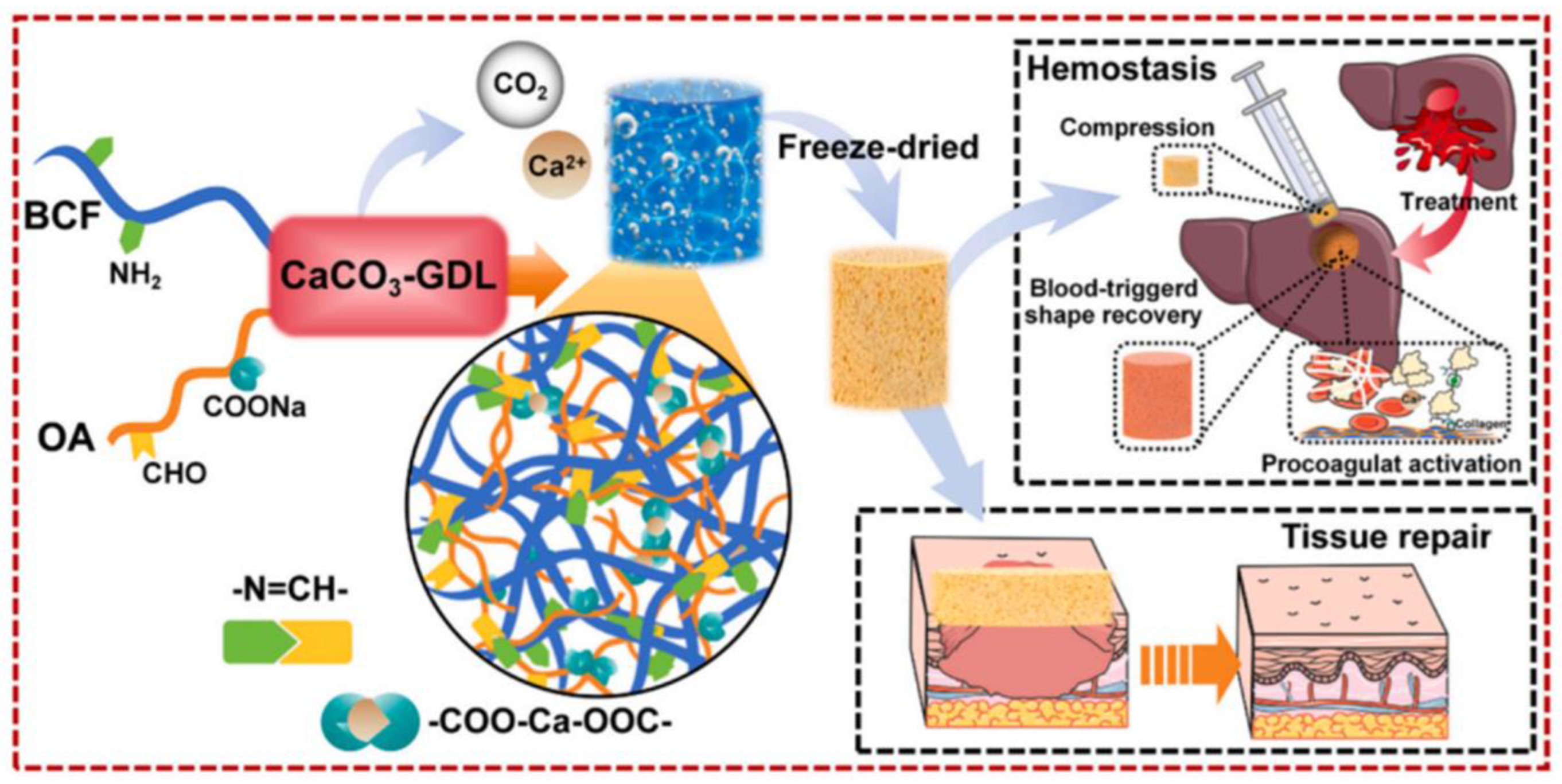
5. Types and Variations of Collagen-Based Wound Dressings
5.1. Films
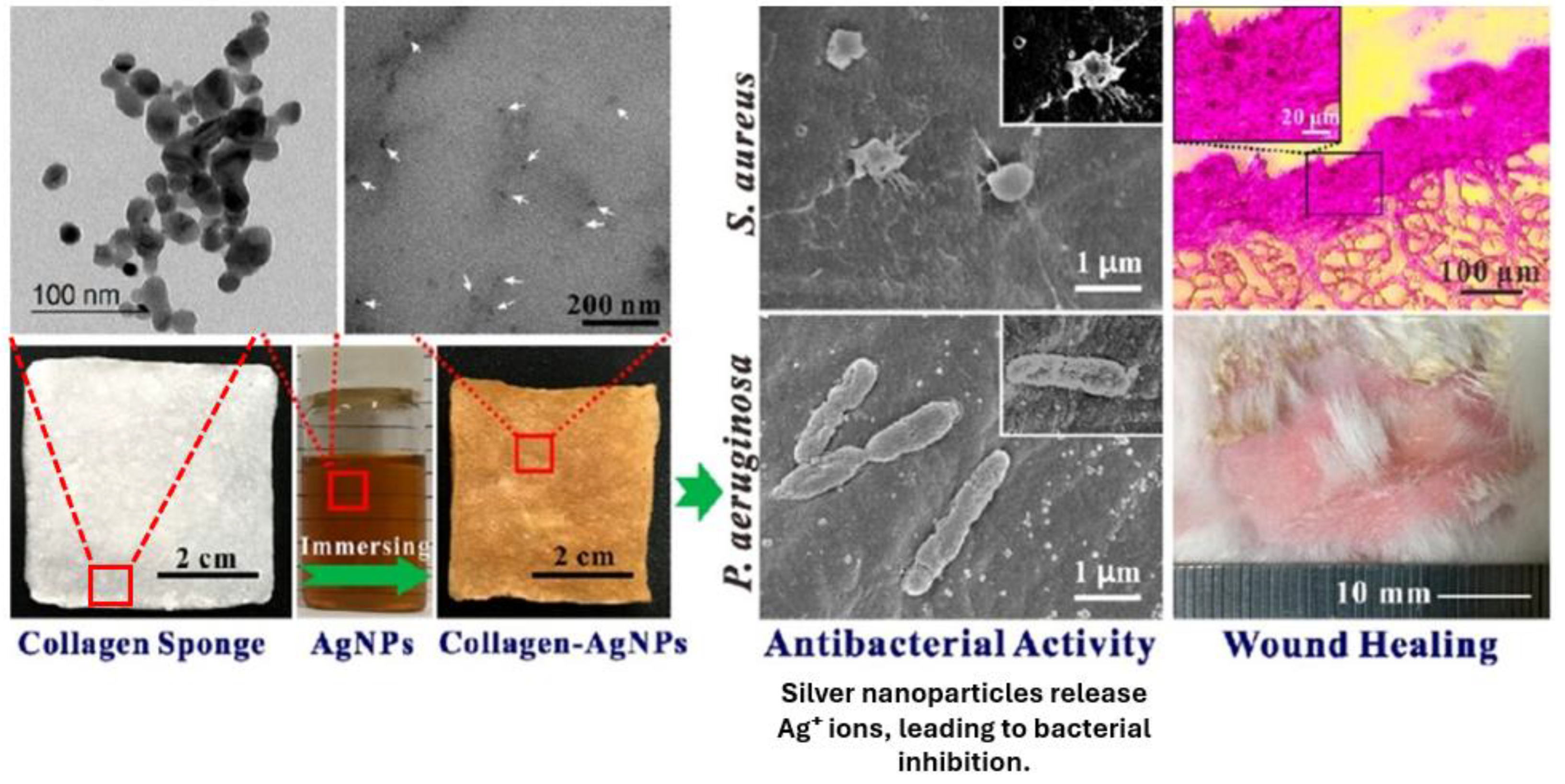
5.2. Sponges
5.3. Hydrogels
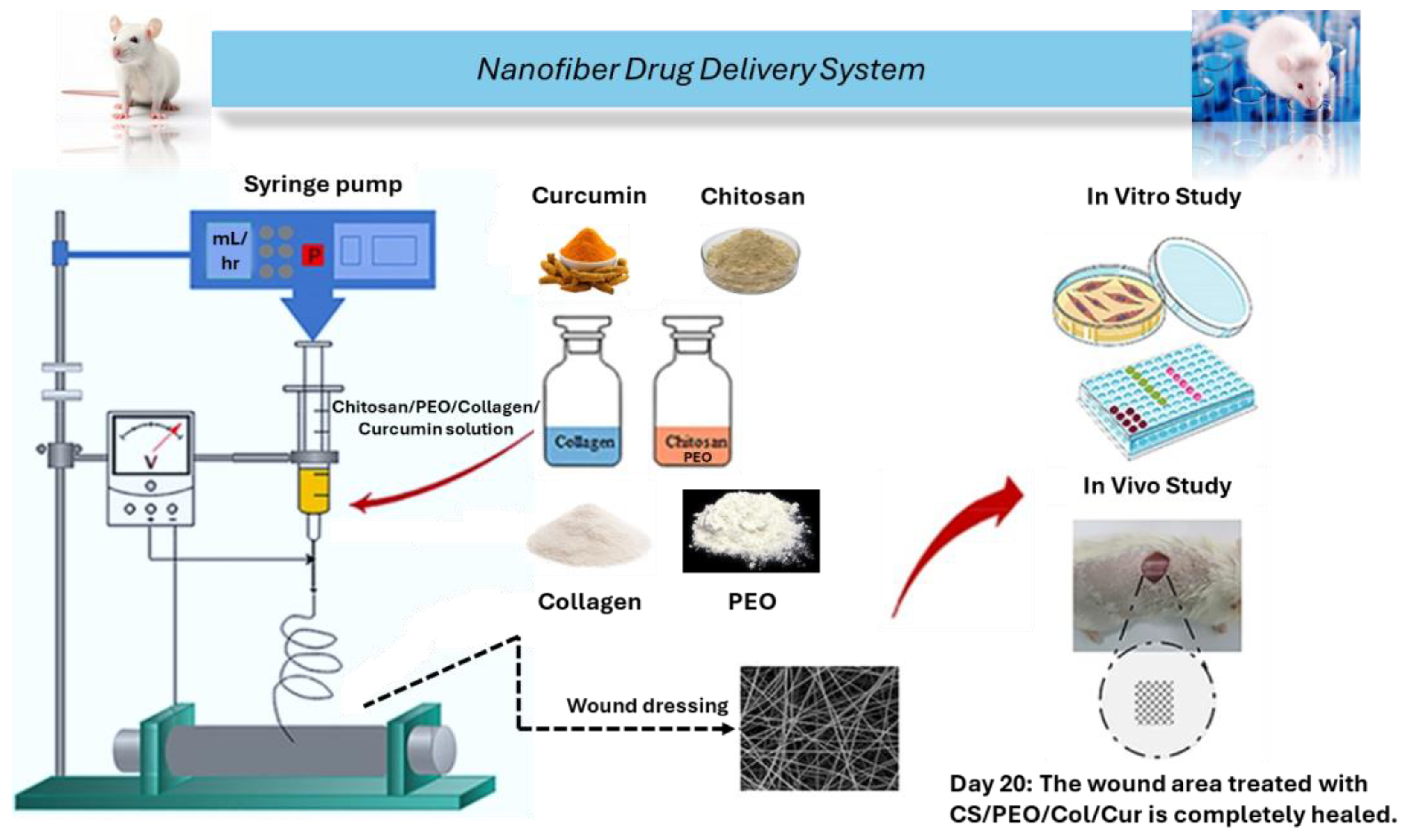
5.4. Nanofibers
6. Fabrication Techniques for Collagen Dressings
7. Clinical Applications and Use Cases
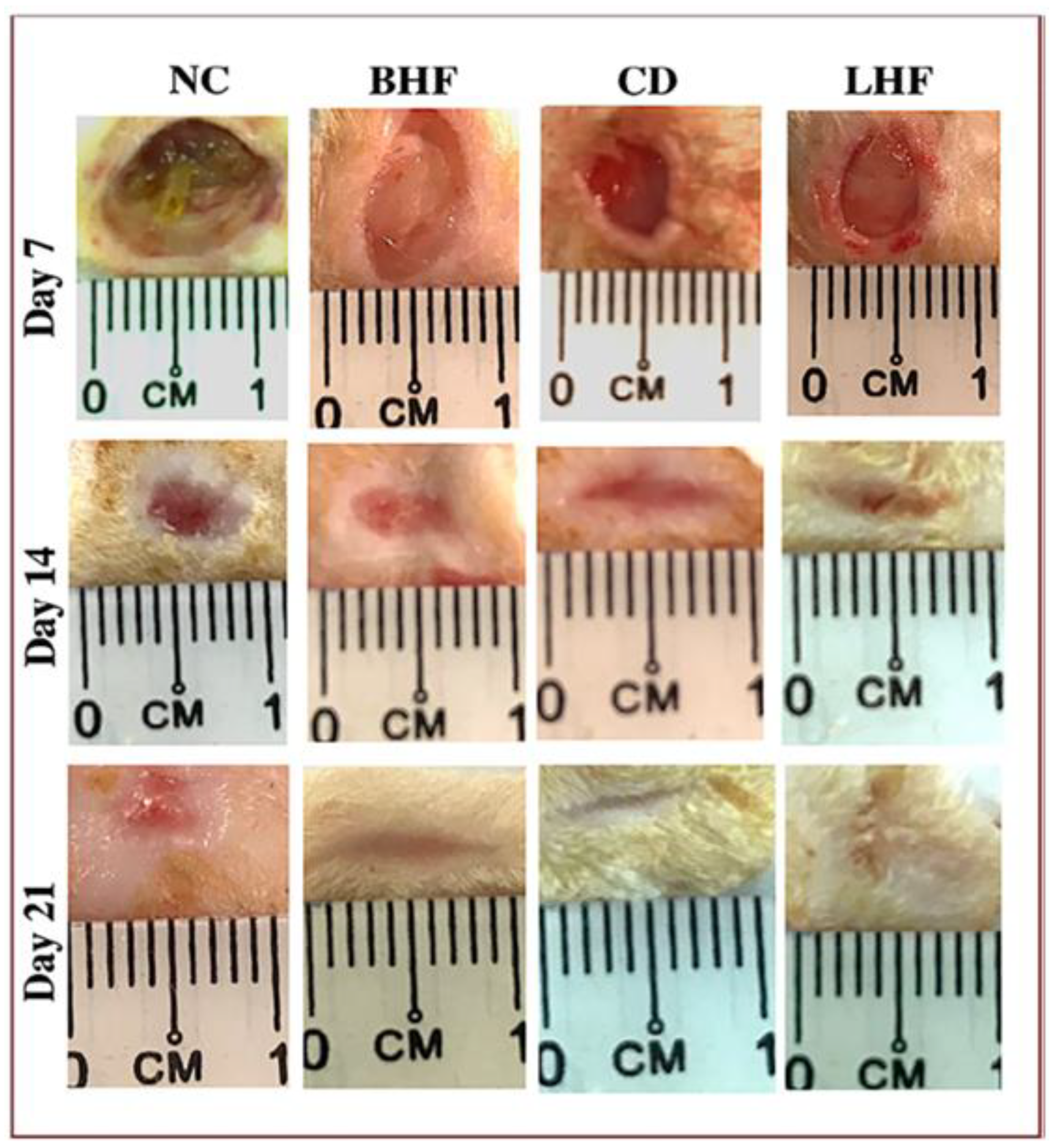
7.1. Clinical Application of Collagen Wound Dressings in Diabetic Foot Ulcers
7.2. Clinical Application of Collagen Wound Dressings in Venous Leg Ulcers
7.3. Clinical Application of Collagen Wound Dressings in Pressure Ulcers
7.4. Clinical Application of Collagen Wound Dressings in Burn Injuries
7.5. Commercially Available Dressings for Collagen Skin Repair
8. Challenges and Limitations
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGrath, J.A.; Uitto, J. Structure and Function of the Skin. In Rook’s Textbook of Dermatology; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 1–50. [Google Scholar]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, 2100475. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Gould, L.J. Opportunities and Challenges of the Management of Chronic Wounds: A Multidisciplinary Viewpoint. Chronic Wound Care Manag. Res. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Tapiwa Chamanga, E. Clinical management of non-healing wounds. Nurs. Stand. (R. Coll. Nurs.) 2018, 32, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Setacci, C.; Benevento, D.; De Donato, G.; Viviani, E.; Bracale, U.M.; Del Guercio, L.; Palasciano, G.; Setacci, F. Focusing on Diabetic Ulcers. Transl. Med. Unisa 2020, 21, 7–9. [Google Scholar]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mazuz, R. Wound Care in the Developing World—Gaps, Opportunities, and Realities. Curr. Dermatol. Rep. 2019, 8, 199–207. [Google Scholar] [CrossRef]
- Fauzian, F.; Garmana, A.N.; Mauludin, R. Applications of nanotechnology-based drug delivery system for delivering natural products into acute and chronic wounds: A review. Biointerface Res. Appl. Chem. 2023, 13, 426. [Google Scholar]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Comotto, M.; Saghazadeh, S.; Bagherifard, S.; Aliakbarian, B.; Kazemzadeh-Narbat, M.; Sharifi, F.; Mousavi Shaegh, S.A.; Arab-Tehrany, E.; Annabi, N.; Perego, P.; et al. Breathable hydrogel dressings containing natural antioxidants for management of skin disorders. J. Biomater. Appl. 2019, 33, 1265–1276. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Al-Mamari, A.; Shahitha, F.; Al-Sibani, M.; Al Saadi, A.; Al Harrasi, A.; Ahmad, A. Novel antibacterial wound healing hydrogels based on HEC/SA/HA using gree n chemistry approach. Lett. Appl. NanoBioSci. 2023, 12, 69. [Google Scholar]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, J.; Lin, H.; Ren, X.; Tian, H.; Liang, Y.; Wang, W.; Wang, Y.; Yin, M.; Huang, Y.; et al. In situ Fabrication of Nano ZnO/BCM Biocomposite Based on MA Modified Bacterial Cellulose Membrane for Antibacterial and Wound Healing. Int. J. Nanomed. 2020, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, R.; Yang, T.; Xing, M.; Luo, G. Preparation of chitin-amphipathic anion/quaternary ammonium salt ecofriendly dressing and its effect on wound healing in mice. Int. J. Nanomed. 2018, 13, 4157–4169. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lee, K.; Shim Jung, O. Gut Bacterial Dysbiosis in Irritable Bowel Syndrome: A Case-Control Study and a Cross-Cohort Analysis Using Publicly Available Data Sets. Microbiol. Spectr. 2023, 11, e02125-22. [Google Scholar] [CrossRef]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen—Structure, properties and application. Eng. Biomater. 2020, 156, 17–23. [Google Scholar] [CrossRef]
- Shenoy, M.; Abdul, N.S.; Qamar, Z.; Bahri, B.M.A.; Al Ghalayini, K.Z.K.; Kakti, A. Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus 2022, 14, e24856. [Google Scholar] [CrossRef]
- D’souza, Z.; Chettiankandy, T.J.; Ahire, M.S.; Thakur, A.; Sonawane, S.G.; Sinha, A. Collagen—Structure, function and distribution in orodental tissues. J. Glob. Oral Health 2020, 2, 134–139. [Google Scholar] [CrossRef]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009, 27, 423–433. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the Applications of Biomedical Compositions Containing Hydroxyapatite and Collagen Modified by Bioactive Components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef]
- Gomes, V.; Salgueiro, S.P. From small to large-scale: A review of recombinant spider silk and collagen bioproduction. Discov. Mater. 2022, 2, 3. [Google Scholar] [CrossRef]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Abu-Rub, M.; Pandit, A.; Zeugolis, D.; Brooks, R.A.; Rushton, N.; Best, S.M.; Cameron, R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011, 7, 3237–3247. [Google Scholar] [CrossRef]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. International Journal of Molecular Sciences 2023, 24, 9841. [Google Scholar] [CrossRef]
- Samad, N.; Sikarwar, A. Collagen: New Dimension in Cosmetic and Healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Saito, T. Biocompatibility of Novel Type I Collagen Purified from Tilapia Fish Scale: An In Vitro Comparative Study. BioMed Res. Int. 2015, 2015, 139476. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- Rahmanian-Schwarz, A.; Held, M.; Knoeller, T.; Stachon, S.; Schmidt, T.; Schaller, H.-E.; Just, L. In vivo biocompatibility and biodegradation of a novel thin and mechanically stable collagen scaffold. J. Biomed. Mater. Res. Part A 2014, 102, 1173–1179. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.D.; John, A.; James, S. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2017, 97, 55–66. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Li, B.; Lareu, R.R.; Chan, C.K.; Raghunath, M. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. J. Biomater. Sci. Polym. Ed. 2008, 19, 1307–1317. [Google Scholar] [CrossRef]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, Y.; Zhu, Z.; Gao, R. Recombinant human collagen digestates exhibit higher protective effect on UVA-damaged skin fibroblasts than animal-derived collagens. J. Funct. Foods 2024, 113, 106035. [Google Scholar] [CrossRef]
- Werkmeister, J.A.; Ramshaw, J.A. Recombinant protein scaffolds for tissue engineering. Biomed. Mater. 2012, 7, 012002. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; Zhao, P.; Xing, L.; Li, J.; Wang, Z.; Ju, S.; Shi, X.; Liu, Y.; Deng, G.; et al. A regulatory perspective on recombinant collagen-based medical devices. Bioact. Mater. 2022, 12, 198–202. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern Collagen Wound Dressings: Function and Purpose. J. Am. Coll. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C.; Elsner, P.; Tittelbach, J. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100253. [Google Scholar] [CrossRef]
- Sachlos, E.; Wahl, D.A.; Triffitt, J.T.; Czernuszka, J.T. The impact of critical point drying with liquid carbon dioxide on collagen–hydroxyapatite composite scaffolds. Acta Biomater. 2008, 4, 1322–1331. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Composition of elastin like polypeptide–collagen composite scaffold influences in vitro osteogenic activity of human adipose derived stem cells. Dent. Mater. 2016, 32, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.S.; Sbravati, N.D.; Janorkar, A.V. Mechanical & Cell Culture Properties of Elastin-Like Polypeptide, Collagen, Bioglass, and Carbon Nanosphere Composites. Ann. Biomed. Eng. 2013, 41, 2042–2055. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Hapach, L.A.; VanderBurgh, J.A.; Miller, J.P.; Reinhart-King, C.A. Manipulation of in vitro collagen matrix architecture for scaffolds of improved physiological relevance. Phys. Biol. 2015, 12, 061002. [Google Scholar] [CrossRef]
- Divakar, P.; Yin, K.; Wegst, U.G.K. Anisotropic freeze-cast collagen scaffolds for tissue regeneration: How processing conditions affect structure and properties in the dry and fully hydrated states. J. Mech. Behav. Biomed. Mater. 2019, 90, 350–364. [Google Scholar] [CrossRef]
- Sprangers, S.; Everts, V. Molecular pathways of cell-mediated degradation of fibrillar collagen. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 75–76, 190–200. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Diegelmann, R.F. What Makes Wounds Chronic. Surg. Clin. N. Am. 2020, 100, 681–693. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Amălinei, C.; Căruntu, I.D.; Giuşcă, S.E.; Bălan, R.A. Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2010, 51, 215–228. [Google Scholar]
- Ricard-Blum, S. Matricryptins derived from collagens and proteoglycans. Front. Biosci. 2011, 16, 674. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef]
- El Masry, M.S.; Chaffee, S.; Das Ghatak, P.; Mathew-Steiner, S.S.; Das, A.; Higuita-Castro, N.; Roy, S.; Anani, R.A.; Sen, C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019, 33, 2144–2155. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 248–257. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Tronci, G. The Application of Collagen in Advanced Wound Dressings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 363–389. [Google Scholar]
- Rangaraj, A.; Harding, K.; Leaper, D. Role of collagen in wound management. Wounds 2011, 7, 54–63. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Song, C.; Yao, L.; Xiao, J. Recent progresses of collagen dressings for chronic skin wound healing. Collagen Leather 2023, 5, 31. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Int. J. Biol. Macromol. 2024, 16, 2668. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Sklenářová, R.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, Z.; Zhang, J.; Shi, G.; Cai, X.; Dou, R.; Tang, J.; Zhang, C.; Zhao, Y.; Chen, J. The potential of collagen-based materials for wound management. Mater. Today Chem. 2024, 41, 102295. [Google Scholar] [CrossRef]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula flower extract loaded collagen film exhibits superior wound healing potential: Preparation, evaluation, in-vitro & in-vivo wound healing study. J. Drug Deliv. Sci. Technol. 2022, 72, 103363. [Google Scholar] [CrossRef]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Aravinthan, A.; Park, J.K.; Hossain, M.A.; Sharmila, J.; Kim, H.J.; Kang, C.W.; Kim, N.S.; Kim, J.H. Collagen-based sponge hastens wound healing via decrease of inflammatory cytokines. 3 Biotech 2018, 8, 487. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Wang, Y.; Xiao, T.; Guo, T.; Ren, Z.; Wu, C.; Wang, Y. Collagen/Curdlan composite sponge for rapid hemostasis and skin wound healing. Int. J. Biol. Macromol. 2024, 273, 133032. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhou, J.; Yu, X.; Wan, J.; Wang, J.; Lei, S.; Zhang, Z.; Zhang, L.; Wang, S. Porous Collagen Sponge Loaded with Large Efficacy-Potentiated Exosome-Mimicking Nanovesicles for Diabetic Skin Wound Healing. ACS Biomater. Sci. Eng. 2024, 10, 975–986. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zeng, X.; Li, G. A bioactive composite sponge based on biomimetic collagen fibril and oxidized alginate for noncompressible hemorrhage and wound healing. Carbohydr. Polym. 2024, 343, 122409. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of Antibacterial Collagen-Based Composite Wound Dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Póvoa, V.C.O.; dos Santos, G.J.V.P.; Picheth, G.F.; Jara, C.P.; da Silva, L.C.E.; de Araújo, E.P.; de Oliveira, M.G. Wound healing action of nitric oxide-releasing self-expandable collagen sponge. ACS Sustain. Chem. Eng. 2020, 14, 807–818. [Google Scholar] [CrossRef]
- Rahmani, S.; Mooney, D. Tissue-Engineered Wound Dressings for Diabetic Foot Ulcers: Medical and Surgical Management; Humana: Cham, Switzerland, 2018; pp. 247–256. [Google Scholar]
- Weller, C.; Team, V. Tissue-Engineered Wound Dressings for Diabetic Foot Ulcers: Medical and Surgical Management. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–134. [Google Scholar]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Li, J.; Zhai, Y.-N.; Xu, J.-P.; Zhu, X.-Y.; Yang, H.-R.; Che, H.-J.; Liu, C.-K.; Qu, J.-B. An injectable collagen peptide-based hydrogel with desirable antibacterial, self-healing and wound-healing properties based on multiple-dynamic crosslinking. Int. J. Biol. Macromol. 2024, 259, 129006. [Google Scholar] [CrossRef]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Guo, Y.; Huang, H.; Zhou, J.; Wang, L.; Bai, J.; Fan, Z. A facile and large-scale synthesis of a PVA/chitosan/collagen hydrogel for wound healing. New J. Chem. 2020, 44, 20776–20784. [Google Scholar] [CrossRef]
- Aguayo-Morales, H.; Cobos-Puc, L.E.; Lopez-Badillo, C.M.; Oyervides-Muñoz, E.; Ramírez-García, G.; Claudio-Rizo, J.A. Collagen-polyurethane-dextran hydrogels enhance wound healing by inhibiting inflammation and promoting collagen fibrillogenesis. J. Biomed. Mater. Res. Part A 2024, 112, 1760–1777. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ng, S.F.; Alavi, T.; Ahmad, W. In-vivo evaluation of Alginate-Pectin hydrogel film loaded with Simvastatin for diabetic wound healing in Streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2021, 171, 308–319. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Doodmani, S.M.; Bagheri, A.; Natouri, O.; Nobakht, A.; Saghebasl, S. Electrospinning-netting of spider-inspired polycaprolactone/collagen nanofiber-nets incorporated with Propolis extract for enhanced wound healing applications. Int. J. Biol. Macromol. 2024, 267, 131452. [Google Scholar] [CrossRef]
- Zhang, S.-y.; Li, X.-y.; Yang, N.; Ling, L.; Zhang, M.; Ye, Q.; Wang, Y. Electrospun Collagen Nanofibers Reduce Inflammation, Inhibit Fibrosis, and Promote Wound Healing on the Ocular Surface. ACS Appl. Nano Mater. 2024, 7, 20267–20278. [Google Scholar] [CrossRef]
- Abdelazim, E.B.; Abed, T.; Goher, S.S.; Alya, S.H.; El-Nashar, H.A.S.; El-Moslamy, S.H.; El-Fakharany, E.M.; Abdul-Baki, E.A.; Shakweer, M.M.; Eissa, N.G.; et al. In vitro and in vivo studies of Syzygium cumini-loaded electrospun PLGA/PMMA/collagen nanofibers for accelerating topical wound healing. RSC Adv. 2024, 14, 101–117. [Google Scholar] [CrossRef]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the Wound-Healing Process by Curcumin-Loaded Chitosan/Collagen Blend Electrospun Nanofibers: In Vitro and In Vivo Studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Roney, M.; Aluwi, M.F.F.M.; Zamri, N.B. An In-Silico Approach to Evaluate Bioactive Molecules of Aloe Vera Leaf Extracts in Inhibiting the Glycogen Synthase Kinase-3β (GSK3-β) Protein for Faster Diabetic Wound Healing Potential. Biointerface Res. Appl. Chem. 2024, 14, 115. [Google Scholar]
- Kusnadi, K.; Herdiana, Y.; Rochima, E.; Putra, O.N.; Mohd Gazzali, A.; Muchtaridi, M. Collagen-Based Nanoparticles as Drug Delivery System in Wound Healing Applications. Int. J. Nanomed. 2024, 19, 11321–11341. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef]
- Vaniushenkova, A.A.; Poberezhniy, D.Y.; Panyukova, N.S.; Morozov, A.N.; Kalenov, S.V.; Belov, A.A. The Co-Use of Various Forms of Silver and Proteases in the Development of New Dressing Biomaterials for Wound Healing. Biointerface Res. Appl. Chem. 2024, 14, 119. [Google Scholar]
- Shtapenko, O.; Syrvatka, V.; Horbay, R.; Dzen, Y.; Slyvchuk, O.; Gromyko, O.; Gevkan, I. Evaluation of Antimicrobial Activity and in Vivo Wound Healing Properties of Copper Nanoparticles and Copper Nanoparticles Nanoemulsion. Biointerface Res. Appl. Chem. 2024, 14, 15. [Google Scholar]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef]
- S, S.; A, S.K.; Nair, P.D.; Thomas, L.V. A nonadherent chitosan-polyvinyl alcohol absorbent wound dressing prepared via controlled freeze-dry technology. Int. J. Biol. Macromol. 2020, 150, 129–140. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Influence of the processing variables on the microstructure and properties of gelatin-based scaffolds by freeze-drying. J. Appl. Polym. Sci. 2019, 136, 47671. [Google Scholar] [CrossRef]
- Katrilaka, C.; Karipidou, N.; Petrou, N.; Manglaris, C.; Katrilakas, G.; Tzavellas, A.N.; Pitou, M.; Tsiridis, E.E.; Choli-Papadopoulou, T.; Aggeli, A. Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering. Materials 2023, 16, 4425. [Google Scholar] [CrossRef]
- Khairnar, S.; Kini, R.; Harwalkar, M.; Salunkhe, K.; Chaudhari, S. A Review on Freeze Drying Process of Pharmaceuticals. Int. J. Res. Pharm. Sci. 2012, 4, 76–94. [Google Scholar]
- Adams, G.; Cook, I.; Ward, K. The Principles of Freeze-Drying. Methods Mol. Biol. 2015, 1257, 121–143. [Google Scholar] [CrossRef]
- Xie, Y.; Kawazoe, N.; Yang, Y.; Chen, G. Preparation of mesh-like collagen scaffolds for tissue engineering. Mater. Adv. 2022, 3, 1556–1564. [Google Scholar] [CrossRef]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin cross-linked porous chitosan–collagen–gelatin scaffolds using chitosan–CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Singh, B.; Verma, S. Polymers in designing the mucoadhesive films: A comprehensive review. Int. J. Green Pharm. 2018, 12, S330–S344. [Google Scholar]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; González-Torres, M.; Alcalá-Alcalá, S.; Bernal-Chávez, S.A.; Morales-Morfin, J.C.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Del Prado-Audelo, M.A.L.; et al. Development of films from natural sources for infections during wound healing. Cell. Mol. Biol. 2021, 67, 96–100. [Google Scholar] [CrossRef]
- Tenorová, K.; Masteiková, R.; Pavloková, S.; Kostelanská, K.; Bernatonienė, J.; Vetchý, D. Formulation and Evaluation of Novel Film Wound Dressing Based on Collagen/Microfibrillated Carboxymethylcellulose Blend. Pharmaceutics 2022, 14, 782. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- Debnath, S.; Agrawal, A.; Jain, N.; Chatterjee, K.; Player, D.J. Collagen as a bio-ink for 3D printing: A critical review. J. Mater. Chem. B 2025, 13, 1890–1919. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-x.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Guerrero, A.; Romero, A. Gelatin vs collagen-based sponges: Evaluation of concentration, additives and biocomposites. J. Polym. Res. 2019, 26, 190. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89A, 363–369. [Google Scholar] [CrossRef]
- Deng, Y.; Kuiper, J. Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Elsevier/Woodhead Publishing: Duxford, UK, 2018. [Google Scholar]
- Wang, W.; Liu, Y.; Liu, A.; Xiao, J.; Wang, K.; Zhao, Y.; Zhang, S.; Zhang, L. Fabrication of acid-swollen collagen fiber-based composite films: Effect of nano-hydroxyapatite on packaging related properties. Int. J. Food Prop. 2017, 20, 968–978. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Pippenger, B.E.; Zhang, Y.; Miron, R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017, 3, 32–40. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, M.K.; Singh, A. Bacterial cellulose: A smart biomaterial for biomedical applications. J. Mater. Res. 2024, 39, 2–18. [Google Scholar] [CrossRef]
- La Monica, F.; Campora, S.; Ghersi, G. Collagen-Based Scaffolds for Chronic Skin Wound Treatment. Gels 2024, 10, 137. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Munish, T.; Ramneesh, G.; Sanjeev, S.; Jasdeep, S.; Jaspal, S.; Nikhil, G. Comparative study of collagen based dressing and standard dressing in diabetic foot ulcer. J. Evol. Med. Dent. Sci. 2015, 4, 3614–3621. [Google Scholar] [CrossRef]
- Djavid, G.E.; Tabaie, S.M.; Tajali, S.B.; Totounchi, M.; Farhoud, A.; Fateh, M.; Ghafghazi, M.; Koosha, M.; Taghizadeh, S. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomised control trial. J. Wound Care 2020, 29, S13–S18. [Google Scholar] [CrossRef]
- Fleischli, J.G.; Laughlin, T.J.; Fleischli, J.W. Equine pericardium collagen wound dressing in the treatment of the neuropathic diabetic foot wound: A pilot study. J. Am. Podiatr. Med. Assoc. 2009, 99, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Romanelli, M.; Mulder, G.; Paggi, B.; Macchia, M.; Panduri, S.; Dini, V. The use of a collagen matrix in hard-to-heal venous leg ulcers. J. Wound Care 2015, 24, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Yoshimura, K.; Niimi, M.; Ito, T.; Aya, R.; Fujitaka, J.; Tada, H.; Teramukai, S.; Murayama, T.; Toyooka, C.; et al. Novel Collagen/Gelatin Scaffold with Sustained Release of Basic Fibroblast Growth Factor: Clinical Trial for Chronic Skin Ulcers. Tissue Eng. Part A 2013, 19, 1931–1940. [Google Scholar] [CrossRef]
- Mościcka, P.; Cwajda-Białasik, J.; Szewczyk, M.T.; Jawień, A. Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 7108. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef]
- Kloeters, O.; Unglaub, F.; de Laat, E.; van Abeelen, M.; Ulrich, D. Prospective and randomised evaluation of the protease-modulating effect of oxidised regenerated cellulose/collagen matrix treatment in pressure sore ulcers. Int. Wound J. 2016, 13, 1231–1236. [Google Scholar] [CrossRef]
- Piatkowski, A.; Ulrich, D.; Seidel, D.; Abel, M.; Pallua, N.; Andriessen, A. Randomised, controlled pilot to compare collagen and foam in stagnating pressure ulcers. J. Wound Care 2012, 21, 505–511. [Google Scholar] [CrossRef]
- Żwierełło, W.; Piorun, K.; Skórka-Majewicz, M.; Maruszewska, A.; Antoniewski, J.; Gutowska, I. Burns: Classification, Pathophysiology, and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 3749. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.A.; Shah, S.; Ranjan, V.; Sarwade, P.; Philipose, A. Comparative study of silver-sulfadiazine-impregnated collagen dressing versus conventional burn dressings in second-degree burns. J. Fam. Med. Prim. Care 2019, 8, 215–219. [Google Scholar] [CrossRef]
- Ben, C.; Liu, X.; Shen, T.; Song, Y.; Li, H.; Pan, B.; Hou, W.; Liu, T.; Luo, P.; Ma, B.; et al. A recombinant human collagen hydrogel for the treatment of partial-thickness burns: A prospective, self-controlled clinical study. Burns 2021, 47, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Newman, M.I.; Baratta, L.G.; Swartz, K. Activated, type I collagen (CellerateRx) and its effectiveness in healing recalcitrant diabetic wounds: A case presentation. Adv. Ski. Wound Care 2008, 21, 370–374. [Google Scholar] [CrossRef]
- Elgharably, H.; Roy, S.; Khanna, S.; Abas, M.; Dasghatak, P.; Das, A.; Mohammed, K.; Sen, C.K. A modified collagen gel enhances healing outcome in a preclinical swine model of excisional wounds. Wound Repair Regen. 2013, 21, 473–481. [Google Scholar] [CrossRef]
- Santhanam, R.; Rameli, M.A.P.; Jeffri, A.A.; Ismail, W.I.W. Bovine Based Collagen Dressings in Wound Care Management. J. Pharm. Res. Int. 2020, 32, 48–63. [Google Scholar] [CrossRef]
- Gruessner, U.; Clemens, M.; Pahlplatz, P.V.; Sperling, P.; Witte, J.; Rosen, H.R. Improvement of perineal wound healing by local administration of gentamicin-impregnated collagen fleeces after abdominoperineal excision of rectal cancer. Am. J. Surg. 2001, 182, 502–509. [Google Scholar] [CrossRef]
- Colenci, R.; Abbade, L.P.F. Fundamental aspects of the local approach to cutaneous ulcers. An. Bras. Dermatol. 2018, 93, 859–870. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Tilbury, M.A.; Wall, J.G.; Zeugolis, D.I. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today. Bio 2020, 7, 100057. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Cziperle, D.J. Avitene™ Microfibrillar Collagen Hemostat for Adjunctive Hemostasis in Surgical Procedures: A Systematic Literature Review. Med. Devices Evid. Res. 2021, 14, 155–163. [Google Scholar] [CrossRef]
- Griswold, J.A.; Cepica, T.; Rossi, L.; Wimmer, J.S.; Merrifield, H.H.; Hester, C.; Sauter, T.; Baker, C.R. A comparison of Xeroform and SkinTemp dressings in the healing of skin graft donor sites. J. Burn Care Rehabil. 1995, 16, 136–140. [Google Scholar] [CrossRef]
- Skelton, H.G.; Smith, K.J.; Turiansky, G.; Couzzo, D.; Lindstrom, J.; Welch, M.L.; Yeager, J.; Wagner, K.F. Helistat absorbable collagen hemostatic sponges in cutaneous surgery in HIV-1+ patients. Military Medical Consortium for the Advancement of Military Medicine (MMCAR). Int. J. Dermatol. 1993, 32, 835–837. [Google Scholar] [CrossRef]
- King, S. Catrix: An easy-to-use collagen treatment for wound healing. Br. J. Community Nurs. 2005, 10, S31–S34. [Google Scholar] [CrossRef]
- Vlassova, N.; Lazarus, G. Use of Apligraf for a devascularized ulcer secondary to mastectomy and radiation. J. Am. Acad. Dermatol. 2011, 65, e132–e134. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Abedi, M.; Shafiee, M.; Afshari, F.; Mohammadi, H.; Ghasemi, Y. Collagen-Based Medical Devices for Regenerative Medicine and Tissue Engineering. Appl. Biochem. Biotechnol. 2024, 196, 5563–5603. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Chen, Y.; Yue, O.; Bai, Z.; Cui, B.; Jiang, H.; Liu, X. A Review of Recent Progress on Collagen-Based Biomaterials. Adv. Healthc. Mater. 2023, 12, 2202042. [Google Scholar] [CrossRef]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Win Naing, M. Production of recombinant collagen: State of the art and challenges. Eng. Biol. 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Salim, N.V.; Madhan, B.; Glattauer, V.; Ramshaw, J.A.M. Comprehensive review on collagen extraction from food by-products and waste as a value-added material. Int. J. Biol. Macromol. 2024, 278, 134374. [Google Scholar] [CrossRef]
- Ogundeji, K.D.; Risenga, P.R.; Thupayagale-Tshweneagae, G. Cost of wound dressing: Implication for enrollment into the National Health Insurance scheme, Nigeria. Curationis 2023, 46, e1–e6. [Google Scholar] [CrossRef]




| Type | Molecular Composition | Tissue Distribution | Supramolecular Structure and Organization |
|---|---|---|---|
| I | [α1(I)]2α2(I) | bone, dermis, tendons, ligaments, and cornea (represent 90% of the total collagen of the human body) | Fibril-forming collagens |
| II | [α1(II)]3 | cartilage, intervertebral disc, notochord, vitreous humor in the eye | |
| III | [α1(III)]3 | skin, vessel wall, and reticular fibers of most tissues (lungs, liver, spleen, etc.) | |
| V | α1(V),α2(V),α3(V) | lung, cornea, bone, and fetal membranes, together with type I collagen | |
| XI | α1(XI)α2(XI)α3(XI) | cartilage, vitreous body | |
| IV | [α1(IV)]2α2(IV); α1–α6 | basement membranes | Basement-membrane collagens |
| VI | α1(VI),α2(VI),α3(VI) | widespread: dermis, cartilage, placenta, lungs, vessel wall, and intervertebral disc | Microfibrillar collagen |
| VII | [α1(VII)]3 | skin, dermal–epidermal junctions, oral mucosa, and cervix | Anchoring fibrils |
| VIII | [α1(VIII)]2α2(VIII) | endothelial cells, Descemet’s membrane | Hexagonal network-forming collagens |
| X | [α3(X)]3 | hypertrophic cartilage | |
| IX | α1(IX)α2(IX)α3(IX) | cartilage, vitreous humor, and cornea | FACIT collagens |
| XII | [α1(XII)]3 | perichondrium, ligaments, and tendon | |
| XIV | [α1(XIV)]3 | dermis, tendon, vessel wall, placenta, lungs, and liver | |
| XIX | [α1(XIX)]3 | human rhabdomyosarcoma | |
| XX | [α1(XX)]3 | corneal epithelium, embryonic skin, sternal cartilage, and tendon | |
| XXI | [α1(XXI)]3 | blood vessel wall | |
| XIII | [α1(XIII)]3 | epidermis, hair follicle, endomysium, intestine, chondrocytes, lungs, and liver | Transmembrane collagens (membrane-associated collagens with interrupted triple helices—MACITs) |
| XVII | [α1(XVII)]3 | dermal–epidermal junctions | Multiplexins |
| XV | [α1(XV)]3 | fibroblasts, smooth muscle cells, and kidney, pancreas | |
| XVI | [α1(XVI)]3 | fibroblasts, amnion, and keratinocytes | |
| XVIII | [α1(XVIII)]3 | lungs, liver |
| Form of Dressing | Composition | Product Name | Advantage | Limitations | Wound Suitable for | Refs. |
|---|---|---|---|---|---|---|
| Gel | Col | CellerateRX | Keep the wound bed wet | Bovine sources and secondary dressings are needed | Partial and full-thickness injuries, including traumatic wounds, surgical wounds, DFUs, and burns | [183] |
| Gel | Col Polypeptides | Stimulen | Keep the wound bed wet | Bovine sources and secondary dressings are needed | Full- and partial-thickness wounds, including PUs, partial-thickness burns, abrasions | [184] |
| Gel | Col | Collatek | Keep the wound bed wet | Bovine sources and secondary dressings are needed | Cuts, abrasions, minor wounds, critical sunburns, partial- and full-thickness wounds, venous stasis ulcers, burns of the first or second degree, ulcers that have many etiologies, surgical wounds | [185] |
| Gel | Type I Col | Collogel | Keep the wound bed wet | Type I bovine collagen, and secondary dressings are needed | Pressure sores, dermal lesions, first-degree burns, donor sites, stretch marks and scar management | [185] |
| Pad | Col fleece, gentamicin salts | Septocoll E | Activate platelets | Skin response | Full and partial-thickness injuries, including infected wounds and bleeding lesions | [186] |
| Pad | Col and Ca alginate | FibracolPlus | Keep the wound bed wet | Secondary dressings are needed | Full and partial thickness injuries, such as burns, DFUs, VLUs, or PUs, abrasions, and dehisced surgical incisions | [187] |
| Pad | Bovine Col, and Manuka Honey | Puracol | No additional debridement is needed | Bovine source and expensive | Full and partial thickness injuries, such as burns, DFUs, VLUs, or PUs, abrasions, and dehisced surgical incisions | [188] |
| Pad | Type I equine Col | Biopad | Free of collagen degradation products | Equine source, time-consuming, high cost | Full and partial thickness injuries, such as DFUs, VLUs, or PUs, abrasions, and dehisced surgical incisions | [189] |
| Micro-fibrillar | Col | Avitene | Adequate hemostasis, absorbable, and minimizes adhesions | Bovine sources, high cost | Lacerated wound, surgical wounds | [190] |
| Sheet | Col | Skintemp® II | Keep the wound bed wet | Bovine sources, limited shelf life, potential for infection | Blisters, second-degree burns, arterial, venous, diabetic neuropathic, pressure ulcers, superficial secondary trauma, dehisced surgical wounds, and abrasions | [191] |
| Sponge | Type I bovine deep flexor tendonCol | Helistat | Effective hemostasis, absorbable | Bovine sources require a dry field | Surgical wounds | [192] |
| Powder | Bovine cartilage | Catrix® | Biodegradable, decreasebleeding | Bovine sources, secondary dressings are needed | Stasis ulcers, DFUs/PUs, first- and second-degree burns, wounds following surgery, cuts, abrasions, irritations, radiation dermatitis, and partial-thickness wounds | [193] |
| Cellularmatrix | Col,polycarbonatemembrane | Apligraf | Resorbable | Bovine source, expensive, and not suitable for infected wounds | Full and partial thickness damage, such as VLUs and DFUs | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. https://doi.org/10.3390/gels11040271
Alberts A, Bratu AG, Niculescu A-G, Grumezescu AM. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels. 2025; 11(4):271. https://doi.org/10.3390/gels11040271
Chicago/Turabian StyleAlberts, Adina, Andreea Gabriela Bratu, Adelina-Gabriela Niculescu, and Alexandru Mihai Grumezescu. 2025. "Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications" Gels 11, no. 4: 271. https://doi.org/10.3390/gels11040271
APA StyleAlberts, A., Bratu, A. G., Niculescu, A.-G., & Grumezescu, A. M. (2025). Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels, 11(4), 271. https://doi.org/10.3390/gels11040271









