Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications
Abstract
1. Introduction
- Biologically active polysaccharides—known for their diverse health benefits, these molecules exhibit antioxidant, antiobesity, antiparasitic, and metabolic syndrome-modulating properties [11].
- Glycosaminoglycans and mucin-type O-glycans, which are essential for maintaining intestinal health [12].
- Sulfated polysaccharides, found in sponges, sea cucumbers, and starfish, distinguished by their high sulfate content.
- Chitin, extracted primarily from crustaceans like shrimp and crabs, widely utilized in biomaterial research.
- Acid mucopolysaccharides, present in several marine organisms and exhibiting notable bioactive properties [11].
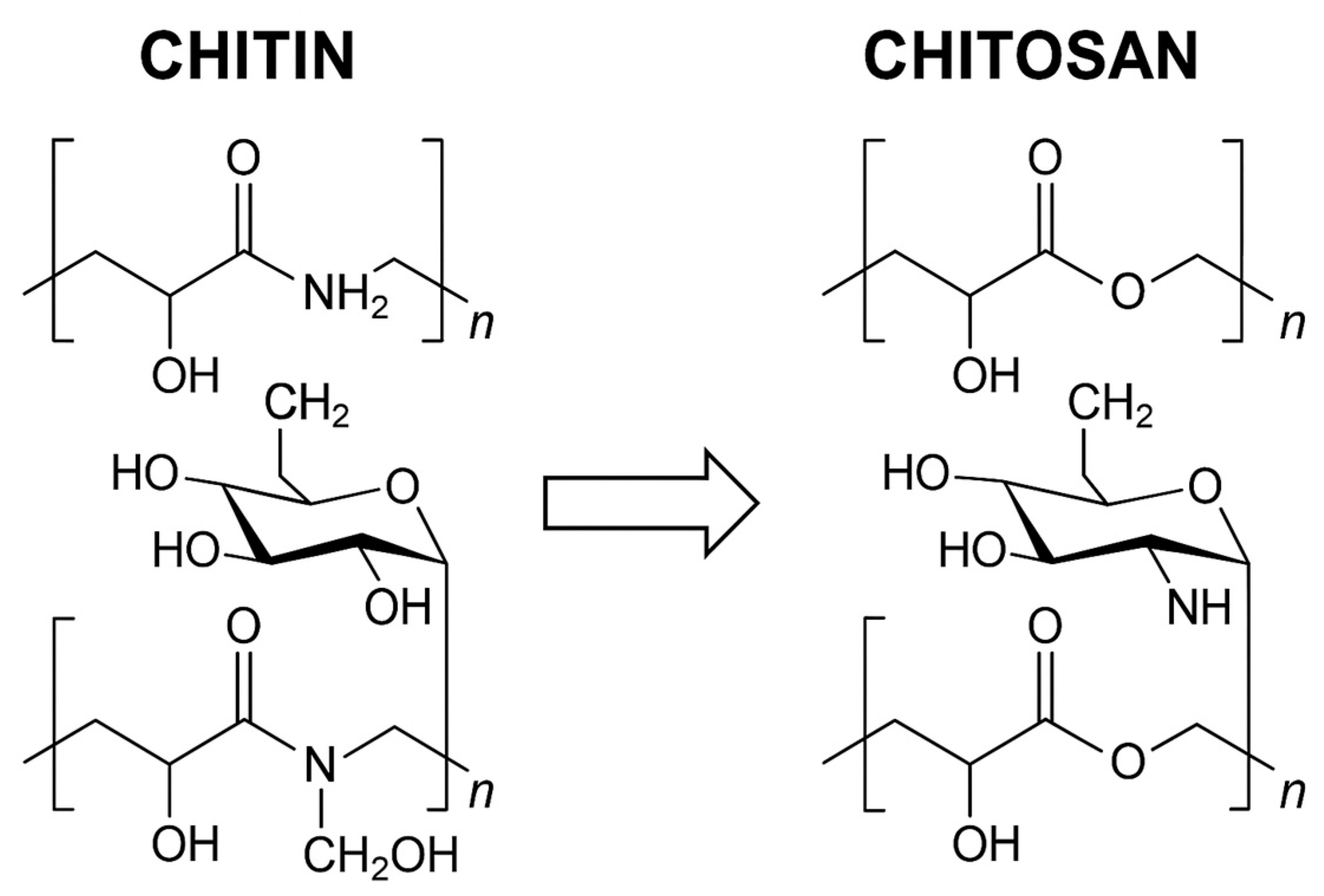
2. Chemical Structure
3. Physicochemical Properties
- α-form CS: most common form, characterized by a highly ordered structure with two antiparallel polysaccharide chains, leading to strong intermolecular hydrogen bonding and high crystallinity [27].
- β-form CS: composed of two parallel polysaccharide chains, exhibiting weaker hydrogen bonding and reduced crystallinity compared to the α form [28].
- γ-form CS: a more complex structure, consisting of three parallel chains, with two aligned in one direction and the third oriented oppositely [29].
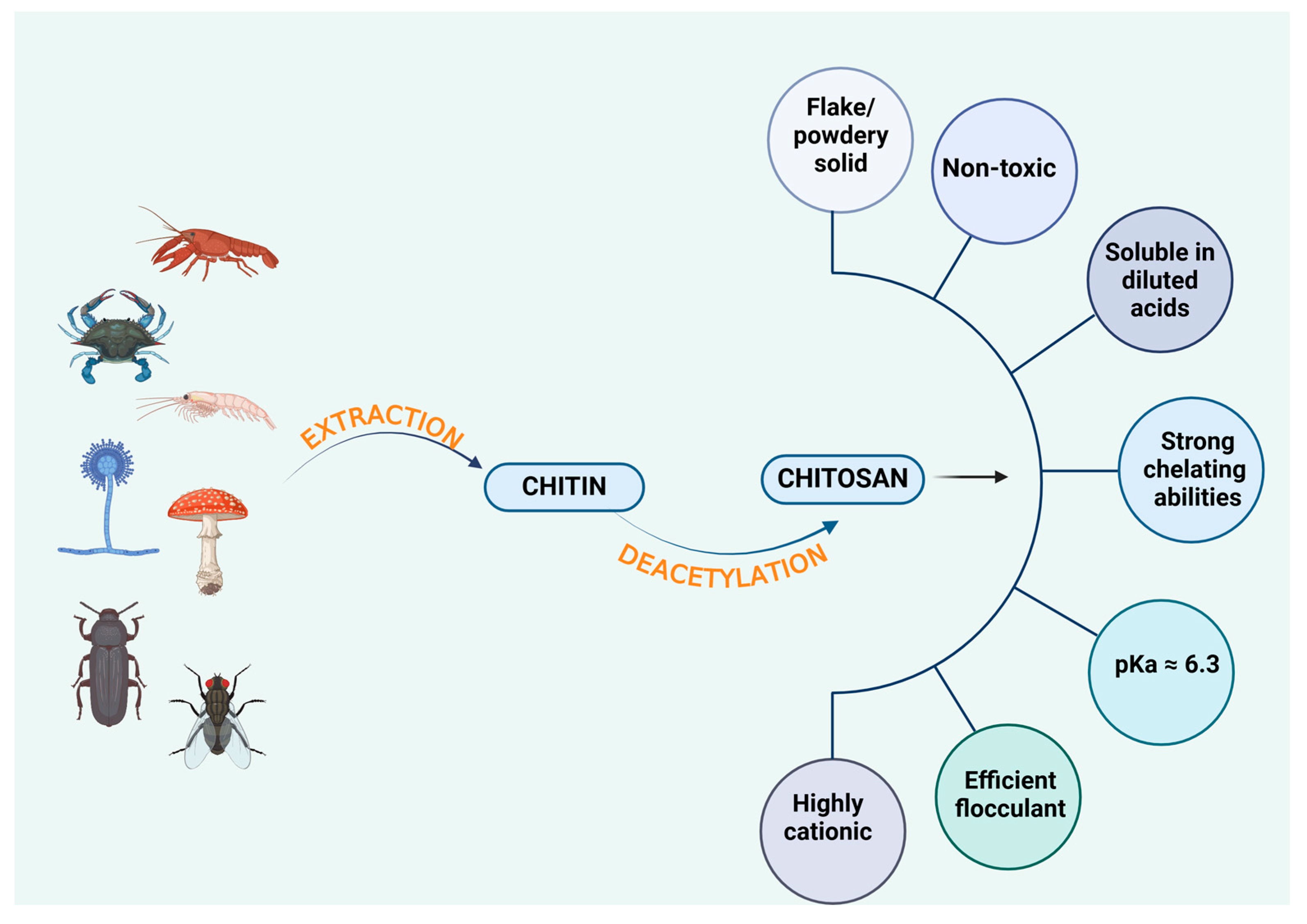
4. Sources of Chitosan
4.1. Crustacean-Derived Chitosan
4.2. Fish-Derived Chitosan
4.3. Fungus-Derived Chitosan
4.4. Insect-Derived Chitosan
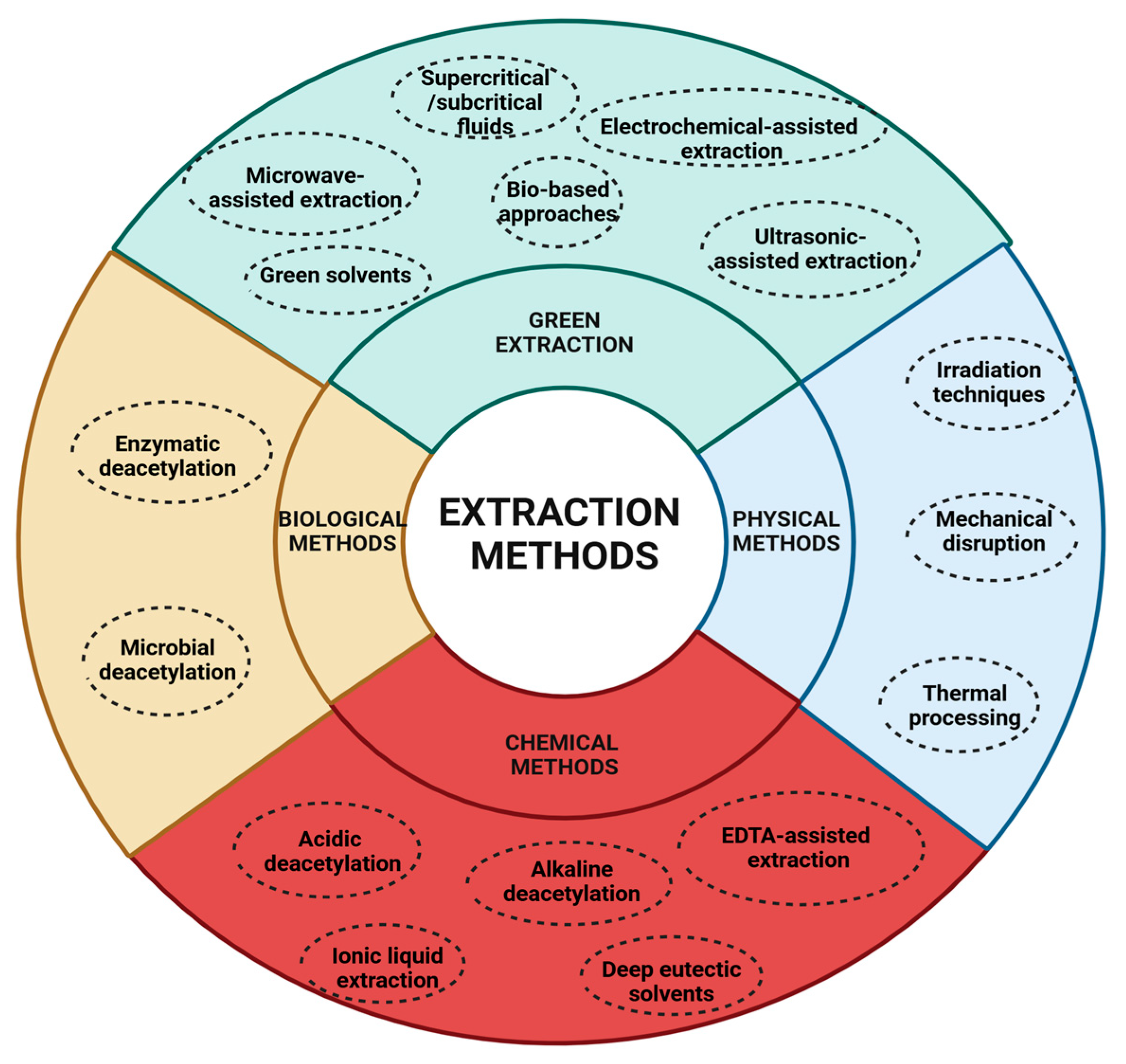
5. Extraction Methods
5.1. Physical Methods
- 1.
- Irradiation Techniques
- 2.
- Mechanical Disruption
- 3.
- Thermal processing
- The application of heat accelerates chitin deacetylation by increasing its reactivity. However, excessive temperatures may lead to CS degradation, potentially compromising its quality [71].
5.2. Chemical Methods
- Acidic deacetylation. Chitin is treated with acids such as HCl or H2SO4 to disrupt its crystalline structure, increasing reactivity. However, this method is less effective in achieving complete deacetylation and may lead to low-molecular-weight CS due to acid hydrolysis of the aminopolysaccharide backbone [73,74].
- Alkaline deacetylation. Chitin is treated with strong bases like NaOH or KOH at high temperatures for extended periods. This process cleaves acetyl–amino linkages, releasing acetate ions and converting chitin into CS [75].
- EDTA-assisted extraction. Ethylenediaminetetraacetic acid (EDTA) helps remove inorganic salts by forming complexes with calcium ions [76]. Zhang et al. [39] demonstrated that EDTA-assisted extraction improved chitin’s crystallinity and degree of acetylation (DA) compared to conventional acid treatments [77]. Despite better working conditions and improved product quality, EDTA extraction still requires alkali for protein removal and shares limitations such as high cost, chemical use, and challenges in waste treatment [78].
- Ionic liquid extraction. Ionic liquids, organic salts in a liquid state below 100 °C, offer a tunable, recyclable, and low-volatility medium for chitin dissolution and deacetylation [79]. Ionic liquids can disrupt hydrogen bonds in chitin, enabling extraction without harsh chemicals [34]. Studies using ammonium-based ionic liquids showed high selectivity and extraction efficiency from shrimp shells. Ionic liquids can assist in demineralization, deproteinization, and even deacetylation steps [80]. However, current research on ionic liquids is limited regarding their deacetylation performance, toxicity, and safety, which restricts their industrial application [78].
- Deep eutectic solvents. Deep eutectic solvents are mixtures of hydrogen bond donors and acceptors that create a eutectic system with a melting point lower than either component [81]. Deep eutectic solvents like choline chloride–lactic acid have been used to extract high-purity chitin (up to 99.3%) in shorter times while also showing low phytotoxicity [82,83]. Deep eutectic solvents are biodegradable, non-toxic, thermally stable, and easy to prepare, making them promising green alternatives to traditional solvents. Compared to ionic liquids, deep eutectic solvents are considered safer and more environmentally friendly. However, large-scale application is still limited due to a lack of industrial studies and long-term safety data [78].
5.3. Biological Methods
- Enzymatic deacetylation. Chitinase and related enzymes, produced by bacteria, fungi, and plants, break glycosidic bonds in chitin, while chitosanase further deacetylates CS, modifying its properties [85]. This method is environmentally friendly and highly selective, but tends to be slower and more expensive due to enzyme costs and the need for controlled conditions [86].
- Microbial deacetylation. Certain bacteria (S. marcescens, P. aeruginosa) and fungi (A. niger, M. rouxii) naturally produce enzymes that deacetylate chitin. This process operates under near-ambient conditions, reducing energy consumption. However, it is slower, requires specific growth conditions, and adds complexity to the extraction process [87].
5.4. Green Extraction Methods
- Supercritical/subcritical fluids. These utilize fluids like supercritical CO2 or water for deacetylation, enhancing purity and process efficiency [89].
- Green solvents. Ionic liquids dissolve and deacetylate chitin under mild conditions, reducing chemical waste. However, challenges in solvent recovery and reuse remain a limitation [90].
- Bio-based approaches. Enzymes or microorganisms deacetylate chitin with minimal waste, aligning with sustainable biorefinery principles [91].
- Microwave-assisted extraction. This accelerates deacetylation while significantly reducing energy consumption [92]. It achieves rapid, uniform heating through dipolar polarization and ionic conduction. Microwave-assisted extraction can reach ~82% deacetylation in just 24 min compared to 6–7 h with conventional methods while maintaining similar product quality. It also improves deproteinization and demineralization and allows control over molecular weight. Optimizing parameters like power, time, solvent concentration, and solid-to-liquid ratio is essential [90].
- Ultrasound-assisted extraction. This utilizes ultrasound-induced cavitation to enhance the solubility of proteins and depolymerize chitin macromolecules, improving extraction efficiency. High-intensity ultrasound (750 W, 20 kHz) reduces extraction time and avoids high temperatures [93]. Ultrasound-assisted extraction is effective as a pretreatment to reduce chitin crystallinity, improving enzymatic hydrolysis. Combined with steam explosion, it significantly lowers the DA without extensive depolymerization [94]. Applying ultrasound in cycles (7.5 min on, 5 min off) further improves chitosan yield, achieving a DA as low as 22.1% [95].
- Electrochemically assisted extraction. This involves electrolysis using inert electrodes and low-molecular-weight salt solutions (NaCl, Na2SO4) to facilitate decellularization, demineralization, and purification steps. By controlling pH through electrode reactions, proteins, pigments, and minerals are removed without harsh chemicals. The method was effectively used to isolate pure 3D sponge chitin, confirmed by N-acetylglucosamine content and chitinase digestion [96].
6. Main Applications of Chitosan
6.1. Biomedical Applications of Chitosan
6.1.1. Hemostatic and Cardiovascular Effects
6.1.2. Antimicrobial Properties
6.1.3. Antioxidant Activity
6.1.4. Antitumor Activity
6.1.5. Anti-Inflammatory Properties
6.1.6. Tissue Engineering
6.1.7. Wound Healing
6.1.8. Drug and Gene Delivery
6.2. Applications in Food and Environmental Science
6.2.1. Food Packaging and Preservation
6.2.2. Food Industry Additives
6.2.3. Wastewater Treatment
6.3. Plant Protection and Growth Enhancement
7. Chitosan as a Gel-Forming Agent
7.1. Physical Gelation
7.1.1. Controlled N-Acylation Gelation
7.1.2. Base-Induced Gelation
7.1.3. Ionic (Ionotropic) Gelation
7.1.4. Thermally Induced Gelation
7.1.5. Electrostatic Interactions
7.1.6. Hydrogen Bonding Interactions
7.1.7. Non-Covalent Chitosan Systems
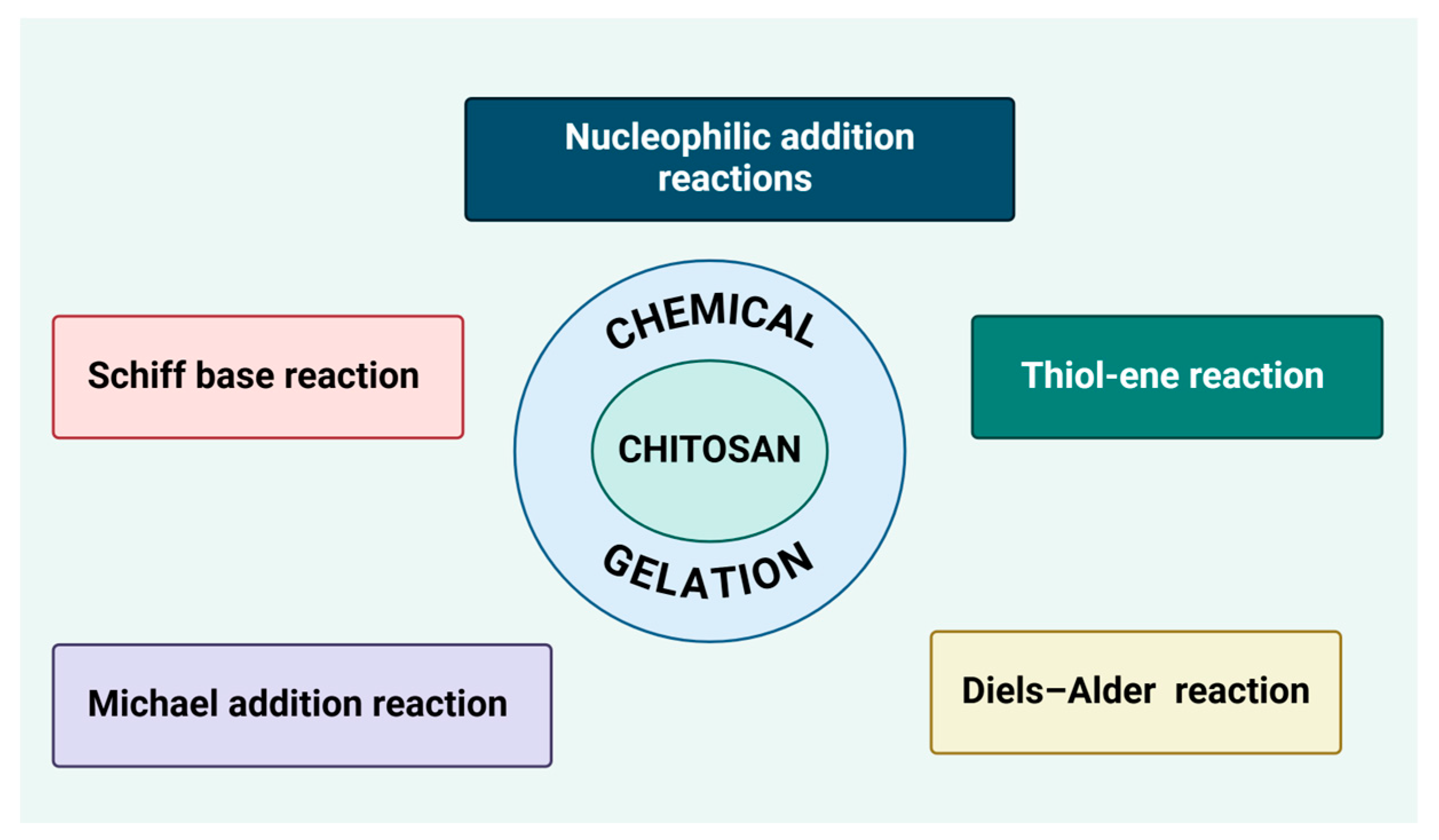
7.2. Chemical Gelation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of Marine Natural Products in Drug Research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The Odyssey of Marine Pharmaceuticals: A Current Pipeline Perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, S.; Parameswaran, P.S. Recent Advances in Marine Drug Research. Biotechnol. Adv. 2013, 31, 1826–1845. [Google Scholar] [CrossRef]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent Advances in Bioactive Polysaccharides from Lycium Barbarum L., Zizyphus Jujuba Mill, Plantago Spp., and Morus Spp.: Structures and Functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance Research in Biomedical Applications on Marine Sulfated Polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of Polysaccharides Extracted from Brown Seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Xu, R.; Wei, S.; Xiong, F.; Cui, W.; Li, B.; Xue, Y.; Xuan, H.; Yuan, H. A Spray-Filming, Tissue-Adhesive, and Bioactive Polysaccharide Self-Healing Hydrogel for Skin Regeneration. Mater. Des. 2022, 217, 110669. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, I.; Dheer, D.; Nagpal, M.; Kumar, P.; Venkatesh, D.N.; Puri, V.; Singh, I. A Propitious Role of Marine Sourced Polysaccharides: Drug Delivery and Biomedical Applications. Carbohydr. Polym. 2023, 308, 120448. [Google Scholar] [CrossRef]
- Guan, X.; Wang, F.; Zhou, B.; Sang, X.; Zhao, Q. The Nutritional Function of Active Polysaccharides from Marine Animals: A Review. Food Biosci. 2024, 58, 103693. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Zhang, Z.; Song, D.; Chen, J.; Ji, C. Progress on the Interaction between Polysaccharides and Gut Microbiota. Food Sci. 2022, 43, 363–373. [Google Scholar] [CrossRef]
- Ray, B.; Schütz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the Amazing Diversity of Natural Source-Derived Polysaccharides: Modern Procedures of Isolation, Engineering, and Optimization of Antiviral Activities. Polymers 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar]
- Khor, E.; Lim, L.Y. Implantable Applications of Chitin and Chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.A.R.; Shaid, A.; Khan, M.A. Cationization of Cotton Fiber by Chitosan and Its Dyeing with Reactive Dye without Salt. Chem. Mater. Eng. 2014, 2, 96–100. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-Based Materials: Preparation, Modification and Application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Jang, M.; Kong, B.; Jeong, Y.; Lee, C.H.; Nah, J. Physicochemical Characterization of A-chitin, Β-chitin, and Γ-chitin Separated from Natural Resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Faria, R.R.; Guerra, R.F.; de Sousa Neto, L.R.; Motta, L.F.; Franca, E. de F Computational Study of Polymorphic Structures of α- and β- Chitin and Chitosan in Aqueous Solution. J. Mol. Graph. Model. 2016, 63, 78–84. [Google Scholar] [CrossRef]
- Subhapradha, N.; Shanmugam, A. Fabrication of β-Chitosan Nanoparticles and Its Anticancer Potential against Human Hepatoma Cells. Int. J. Biol. Macromol. 2017, 94, 194–201. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and Its Derivatives: Preparation and Antibacterial Properties. Materials 2023, 16, 6076. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Cabezudo, S.; Caba, K. de la Environmental Assessment of Chitosan-Based Films. J. Clean. Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical Properties and Characterization of Chitosan Synthesized from Fish Scales, Crab and Shrimp Shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and Characterization of Chitosan Extracted from Shrimp Shells Waste and Chitosan Film: Application for Eriochrome Black T Removal from Aqueous Solutions. Appl. Water Sci. 2019, 9, 91. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Liaw, B.-S.; Chang, T.-T.; Chang, H.-K.; Liu, W.-K.; Chen, P.-Y. Fish Scale-Extracted Hydroxyapatite/Chitosan Composite Scaffolds Fabricated by Freeze Casting—An Innovative Strategy for Water Treatment. J. Hazard. Mater. 2020, 382, 121082. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Mane, S.; Pathan, E.; Tupe, S.; Deshmukh, S.; Kale, D.; Ghormade, V.; Chaudhari, B.; Deshpande, M. Isolation and Characterization of Chitosans from Different Fungi with Special Emphasis on Zygomycetous Dimorphic Fungus Benjaminiella Poitrasii: Evaluation of Its Chitosan Nanoparticles for the Inhibition of Human Pathogenic Fungi. Biomacromolecules 2022, 23, 808–815. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current Trends in Fungal Biosynthesis of Chitin and Chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- ur Rehman, K.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; ur Rehman, R.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Faqir, Y.; Tan, C.; Khaliq, G. Terrestrial Insects as a Promising Source of Chitosan and Recent Developments in Its Application for Various Industries. Food Chem. 2022, 373, 131407. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of Chitin and Chitosan Derived from Hermetia Illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Mahdy Samar, M.; El-Kalyoubi, M.H.; Khalaf, M.M.; Abd El-Razik, M.M. Physicochemical, Functional, Antioxidant and Antibacterial Properties of Chitosan Extracted from Shrimp Wastes by Microwave Technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- Arbia, W.; Adour, L.; Amrane, A.; Lounici, H. Optimization of Medium Composition for Enhanced Chitin Extraction from Parapenaeus Longirostris by Lactobacillus Helveticus Using Response Surface Methodology. Food Hydrocoll. 2013, 31, 392–403. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Afroz, M.M.; Kashem, M.N.H.; Piash, K.M.P.S.; Islam, N. Saccharomyces Cerevisiae as an Untapped Source of Fungal Chitosan for Antimicrobial Action. Appl. Biochem. Biotechnol. 2021, 193, 3765–3786. [Google Scholar] [CrossRef]
- Berger, L.; Stamford, T.; Stamford-Arnaud, T.; De Alcântara, S.; Da Silva, A.; Da Silva, A.; Do Nascimento, A.; De Campos-Takaki, G. Green Conversion of Agroindustrial Wastes into Chitin and Chitosan by Rhizopus Arrhizus and Cunninghamella Elegans Strains. Int. J. Mol. Sci. 2014, 15, 9082–9102. [Google Scholar] [CrossRef]
- Montenegro Stamford, T.C.; Montenegro Stamford, T.L.; Pereira Stamford, N.; De Barros Neto, B.; De Campos-Takaki, G.M. Growth of Cunninghamella Elegans UCP 542 and Production of Chitin and Chitosan Using Yam Bean Medium. Electron. J. Biotechnol. 2007, 10, 61–68. [Google Scholar] [CrossRef]
- Krishnaveni, B.; Ragunathan, R. Extraction and Characterization of Chitin and Chitosan from Aspergillus Terreus Sps, Synthesis of Their Bionanocomposites and Study of Their Productive Applications. J. Chem. Pharm. Res. 2015, 7, 115–132. [Google Scholar]
- Gonil, P.; Sajomsang, W. Applications of Magnetic Resonance Spectroscopy to Chitin from Insect Cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522. [Google Scholar] [CrossRef]
- Song, Y.; Kim, M.; Moon, C.; Seo, D.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.; Kim, S.; Kim, Y.W.; et al. Extraction of Chitin and Chitosan from Larval Exuvium and Whole Body of Edible Mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia Parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the Physicochemical, Rheological, and Morphologic Properties of Chitosan from Four Insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Shih, I.-L.; Tzeng, Y.-M.; Wang, S.-L. Production and Purification of Protease from a Bacillus Subtilis That Can Deproteinize Crustacean Wastes. Enzym. Microb. Technol. 2000, 26, 406–413. [Google Scholar] [CrossRef]
- Mehranian, M.; Pourabad, R.F.; Bashir, N.S.; Taieban, S. Physicochemical Characterization of Chitin from the Mediterranean Flour Moth, Ephestia Kuehniella Zeller (Lepidoptera: Pyralidae). J. Macromol. Sci. Part A 2017, 54, 720–726. [Google Scholar] [CrossRef]
- Kaya, M.; Bitim, B.; Mujtaba, M.; Koyuncu, T. Surface Morphology of Chitin Highly Related with the Isolated Body Part of Butterfly (Argynnis Pandora ). Int. J. Biol. Macromol. 2015, 81, 443–449. [Google Scholar] [CrossRef]
- Kim, M.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Kang, S.H.; Kim, S.; Jung, W. Extraction of Chitin and Chitosan from Housefly, Musca Domestica, Pupa Shells. Entomol. Res. 2016, 46, 324–328. [Google Scholar] [CrossRef]
- Adhiksana, A.; Wahyudi, W.; Arifin, Z.; Irwan, M. Effect of microwave irradiation time to deacetylation process of chitin from shrimp shells. J. Kim. Ris. 2023, 8, 1–7. [Google Scholar] [CrossRef]
- Tahtat, D.; Uzun, C.; Mahlous, M.; Güven, O. Beneficial Effect of Gamma Irradiation on the N-Deacetylation of Chitin to Form Chitosan. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 425–428. [Google Scholar] [CrossRef]
- Akopova, T.A.; Popyrina, T.N.; Demina, T.S. Mechanochemical Transformations of Polysaccharides: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10458. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Effendy, M.; Clausse, D.; Saleh, K.; Guénin, E. Heterogeneous Deacetylation Reaction of Chitin under Low-Frequency Ultrasonic Irradiation. Carbohydr. Polym. 2021, 267, 118180. [Google Scholar] [CrossRef]
- Hasan, S.; Boddu, V.M.; Viswanath, D.S.; Ghosh, T.K. Preparation of Chitin and Chitosan. In Chitin and Chitosan: Science and Engineering; Ghosh, T.K., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 17–50. [Google Scholar]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Liu, Y.; Zhang, T. Review on Preparation and Adsorption Properties of Chitosan and Chitosan Composites. Polym. Bull. 2022, 79, 2633–2665. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Pires, C.T.G.V.M.T.; Vilela, J.A.P.; Airoldi, C. The Effect of Chitin Alkaline Deacetylation at Different Condition on Particle Properties. Procedia Chem. 2014, 9, 220–225. [Google Scholar] [CrossRef]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent Advances in Extraction of Chitin and Chitosan. World J. Microbiol. Biotechnol. 2023, 39, 28. [Google Scholar] [CrossRef]
- Ramírez, M.Á.; González, P.; Fagundo, J.R.; Suarez, M.; Melian, C.; Rodríguez, T.; Peniche, C. Chitin Preparation by Demineralizing Deproteinized Lobster Shells with CO2 and a Cationite. J. Renew. Mater. 2017, 5, 30–37. [Google Scholar] [CrossRef]
- Yi, K.; Miao, S.; Yang, B.; Li, S.; Lu, Y. Harnessing the Potential of Chitosan and Its Derivatives for Enhanced Functionalities in Food Applications. Foods 2024, 13, 439. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Advances in Processing Chitin as a Promising Biomaterial from Ionic Liquids. In Advances in Biochemical Engineering/Biotechnology; Springer: Singapore, 2018; pp. 177–198. [Google Scholar]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and Chitosan Production from Shrimp Shells Using Ammonium-Based Ionic Liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Şahin, S. Tailor-Designed Deep Eutectic Liquids as a Sustainable Extraction Media: An Alternative to Ionic Liquids. J. Pharm. Biomed. Anal. 2019, 174, 324–329. [Google Scholar] [CrossRef]
- Sun, X.; Wei, Q.; Yang, Y.; Xiao, Z.; Ren, X. In-Depth Study on the Extraction and Mechanism of High-Purity Chitin Based on NADESs Method. J. Environ. Chem. Eng. 2022, 10, 106859. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Radojčić Redovniković, I.; Duarte, A.R.C.; Matias, A.A.; Paiva, A. Low-Phytotoxic Deep Eutectic Systems as Alternative Extraction Media for the Recovery of Chitin from Brown Crab Shells. ACS Omega 2021, 6, 28729–28741. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Somashekar, D.; Joseph, R. Chitosanases—Properties and Applications: A Review. Bioresour. Technol. 1996, 55, 35–45. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Mouyna, I.; Dellière, S.; Beauvais, A.; Gravelat, F.; Snarr, B.; Lehoux, M.; Zacharias, C.; Sun, Y.; de Jesus Carrion, S.; Pearlman, E.; et al. What Are the Functions of Chitin Deacetylases in Aspergillus Fumigatus? Front. Cell. Infect. Microbiol. 2020, 10, 28. [Google Scholar] [CrossRef]
- Águila-Almanza, E.; Salgado-Delgado, R.; Vargas-Galarza, Z.; García-Hernández, E.; Hernández-Cocoletzi, H. Enzymatic Depolimerization of Chitosan for the Preparation of Functional Membranes. J. Chem. 2019, 2019, 5416297. [Google Scholar] [CrossRef]
- Hajiali, F.; Vidal, J.; Jin, T.; de la Garza, L.C.; Santos, M.; Yang, G.; Moores, A. Extraction of Chitin from Green Crab Shells by Mechanochemistry and Aging. ACS Sustain. Chem. Eng. 2022, 10, 11348–11357. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and Efficient Extraction of Chitin and Chitosan for Scale-up Production: Effect of Process Parameters on Deacetylation Degree and Molecular Weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef]
- Villa-Lerma, G.; González-Márquez, H.; Gimeno, M.; López-Luna, A.; Bárzana, E.; Shirai, K. Ultrasonication and Steam-Explosion as Chitin Pretreatments for Chitin Oligosaccharide Production by Chitinases of Lecanicillium Lecanii. Bioresour. Technol. 2013, 146, 794–798. [Google Scholar] [CrossRef]
- Birolli, W.G.; de Moura Delezuk, J.A.; Campana-Filho, S.P. Ultrasound-Assisted Conversion of Alpha-Chitin into Chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Nowacki, K.; Stępniak, I.; Langer, E.; Tsurkan, M.; Wysokowski, M.; Petrenko, I.; Khrunyk, Y.; Fursov, A.; Bo, M.; Bavestrello, G.; et al. Electrochemical Approach for Isolation of Chitin from the Skeleton of the Black Coral Cirrhipathes Sp. (Antipatharia). Mar. Drugs 2020, 18, 297. [Google Scholar] [CrossRef]
- Farkaš, V. Fungal Cell Walls: Their Structure, Biosynthesis and Biotechnological Aspects. Acta Biotechnol. 1990, 10, 225–238. [Google Scholar] [CrossRef]
- Fleet, G.H.; Phaff, H.J. Fungal Glucans—Structure and Metabolism. In Plant Carbohydrates II; Springer: Berlin/Heidelberg, Germany, 1981; pp. 416–440. [Google Scholar]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly Curable Chitosan–PEG Hydrogels as Tissue Adhesives for Hemostasis and Wound Healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Nayal, A.; Malhotra, S.; Koul, V. Dual Functionalized Chitosan Based Composite Hydrogel for Haemostatic Efficacy and Adhesive Property. Carbohydr. Polym. 2020, 247, 116757. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Mamadouba, B.; Xia, W. A Comparative Study on Hypolipidemic Activities of High and Low Molecular Weight Chitosan in Rats. Int. J. Biol. Macromol. 2012, 51, 504–508. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B.H. Antimicrobial Activity of Chitosan and a Chitosan Oligomer against Bacterial Pathogens of Warmwater Fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Schollmeyer, E. Anticandidal Action of Fungal Chitosan against Candida Albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U. Antifungal Effect of High- and Low-Molecular-Weight Chitosan Hydrochloride, Carboxymethyl Chitosan, Chitosan Oligosaccharide and N-Acetyl-d-Glucosamine against Candida Albicans, Candida Krusei and Candida Glabrata. Int. J. Pharm. 2007, 353, 139–148. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A Potential Biopolymer for Wound Management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and Antitumor Activities of Chitosan from Shiitake Stipes, Compared to Commercial Chitosan from Crab Shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Ling, Y.; Luo, Y.; Luo, J.; Wang, X.; Sun, R. Novel Antibacterial Paper Based on Quaternized Carboxymethyl Chitosan/Organic Montmorillonite/Ag NP Nanocomposites. Ind. Crops Prod. 2013, 51, 470–479. [Google Scholar] [CrossRef]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-Loaded Chitosan Hydrogel with Superior Dual Functions: Antibacterial Efficacy and Osteoblastic Cell Responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- El Shafei, A.; Abou-Okeil, A. ZnO/Carboxymethyl Chitosan Bionano-Composite to Impart Antibacterial and UV Protection for Cotton Fabric. Carbohydr. Polym. 2011, 83, 920–925. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.-E.; Wang, N.; Liu, X.; An, Q.; Ye, X.-M.; Zhao, Z.-T.; Zhao, M.; Han, Y.; et al. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxid. Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef]
- Li, J.-E.; Wang, W.-J.; Zheng, G.-D.; Li, L.-Y. Physicochemical Properties and Antioxidant Activities of Polysaccharides from Gynura Procumbens Leaves by Fractional Precipitation. Int. J. Biol. Macromol. 2017, 95, 719–724. [Google Scholar] [CrossRef]
- Feng, T.; Du, Y.; Li, J.; Wei, Y.; Yao, P. Antioxidant Activity of Half N-Acetylated Water-Soluble Chitosan in Vitro. Eur. Food Res. Technol. 2007, 225, 133. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative Activity of Chitosans with Varying Molecular Weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Kan, J.; Tang, Y.; Jin, C. Preparation, Characterization and Antioxidant Activity of Phenolic Acids Grafted Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Liu, M.; Min, L.; Zhu, C.; Rao, Z.; Liu, L.; Xu, W.; Luo, P.; Fan, L. Preparation, Characterization and Antioxidant Activity of Silk Peptides Grafted Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2017, 104, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced Antitumor Immunity by Targeting Dendritic Cells with Tumor Cell Lysate-Loaded Chitosan Nanoparticles Vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Dong, X.; Yu, J.; Meng, F.; Feng, Y.; Ji, H.; Liu, A. Antitumor Effects of Seleno-Short-Chain Chitosan (SSCC) against Human Gastric Cancer BGC-823 Cells. Cytotechnology 2019, 71, 1095–1108. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic Acid-Coated Chitosan Nanoparticles Induce ROS-Mediated Tumor Cell Apoptosis and Enhance Antitumor Efficiency by Targeted Drug Delivery via CD44. J. Nanobiotechnology 2017, 15, 7. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Qiao, J.; Zhang, W.; Peng, Y.; Liu, W.; Han, B. Antitumor Evaluation of Carboxymethyl Chitosan Based Norcantharidin Conjugates against Gastric Cancer as Novel Polymer Therapeutics. Int. J. Biol. Macromol. 2019, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, B.; Li, H.; Li, X.; Yang, Y.; Liu, W. Preparation and Anti-Tumor Metastasis of Carboxymethyl Chitosan. Carbohydr. Polym. 2015, 125, 53–60. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic Carboxymethyl Chitosan-Quercetin Conjugate with P-Gp Inhibitory Properties for Oral Delivery of Paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Jena, S.K.; Samal, S.K.; Kaur, S.; Chand, M.; Sangamwar, A.T. Potential of Amphiphilic Graft Copolymer α-Tocopherol Succinate-g-Carboxymethyl Chitosan in Modulating the Permeability and Anticancer Efficacy of Tamoxifen. Eur. J. Pharm. Sci. 2017, 101, 149–159. [Google Scholar] [CrossRef]
- Wang, G.; Li, R.; Parseh, B.; Du, G. Prospects and Challenges of Anticancer Agents’ Delivery via Chitosan-Based Drug Carriers to Combat Breast Cancer: A Review. Carbohydr. Polym. 2021, 268, 118192. [Google Scholar] [CrossRef]
- Khorasani, M.A.; Naghib, S.M. A Review of Chitosan-Based Multifunctional Nanocomposites for Drug/Gene/Protein Delivery and Gene Therapy in Cancer Treatments: Promises, Challenges and Outlooks. Int. J. Biol. Macromol. 2025, 306, 141394. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Liu, J. Multi-Functional Chitosan-Based Nanoparticles for Drug Delivery: Recent Advanced Insight into Cancer Therapy. Carbohydr. Polym. 2023, 315, 120972. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-Based Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, Y.-Y.; Wu, G.-J.; Huang, C.-H.; Tsai, G.J. Effect of Chitosan Molecular Weight on Anti-Inflammatory Activity in the RAW 264.7 Macrophage Model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and Inhibitory Enzyme Activity of N-(Benzyl) and Quaternary N-(Benzyl) Chitosan Derivatives on Plant Pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Águas, A.P.; Barbosa, J.N. Macrophage Polarization Following Chitosan Implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on Physical Properties and Nerve Cell Affinity of Composite Films from Chitosan and Gelatin Solutions. Biomaterials 2003, 24, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and Histological Evaluation of Biomimetic Three-Dimensional Hydroxyapatite/Chitosan-Gelatin Network Composite Scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.; Endres, M.; Ringe, J.; Bhonde, R.; Sittinger, M. Biocompatible Hydrogel Supports the Growth of Respiratory Epithelial Cells: Possibilities in Tracheal Tissue Engineering. J. Biomed. Mater. Res. 2001, 56, 120–127. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite Chitosan Film Containing Graphene Oxide/Hydroxyapatite/Gold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zia, I.; Jolly, R.; Mirza, S.; Rehman, A.; Shakir, M. Nanocomposite Materials Developed from Nano-hydroxyapatite Impregnated Chitosan/Κ-Carrageenan for Bone Tissue Engineering. ChemistrySelect 2022, 7, e202103234. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Alizadeh Sardroud, H.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of Chitosan/Alginate/Hydroxyapatite Hybrid Scaffolds Using 3D Printing and Impregnating Techniques for Potential Cartilage Regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; McLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in Vitro Assessment of a Bi-Layered Chitosan-Nano-Hydroxyapatite Osteochondral Scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.-K. Preparation and Characterization of Chitosan-Natural Nano Hydroxyapatite-Fucoidan Nanocomposites for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef]
- Gomes, S.; Rodrigues, G.; Martins, G.; Henriques, C.; Silva, J.C. Evaluation of Nanofibrous Scaffolds Obtained from Blends of Chitosan, Gelatin and Polycaprolactone for Skin Tissue Engineering. Int. J. Biol. Macromol. 2017, 102, 1174–1185. [Google Scholar] [CrossRef]
- Badhe, R.V.; Bijukumar, D.; Chejara, D.R.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A Composite Chitosan-Gelatin Bi-Layered, Biomimetic Macroporous Scaffold for Blood Vessel Tissue Engineering. Carbohydr. Polym. 2017, 157, 1215–1225. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Yan, H.; Rafique, M.; Li, S.; Shan, X.; Wu, L.; Qiao, M.; Kong, D.; Wang, L. Anti-Infective and Pro-Coagulant Chitosan-Based Hydrogel Tissue Adhesive for Sutureless Wound Closure. Biomacromolecules 2020, 21, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled Alkylated Chitosan Sponge to Treat Noncompressible Hemorrhages and Facilitate Wound Healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef] [PubMed]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and Its Derivatives for Controlled Drug Release Applications—A Review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Zhang, T.; Yu, Y.; Zhang, Y.; Zhai, J.; Huang, H.; Wei, S.; Ding, L.; Liu, B. A Study on the Preparation of Chitosan-Tripolyphosphate Nanoparticles and Its Entrapment Mechanism for Egg White Derived Peptides. Food Chem. 2019, 286, 530–536. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Bao, X.; Xu, G.; Yao, P. Fatty Acid and Quaternary Ammonium Modified Chitosan Nanoparticles for Insulin Delivery. Colloids Surf. B Biointerfaces 2018, 170, 136–143. [Google Scholar] [CrossRef]
- Tsai, L.-C.; Chen, C.-H.; Lin, C.-W.; Ho, Y.-C.; Mi, F.-L. Development of Mutlifunctional Nanoparticles Self-Assembled from Trimethyl Chitosan and Fucoidan for Enhanced Oral Delivery of Insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Sun, Y.; Kou, Y.; Shen, X.; Huo, Y.; Liu, C.; Sun, Z.; Zhang, X.; Mao, S. Effect of Glyceryl Monocaprylate–Modified Chitosan on the Intranasal Absorption of Insulin in Rats. J. Pharm. Sci. 2019, 108, 3623–3629. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, L.; Ye, S.; Li, G.; Zhang, M. Construction of an Environmentally Friendly Octenylsuccinic Anhydride Modified PH-Sensitive Chitosan Nanoparticle Drug Delivery System to Alleviate Inflammation and Oxidative Stress. Carbohydr. Polym. 2020, 236, 115972. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of PH-Responsive Fucoidan/Chitosan Nanoparticles to Improve Oral Quercetin Delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef]
- Kheiri, K.; Sohrabi, N.; Mohammadi, R.; Amini-Fazl, M.S. Preparation and Characterization of Magnetic Nanohydrogel Based on Chitosan for 5-Fluorouracil Drug Delivery and Kinetic Study. Int. J. Biol. Macromol. 2022, 202, 191–198. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl Chitosan: An Advanced Polymer with Myriad of Opportunities in Nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, N.; Chen, J.; Ji, J.; Liu, X.; Wang, J.; Zhu, J.; Ma, Y. Temperature and PH Sensitive Composite for Rapid and Effective Removal of Sulfonylurea Herbicides in Aqueous Solution. Environ. Pollut. 2019, 255, 113150. [Google Scholar] [CrossRef]
- Sousa, Â.; Almeida, A.M.; Faria, R.; Konate, K.; Boisguerin, P.; Queiroz, J.A.; Costa, D. Optimization of Peptide-Plasmid DNA Vectors Formulation for Gene Delivery in Cancer Therapy Exploring Design of Experiments. Colloids Surf. B Biointerfaces 2019, 183, 110417. [Google Scholar] [CrossRef] [PubMed]
- Mai, Q.; Shen, S.; Liu, Y.; Tang, C.; Yin, C. PEG Modified Trimethyl Chitosan Based Nanoparticles for the Codelivery of Doxorubicin and ISur-PDNA. Mater. Lett. 2019, 238, 143–146. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and Characterization of Porous Chitosan Microspheres and Adsorption Performance for Hexavalent Chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Xie, Y.; Chen, J.; Dou, Y. Apoptosis of A549 Cells by Small Interfering RNA Targeting Survivin Delivery Using Poly-β-Amino Ester/Guanidinylated O-Carboxymethyl Chitosan Nanoparticles. Asian J. Pharm. Sci. 2020, 15, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for Gene Delivery: Methods for Improvement and Applications. Adv. Colloid Interface Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef]
- Arami, S.; Rashidi, M.; Mahdavi, M.; Fathi, M.; Entezami, A. Synthesis and Characterization of Fe3O4-PEG-LAC-Chitosan-PEI Nanoparticle as a Survivin SiRNA Delivery System. Hum. Exp. Toxicol. 2017, 36, 227–237. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, T.; Chen, J.; Cui, Y.; Zhang, X.; Lee, R.J.; Sun, F.; Li, Y.; Teng, L. PLGA/PCADK Composite Microspheres Containing Hyaluronic Acid–Chitosan SiRNA Nanoparticles: A Rational Design for Rheumatoid Arthritis Therapy. Int. J. Pharm. 2021, 596, 120204. [Google Scholar] [CrossRef]
- Porta, R.; Mariniello, L.; Di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase Crosslinked Pectin- and Chitosan-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric Acid-Incorporated Fish Gelatin/Chitosan Composite Films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Li, P.; Tan, H.; Xu, D.; Yin, F.; Cheng, Y.; Zhang, X.; Liu, Y.; Wang, F. Effect and Mechanisms of Curdlan Sulfate on Inhibiting HBV Infection and Acting as an HB Vaccine Adjuvant. Carbohydr. Polym. 2014, 110, 446–455. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double Network Hydrophobic Starch Based Amphoteric Hydrogel as an Effective Adsorbent for Both Cationic and Anionic Dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Tian, B.; Huang, X.; Bi, J.; Wang, T.; Hao, H. Application of N-Doped MoS 2 Nanocrystals for Removal of Azo Dyes in Wastewater. Chem. Eng. Technol. 2018, 41, 1180–1187. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- de Farias, B.S.; Vidal, É.M.; Ribeiro, N.T.; da Silveira, N.; da Silva Vaz, B.; Kuntzler, S.G.; de Morais, M.G.; Cadaval, T.R.S.; de Almeida Pinto, L.A. Electrospun Chitosan/Poly(Ethylene Oxide) Nanofibers Applied for the Removal of Glycerol Impurities from Biodiesel Production by Biosorption. J. Mol. Liq. 2018, 268, 365–370. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Panda, K.; Acharya, K. Chitosan-Induced Immunity in Camellia Sinensis (L.) O. Kuntze against Blister Blight Disease Is Mediated by Nitric-Oxide. Plant Physiol. Biochem. 2017, 115, 298–307. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered Chitosan Based Nanomaterials: Bioactivities, Mechanisms and Perspectives in Plant Protection and Growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Gabriel Paulraj, M.; Ignacimuthu, S.; Gandhi, M.R.; Shajahan, A.; Ganesan, P.; Packiam, S.M.; Al-Dhabi, N.A. Comparative Studies of Tripolyphosphate and Glutaraldehyde Cross-Linked Chitosan-Botanical Pesticide Nanoparticles and Their Agricultural Applications. Int. J. Biol. Macromol. 2017, 104, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Lelu, S.; Reitan, N.K.; de Lange Davies, C.; Artursson, P.; Vårum, K.M. Molecular Design of Chitosan Gene Delivery Systems with an Optimized Balance between Polyplex Stability and Polyplex Unpacking. Biomaterials 2010, 31, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and Interactions in Covalently and Ionically Crosslinked Chitosan Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The Versatile Biopolymer Chitosan: Potential Sources, Evaluation of Extraction Methods and Applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-Solubility of Partially N-Acetylated Chitosans as a Function of PH: Effect of Chemical Composition and Depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Salt-Assisted Mechanistic Analysis of Chitosan/Tripolyphosphate Micro- and Nanogel Formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Chitosan Gels: 1. Study of Reaction Variables. Int. J. Biol. Macromol. 1980, 2, 73–77. [Google Scholar] [CrossRef]
- Vachoud, L.; Zydowicz, N.; Domard, A. Formation and Characterisation of a Physical Chitin Gel. Carbohydr. Res. 1997, 302, 169–177. [Google Scholar] [CrossRef]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan Hydrogels for the Regeneration of Infarcted Myocardium: Preparation, Physicochemical Characterization, and Biological Evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical Chitosan Microhydrogels as Scaffolds for Spinal Cord Injury Restoration and Axon Regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amédée, J.; Montembault, A.; David, L.; et al. Physicochemical Modulation of Chitosan-based Hydrogels Induces Different Biological Responses: Interest for Tissue Engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-Based Networks from Homogeneous Chitosan-Tripolyphosphate Hydrogels: Synthesis and Characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the Ionotropic Gelation of Chitosan Using Tripolyphosphate and Pyrophosphate as Cross-Linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the Correlation between the Microscopic Structure and Properties of Phosphate-Cross-Linked Chitosan Gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-Containing Antimicrobial Membrane Based on Chitosan-TPP Hydrogel for the Treatment of Wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic Hydrogel Based on Chitosan Cross-Linked with 6-Phosphogluconic Trisodium Salt as a Drug Delivery System. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. On the Kinetics of Chitosan/Tripolyphosphate Micro- and Nanogel Aggregation and Their Effects on Particle Polydispersity. J. Colloid Interface Sci. 2017, 486, 27–37. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors Affecting the Stability of Chitosan/Tripolyphosphate Micro- and Nanogels: Resolving the Opposing Findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Abul Kalam, M.; Khan, A.A.; Khan, S.; Almalik, A.; Alshamsan, A. Optimizing Indomethacin-Loaded Chitosan Nanoparticle Size, Encapsulation, and Release Using Box–Behnken Experimental Design. Int. J. Biol. Macromol. 2016, 87, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of Aspirin and Probucol in Combination Loaded Chitosan Nanoparticles and in Vitro Release Study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Manzoor, S.; Rehman, A.; Rahman, A.; Nasir, H.; Imran, M. Antihypertensive Nano-Ceuticales Based on Chitosan Biopolymer: Physico-Chemical Evaluation and Release Kinetics. Carbohydr. Polym. 2016, 142, 268–274. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors Determining the Stability, Size Distribution, and Cellular Accumulation of Small, Monodisperse Chitosan Nanoparticles as Candidate Vectors for Anticancer Drug Delivery: Application to the Passive Encapsulation of [14C]-Doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Vårum, K.M.; Marsich, E. Butyrate-Loaded Chitosan/Hyaluronan Nanoparticles: A Suitable Tool for Sustained Inhibition of ROS Release by Activated Neutrophils. Macromol. Biosci. 2017, 17, 1700214. [Google Scholar] [CrossRef]
- Parajó, Y.; D’Angelo, I.; Welle, A.; Garcia-Fuentes, M.; Alonso, M.J. Hyaluronic Acid/Chitosan Nanoparticles as Delivery Vehicles for VEGF and PDGF-BB. Drug Deliv. 2010, 17, 596–604. [Google Scholar] [CrossRef]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic Acid (HA) Presentation as a Tool to Modulate and Control the Receptor-Mediated Uptake of HA-Coated Nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic Acid-Chitosan Nanoparticles for Co-Delivery of MiR-34a and Doxorubicin in Therapy against Triple Negative Breast Cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Alradwan, I.; Majrashi, M.A.; Alsaffar, B.A.; Algarni, A.T.; Alsuabeyl, M.S.; Alrabiah, H.; Tirelli, N.; Alhasan, A.H. Cellular Responses of Hyaluronic Acid-Coated Chitosan Nanoparticles. Toxicol. Res. 2018, 7, 942–950. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Chen, D.; Yuan, Y.; Wang, S.; Li, C.; Yan, Y.; Liu, Q.; Shao, L.; Huang, L.; et al. Enhanced Treatment Effects of Tilmicosin Against Staphylococcus Aureus Cow Mastitis by Self-Assembly Sodium Alginate-Chitosan Nanogel. Pharmaceutics 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; El-Banna, S.T.; El-Bahy, G.S.; Abdelrazek, E.M.; Kamal, M. Alginate Coated Chitosan Nanogel for the Controlled Topical Delivery of Silver Sulfadiazine. Carbohydr. Polym. 2017, 177, 194–202. [Google Scholar] [CrossRef]
- Hesan, M.; Gholipour-Kanani, A.; Lotfi, M.; Shafiee, M. The Synthesis and Characterization of Core-Shell Nanogels Based on Alginate and Chitosan for the Controlled Delivery of Mupirocin. Biochem. Eng. J. 2023, 190, 108742. [Google Scholar] [CrossRef]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling Concept Combining Chitosan and Alginate—Proof of Principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef]
- Rodrigues, S.; da Costa, A.M.R.; Grenha, A. Chitosan/Carrageenan Nanoparticles: Effect of Cross-Linking with Tripolyphosphate and Charge Ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef]
- Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remuñán-López, C.; Grenha, A. Hybrid Nanosystems Based on Natural Polymers as Protein Carriers for Respiratory Delivery: Stability and Toxicological Evaluation. Carbohydr. Polym. 2015, 123, 369–380. [Google Scholar] [CrossRef]
- Guyot, C.; Cerruti, M.; Lerouge, S. Injectable, Strong and Bioadhesive Catechol-Chitosan Hydrogels Physically Crosslinked Using Sodium Bicarbonate. Mater. Sci. Eng. C 2021, 118, 111529. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, 1800069. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Qi, P.; Takahashi, A.; Ohta, S.; Ito, T. Prevention of Postoperative Peritoneal Adhesions in Rats with Sidewall Defect-Bowel Abrasions Using Metal Ion-Crosslinked N-Succinyl Chitosan Hydrogels. React. Funct. Polym. 2019, 145, 104374. [Google Scholar] [CrossRef]
- Wang, M.; Muhammad, T.; Gao, H.; Liu, J.; Liang, H. Targeted PH-Responsive Chitosan Nanogels with Tanshinone IIA for Enhancing the Antibacterial/Anti-Biofilm Efficacy. Int. J. Biol. Macromol. 2023, 237, 124177. [Google Scholar] [CrossRef]
- Li, S.-N.; Li, B.; Yu, Z.-R.; Li, Y.; Guo, K.-Y.; Gong, L.-X.; Feng, Y.; Jia, D.; Zhou, Y.; Tang, L.-C. Constructing Dual Ionically Cross-Linked Poly(Acrylamide-Co-Acrylic Acid) /Chitosan Hydrogel Materials Embedded with Chitosan Decorated Halloysite Nanotubes for Exceptional Mechanical Performance. Compos. Part B Eng. 2020, 194, 108046. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Boisclair, J.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Chitosan/Glucose 1-Phosphate as New Stable in Situ Forming Depot System for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2014, 88, 361–373. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological Study of Chitosan/Polyol-Phosphate Systems: Influence of the Polyol Part on the Thermo-Induced Gelation Mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Hu, X.; Dai, L.; Zhu, J.; Man, Z.; Chen, H.; Zhou, C.; Ao, Y. Directing Chondrogenic Differentiation of Mesenchymal Stem Cells with a Solid-Supported Chitosan Thermogel for Cartilage Tissue Engineering. Biomed. Mater. 2014, 9, 035008. [Google Scholar] [CrossRef]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive Chitosan/Phosphate Hydrogel-Composites Fortified with Ag versus Ag@Pd for Biomedical Applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Wang, X.; Zhang, R.; Liang, F. Mussel-mimetic Chitosan Based Injectable Hydrogel with Fast-Crosslinking and Water-Resistance as Tissue Adhesive. Int. J. Adhes. Adhes. 2023, 124, 103382. [Google Scholar] [CrossRef]
- Costalat, M.; David, L.; Delair, T. Reversible Controlled Assembly of Chitosan and Dextran Sulfate: A New Method for Nanoparticle Elaboration. Carbohydr. Polym. 2014, 102, 717–726. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Jeong, J.; Kim, J.-M.; Kim, M.S.; Jung, S. A PH-Sensitive Drug Delivery Using Biodegradable Succinoglycan/Chitosan Hydrogels with Synergistic Antibacterial Activity. Int. J. Biol. Macromol. 2023, 242, 124888. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Trad, M.; Miled, W.; Benltoufa, S.; Boughattas, A.; Benslama, R.; Fayala, F.; Bakhrouf, A. Chitosan Hydrogel-coated Cotton Fabric: Antibacterial, PH-responsiveness, and Physical Properties. J. Appl. Polym. Sci. 2018, 135, 46645. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Nikolakis, S.-P.; Pamvouxoglou, A.; Koutsopoulou, E. Physicochemical Properties of Electrostatically Crosslinked Carrageenan/Chitosan Hydrogels and Carrageenan/Chitosan/Laponite Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2023, 225, 565–573. [Google Scholar] [CrossRef]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.-H.; Jo, Y.-J.; Seo, J.-W.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Dual Cross-Linked Chitosan/Alginate Hydrogels Prepared by Nb-Tz ‘Click’ Reaction for PH Responsive Drug Delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Paluch, K.J.; Inkielewicz-Stępniak, I.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Exploring the Assembly Process and Properties of Novel Crosslinker-Free Hyaluronate-Based Polyelectrolyte Complex Nanocarriers. Int. J. Pharm. 2012, 436, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Delair, T. Stabilization of Chitosan/Hyaluronan Colloidal Polyelectrolyte Complexes in Physiological Conditions. Carbohydr. Polym. 2015, 119, 149–158. [Google Scholar] [CrossRef]
- Zucca, G.; Vigani, B.; Valentino, C.; Ruggeri, M.; Marchesi, N.; Pascale, A.; Giovilli, G.; Malavasi, L.; Sandri, G.; Rossi, S. Chondroitin Sulphate-Chitosan Based Nanogels Loaded with Naringenin-β-Cyclodextrin Complex as Potential Tool for the Treatment of Diabetic Retinopathy: A Formulation Study. Int. J. Nanomed. 2025, 20, 907–932. [Google Scholar] [CrossRef]
- Ali, S.M.A.; Khan, J.; Shahid, R.; Shabbir, S.; Ayoob, M.F.; Imran, M. Chitosan-Carrageenan Microbeads Containing Nano-Encapsulated Curcumin: Nano-in-Micro Hydrogels as Alternative-Therapeutics for Resistant Pathogens Associated with Chronic Wounds. Int. J. Biol. Macromol. 2024, 278, 134841. [Google Scholar] [CrossRef]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile Synthesis of PH-Responsive Sodium Alginate/Carboxymethyl Chitosan Hydrogel Beads Promoted by Hydrogen Bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, G.; Wang, J.; Zhang, R.; Zhong, S.; Wang, J.; Cui, X. Injectable Chitosan-Based Self-Healing Supramolecular Hydrogels with Temperature and PH Dual-Responsivenesses. Int. J. Biol. Macromol. 2023, 227, 1038–1047. [Google Scholar] [CrossRef]
- Enache, A.-C.; Cojocaru, C.; Samoila, P.; Bele, A.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V. Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids. Gels 2022, 8, 495. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A Novel Tetraethylenepentamine Crosslinked Chitosan Oligosaccharide Hydrogel for Total Adsorption of Cr(VI). Carbohydr. Polym. 2019, 224, 115154. [Google Scholar] [CrossRef] [PubMed]
- Soto Garcia, P.; Sabino Leocádio Antunes, B.; Komatsu, D.; de Alencar Hausen, M.; Dicko, C.; de Rezende Duek, E.A. Mechanical and Rheological Properties of Pluronic F127 Based-Hydrogels Loaded with Chitosan Grafted with Hyaluronic Acid and Propolis, Focused to Atopic Dermatitis Treatment. Int. J. Biol. Macromol. 2025, 307, 141942. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, H.; Amiri, S.; Amiri, F.; Moradi, S.; Zarrintaj, P. Antibacterial Biocomposite Based on Chitosan/Pluronic/Agarose Noncovalent Hydrogel: Controlled Drug Delivery by Alginate/Tetracycline Beads System. J. Funct. Biomater. 2024, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhang, Y.; Chen, Y.; Huo, L.; Wen, F.; Cai, J.; Gong, Q.; Li, P. Intelligent Response Agarose/Chitosan Hydrogel Wound Dressing: Synergistic Chemotherapy, PDT and PTT Effects against Bacterial Infection. Int. J. Biol. Macromol. 2025, 298, 139927. [Google Scholar] [CrossRef]
- Mahmoudi, C.; Tahraoui Douma, N.; Mahmoudi, H.; Iurciuc (Tincu), C.E.; Popa, M. Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 7839. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Solanki, R.; Dhanka, M.; Thareja, P.; Bhatia, D. Self-Healing, Injectable Chitosan-Based Hydrogels: Structure, Properties and Biological Applications. Mater. Adv. 2024, 5, 5365–5393. [Google Scholar] [CrossRef]
- Abdalla, T.H.; Nasr, A.S.; Bassioni, G.; Harding, D.R.; Kandile, N.G. Fabrication of Sustainable Hydrogels-Based Chitosan Schiff Base and Their Potential Applications. Arab. J. Chem. 2022, 15, 103511. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Salagierski, S.; Drozdowska, M.; Serafim, A.; Stancu, I.-C.; Szatkowski, P.; Kopec, A.; Rajzer, I.; Douglas, T.E.L.; et al. Newly Crosslinked Chitosan- and Chitosan-Pectin-Based Hydrogels with High Antioxidant and Potential Anticancer Activity. Carbohydr. Polym. 2022, 290, 119486. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized Hydroxypropyl Cellulose/Carboxymethyl Chitosan Hydrogels Permit pH-Responsive, Targeted Drug Release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, Y.; Chen, S.; Lin, Y.; Yue, Y. A Schiff Base Hydrogel Dressing Loading Extracts from Periplaneta Americana for Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 230, 123256. [Google Scholar] [CrossRef]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with PH-Regulated Recovery of Self-Healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar] [CrossRef]
- Ma, L.; Su, W.; Ran, Y.; Ma, X.; Yi, Z.; Chen, G.; Chen, X.; Deng, Z.; Tong, Q.; Wang, X.; et al. Synthesis and Characterization of Injectable Self-Healing Hydrogels Based on Oxidized Alginate-Hybrid-Hydroxyapatite Nanoparticles and Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2020, 165, 1164–1174. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in Situ Forming Biodegradable Chitosan–Hyaluronic Acid Based Hydrogels for Cartilage Tissue Engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-Chitosan Oligosaccharide-ZnO Composite Hydrogel for Accelerating Wound Healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Oh, G.-W.; Kim, S.-C.; Kim, T.-H.; Jung, W.-K. Characterization of an Oxidized Alginate-Gelatin Hydrogel Incorporating a COS-Salicylic Acid Conjugate for Wound Healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef]
- Li, C.-J.; Trost, B.M. Green Chemistry for Chemical Synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef] [PubMed]
- Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef]
- Atmani, Z.; Heinze, T.; Gericke, M. Development of Tailored Polysaccharide Gels through Selective Diels–Alder Crosslinking. Cellulose 2025, 32, 187–209. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, L.; Yang, C.; Wang, Q.; Wang, P.; Xu, B.; Yu, Y. Disulfide Bond Network Crosslinked Flexible Multifunctional Chitosan Coating on Fabric Surface Prepared by the Chitosan Grafted with Thioctic Acid. Int. J. Biol. Macromol. 2024, 263, 130431. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Zhu, Q.; Zhang, H. Characterization of Chitosan-Gelatin Cryogel Templates Developed by Chemical Crosslinking and Oxidation Resistance of Camellia Oil Cryogel-Templated Oleogels. Carbohydr. Polym. 2023, 315, 120971. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a Food Packaging Antibacterial Hydrogel Based on Gelatin, Chitosan, and 3-Phenyllactic Acid for the Shelf-Life Extension of Chilled Chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Zhang, H. Comment on Lu et al (2022) “The Preventive and Relieving Effects of Ginger on Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis of Randomized Controlled Trials”. Int. J. Nurs. Stud. 2022, 128, 104192. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Hu, B.; Wang, Z.; Gu, Q.; Wang, W.; Zhu, C.; Wang, S. Borate Bonds-Containing PH-Responsive Chitosan Hydrogel for Postoperative Tumor Recurrence and Wound Infection Prevention. Carbohydr. Polym. 2024, 339, 122262. [Google Scholar] [CrossRef]
- Nangia, S.; Warkar, S.; Katyal, D. A Review on Environmental Applications of Chitosan Biopolymeric Hydrogel Based Composites. J. Macromol. Sci. Part A 2018, 55, 747–763. [Google Scholar] [CrossRef]
| Source | Yield (%) of Chitin | References |
|---|---|---|
| Shrimp shells (shrimp waste) | 33.37%–36.43% (depending on particle size of shrimp waste) | [52] |
| Shrimp shells (P. longirostris) | 26.98% | [53] |
| Shrimp shells (P. durarum) | 23.72% | [54] |
| Crabs’ shells | 16.73% | [54] |
| Fungi (S. cerevisiae) | 2% | [55] |
| Fungi (R. arrhizus) | 8.3% | [56] |
| Fungi (C. elegans) | 6.6% | [57] |
| Fungi (A. terreus) | 34% | [58] |
| Insect cuticles (cicada sloughs) | 36.6% | [59] |
| Insects (T. molitor) | 17.32% | [60] |
| Insects (H. parallela) | 15% | [61] |
| Insects (B. mori) | 2.59%–20% | [62,63] |
| Insects (E. kuehniella) | 9.5%–10.5% | [64] |
| Insects (A. pandora) | 22% | [65] |
| Fungi (M. domestica) | 8.02% | [66] |
| Extraction Method | Advantages | Limitations | Environmental Impact | Scalability |
|---|---|---|---|---|
| Physical | Enhances reactivity; reduces processing time when combined with other methods | Requires high energy input; may alter chitin structure | Moderate to high (depending on energy source) | Moderate |
| Chemical | High yield and efficiency; widely used industrially | Uses harsh chemicals; produces hazardous waste; random deacetylation | High (due to chemical usage and waste) | High |
| Biological | Eco-friendly; selective deacetylation; high-quality chitosan | Slow process; enzyme cost; sensitive to process conditions | Low | Low to moderate |
| Green (e.g., supercritical fluids, ionic liquids) | Environmentally sustainable; minimal hazardous waste; high purity | Requires specialized equipment; high initial cost | Low | Currently low to moderate; promising for scale-up |
| Application Area | Specific Examples | Key Benefits |
|---|---|---|
| Biomedical | Wound dressings, hemostatic agents, tissue engineering scaffolds, drug/gene delivery | Biocompatibility, biodegradability, mucoadhesiveness, controlled release |
| Pharmaceutical | Nanoparticles for oral, ocular, and parenteral drug delivery; vaccine adjuvants | Enhances bioavailability, sustained release, low toxicity |
| Food industry | Edible films/coatings, preservatives, fat replacers | Antimicrobial, antioxidant, non-toxic, extends shelf life |
| Environmental | Water purification, dye adsorption, heavy metal removal | Chelating ability, biodegradability, flocculation, low cost |
| Agriculture | Biopesticides, plant growth promoters, seed coatings | Enhances resistance, reduces chemical pesticide use, and promotes sustainable farming |
| Cosmetics | Moisturizers, antiaging creams, shampoos | Film-forming, moisturizing, antimicrobial, stabilizer |
| Main Components | Characteristics | Reference |
|---|---|---|
| CS, acyl anhydrides (acetic, propionic, butyric, valeric, hexanoic), aqueous acetic acid, methanol |
| [184] |
| CS, acyl anhydrides (acetic, propionic, butyric, valeric, hexanoic), aqueous acetic acid, ethanol, 1,2-propanediol |
| [185] |
| Main Components | Characteristics | Reference |
|---|---|---|
| CS, NaOH |
| [186] |
| CS, NH3 |
| |
| CS, NH3, acetic acid, deionized water |
| |
| CS, NH3, acetic acid, 1,2-propanediol |
| [187] |
| CS, NaOH, acetic acid, 1,2-propanediol |
| [188] |
| Main Components | Cross-Linker | Characteristics | Reference |
|---|---|---|---|
| CS, acetic acid, TPP, NaCl, glycerol | TPP |
| [189] |
| CS, acetic acid, TPP or PPi, NaCl | TPP or PPi |
| [190] |
| CS, TPP or PPi, glycerol, acetic acid, NaCl | TPP or PPi |
| [191] |
| CS, TPP, Chitlac–Ag, glycerol | TPP |
| [192] |
| CS, 6-PG−Na+, acetic acid, NaCl | 6-PG−Na+ |
| [193] |
| CS, TPP, acetic acid, NaCl, deionized water | TPP |
| [183] |
| CS, TPP, NaCl, glucosamine | TPP |
| [194] |
| CS, TPP, NaCl | TPP |
| [195] |
| CS, TPP, NaCl, trehalose or mannitol or PEG | TPP |
| [196] |
| CS, TPP, indomethacin | TPP |
| [197] |
| CS, TPP, ASA, PRO | TPP |
| [198] |
| CS, TPP, captopril, amlodipine, valsartan | TPP |
| [199] |
| CS, TPP, DOX | TPP |
| [200] |
| CS, HA, butyrate, TPP | TPP |
| [201] |
| CS, HA, TPP, VEGF or PDGF-BB, BSA or heparin sodium salt | TPP |
| [202] |
| CS, HA, TPP | TPP |
| [203] |
| CS, HA, TPP, DOX, miR-34a | TPP |
| [205] |
| CS, TPP, HA, Alg | TPP |
| [206] |
| CS, TPP, HA, NaNO2, HCl, NaOH | TPP |
| [207] |
| CS, SA, CaCO3, GDL, tilmicosin | Ca2+ |
| [208] |
| CS, SA, TPP, SSD, acetic acid | TPP |
| [209] |
| CS, Alg, D-GDL, NaHCO3 | Alg (poly-M or poly-G) |
| [211] |
| CS, SA, TPP, CaCl2, mupirocin, acetic acid | TPP (for CS) CaCl2 (for Alg) |
| [210] |
| CS, κ-carrageenan, TPP, acetic acid | TPP |
| [212] |
| CS, κ-carrageenan, TPP, BSA, acetic acid | TPP |
| [213] |
| CS, sodium bicarbonate, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) | - |
| [214] |
| CS, Alg, CaCl2, epidermal growth factor powder | CaCl2 |
| [215] |
| CH-Su, Fe3+/Al3+/Ca2+, PBS | Fe3+/Al3+/Ca2+ |
| [216] |
| CS, TA, TPP | TPP |
| [217] |
| CS, AAm, AAc, APS, halloysite nanotubes, HSiv, Fe(NO3)3·9H2O | Fe3+ |
| [218] |
| Main Components | Cross-Linker | Characteristics | Reference |
|---|---|---|---|
| CS, G1-P, HCl, PBS | G1-P |
| [219] |
| CS, β-GP or G1-P or G6-P, HCl, Milli-Q water | β-GP, G1-P, or G6-P |
| [220] |
| CS, β-GP, DBM, acetic acid | β-GP |
| [221] |
| CS, β-GP, HA, β-TCP, silver or silver–palladium NPs | β-GP |
| [222] |
| CS, thermosensitive polymer, NaHCO3 | - |
| [223] |
| Main Components | Characteristics | Reference |
|---|---|---|
| CS, dextran sulfate, acetic acid, NaCl |
| [224] |
| CS, SG, 5-FU |
| [225] |
| CS, two-component silicone elastomer, sulfate |
| [226] |
| CS, Na2S2O4, monochloroacetic acid, Na2CO3, reactive dye (Supra rouge S-PX) |
| [227] |
| CS, carrageenan, laponite |
| [228] |
| CS, Alg, acetic acid |
| [229] |
| CS, HA |
| [230] |
| CS, HA, Zn2+ |
| [231] |
| Main Components | Cross- Linker | Characteristics | Reference |
|---|---|---|---|
| CS, sodium Alg, citric acid | - |
| [234] |
| CS, orotic acid, 2,6-diaminopurine | - |
| [235] |
| CS, micronized nystatin, succinic anhydride, diepoxy-functionalized siloxane, lactic acid, glycerine, nystatin | - |
| [236] |
| CS, TEPA, epichlorohydrin, Cr (VI) | TEPA |
| [237] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blebea, N.-M.; Pușcașu, C.; Vlad, R.-A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. https://doi.org/10.3390/gels11040275
Blebea N-M, Pușcașu C, Vlad R-A, Hancu G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels. 2025; 11(4):275. https://doi.org/10.3390/gels11040275
Chicago/Turabian StyleBlebea, Nicoleta-Mirela, Ciprian Pușcașu, Robert-Alexandru Vlad, and Gabriel Hancu. 2025. "Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications" Gels 11, no. 4: 275. https://doi.org/10.3390/gels11040275
APA StyleBlebea, N.-M., Pușcașu, C., Vlad, R.-A., & Hancu, G. (2025). Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels, 11(4), 275. https://doi.org/10.3390/gels11040275









