Crystallization of Vanillin Isomers in Carboxymethyl Chitosan Gels
Abstract
1. Introduction
2. Results and Discussions
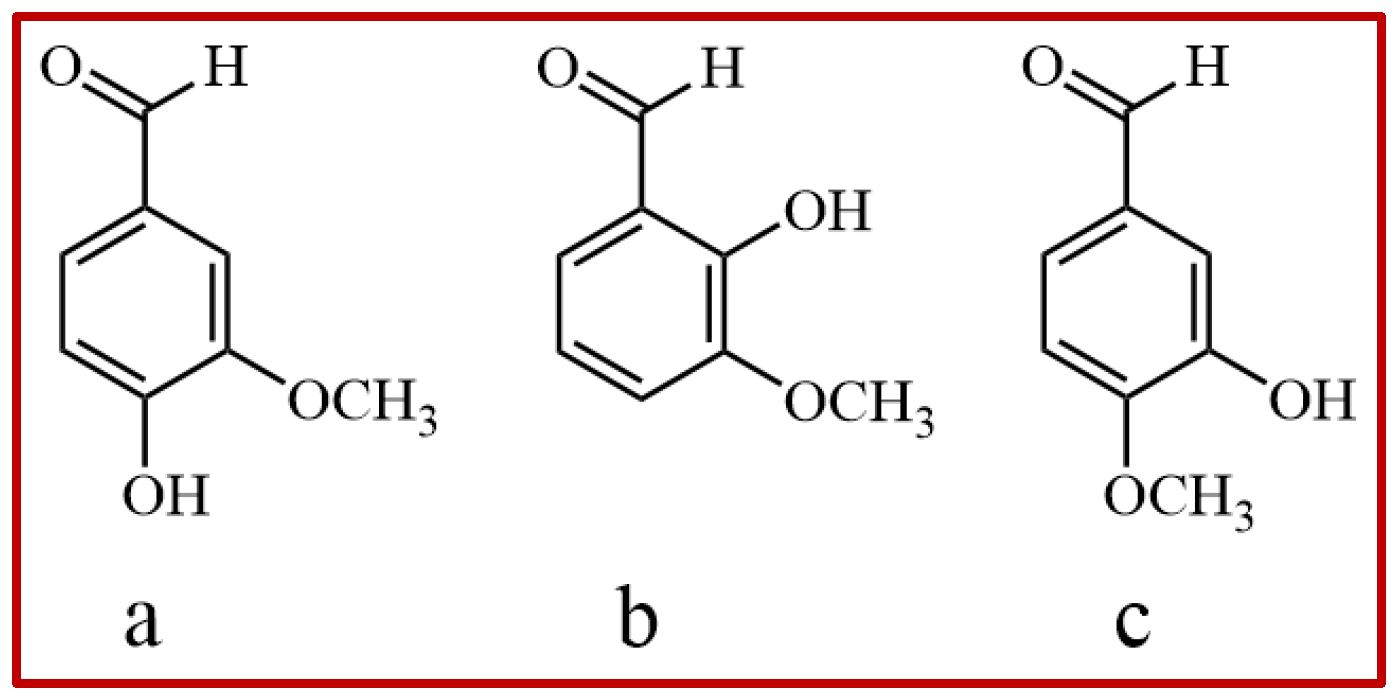
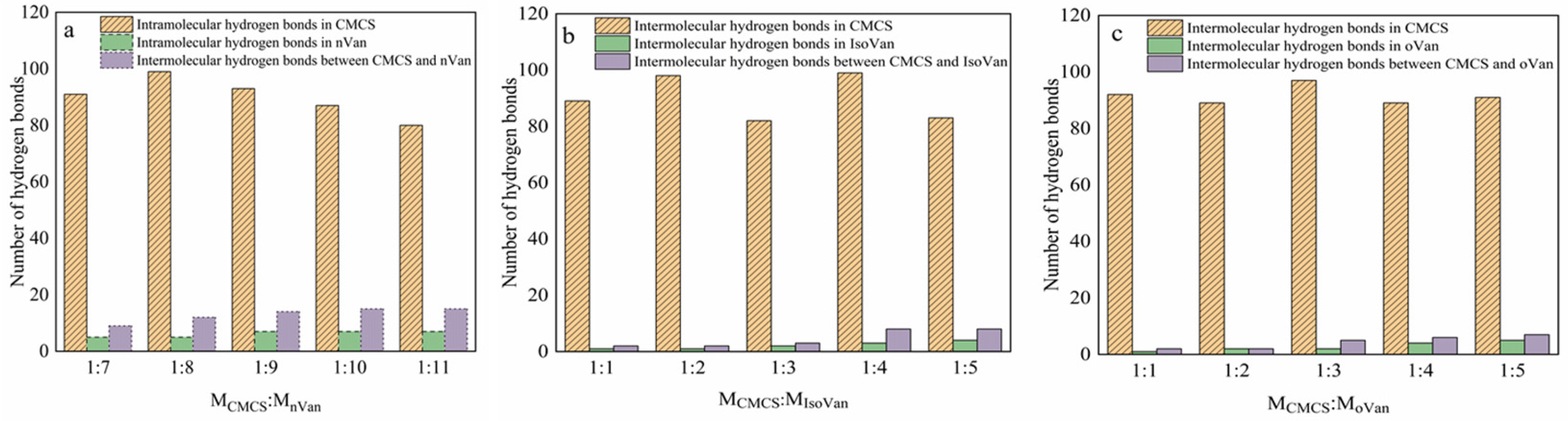
2.1. Hydrogen Bonds from Molecular Simulation
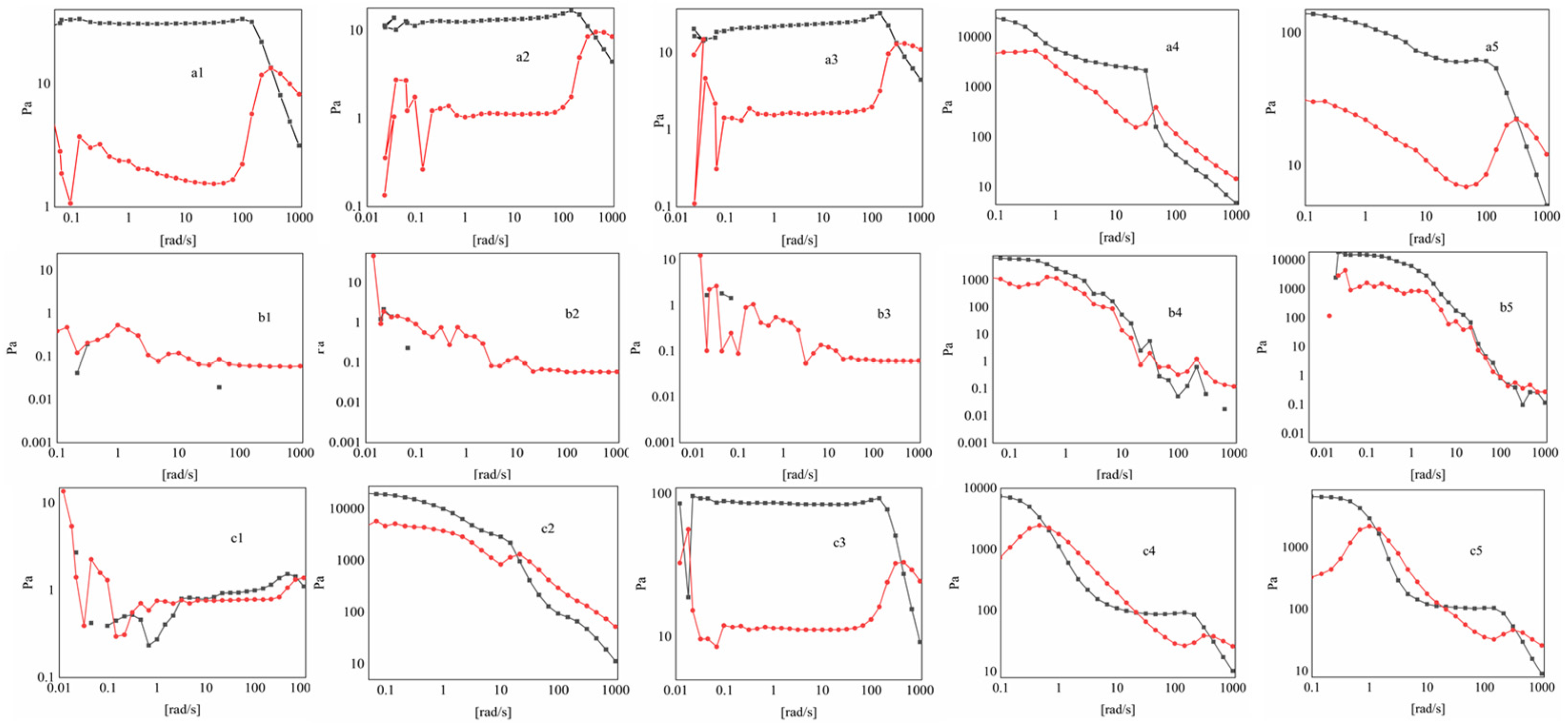
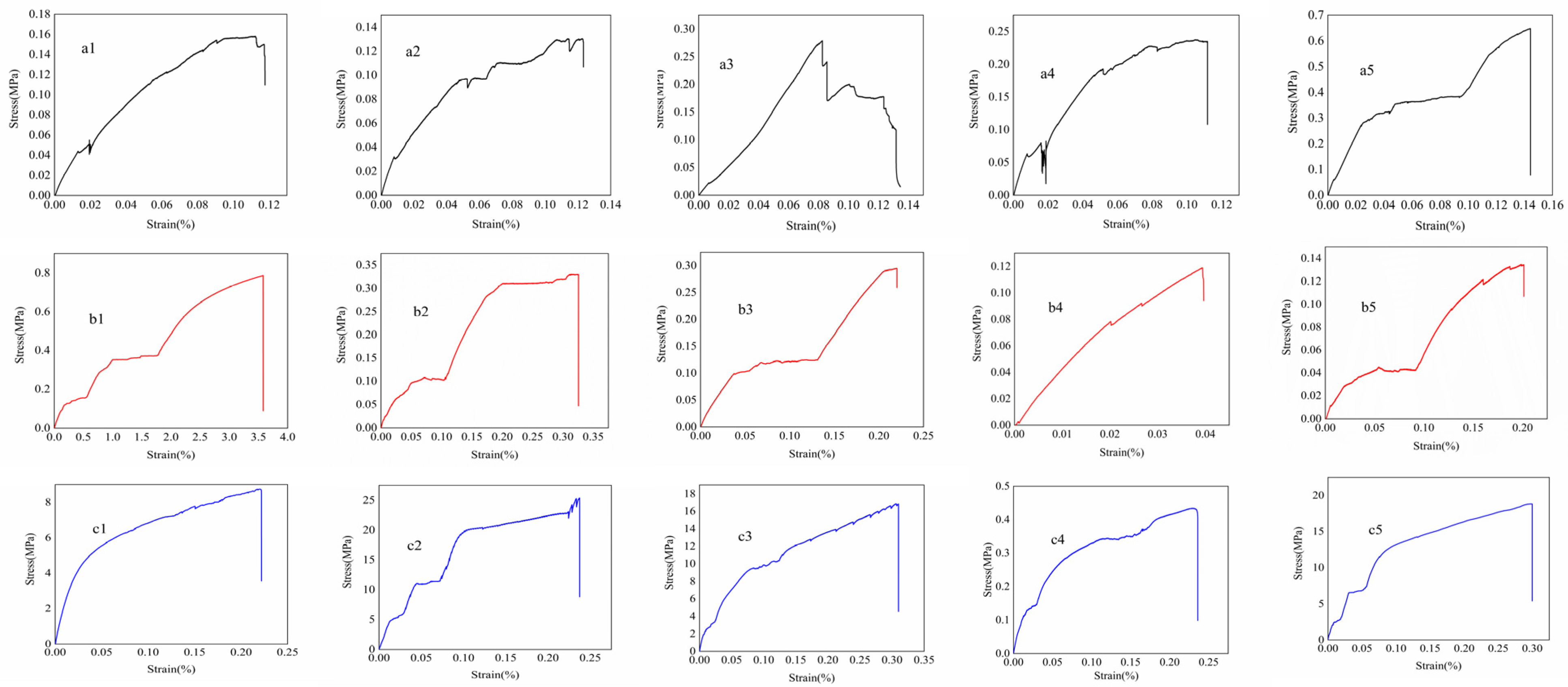
2.2. Transformation of Physical Chemical Performances During Sol–Gel Conversion
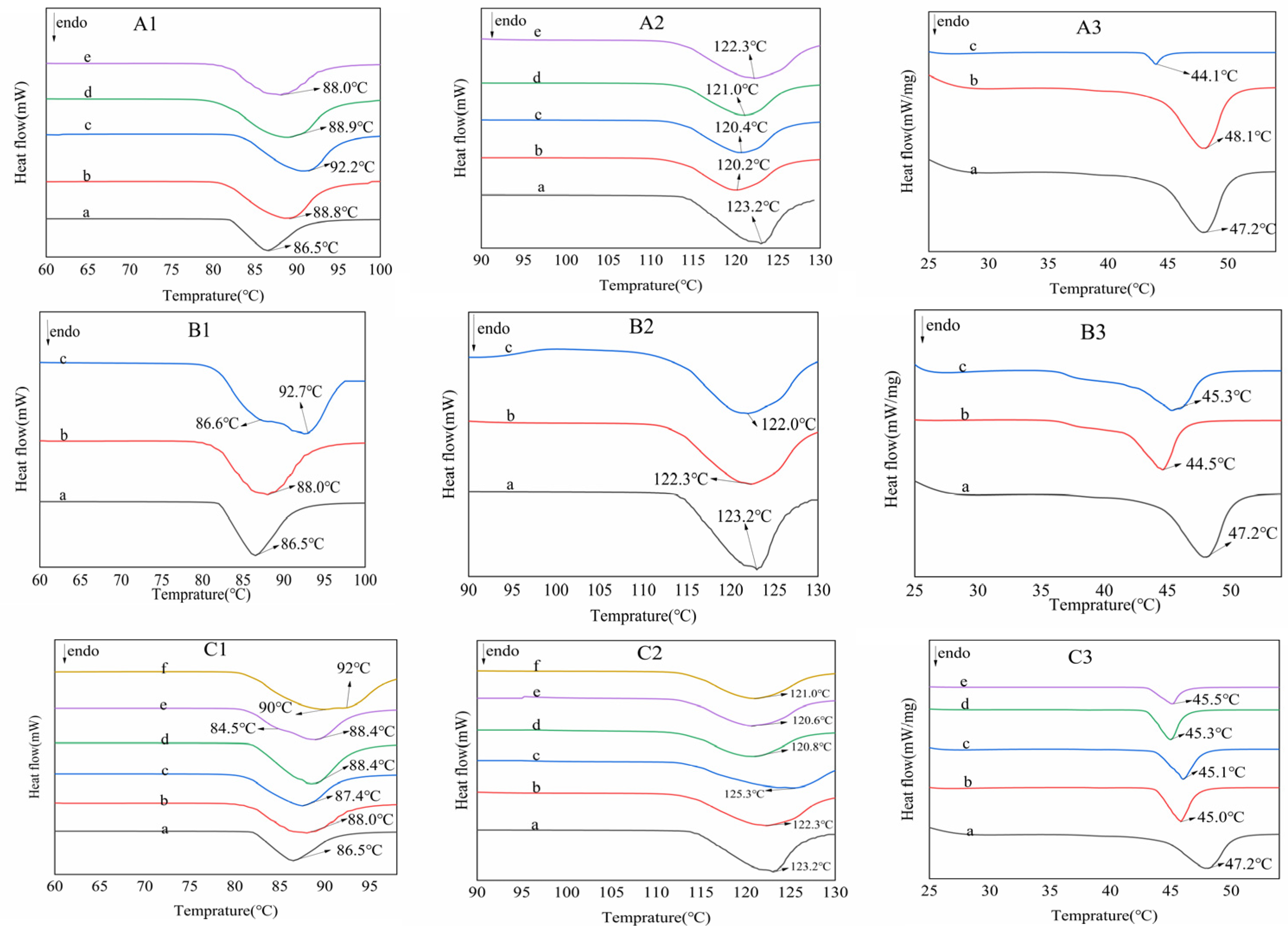
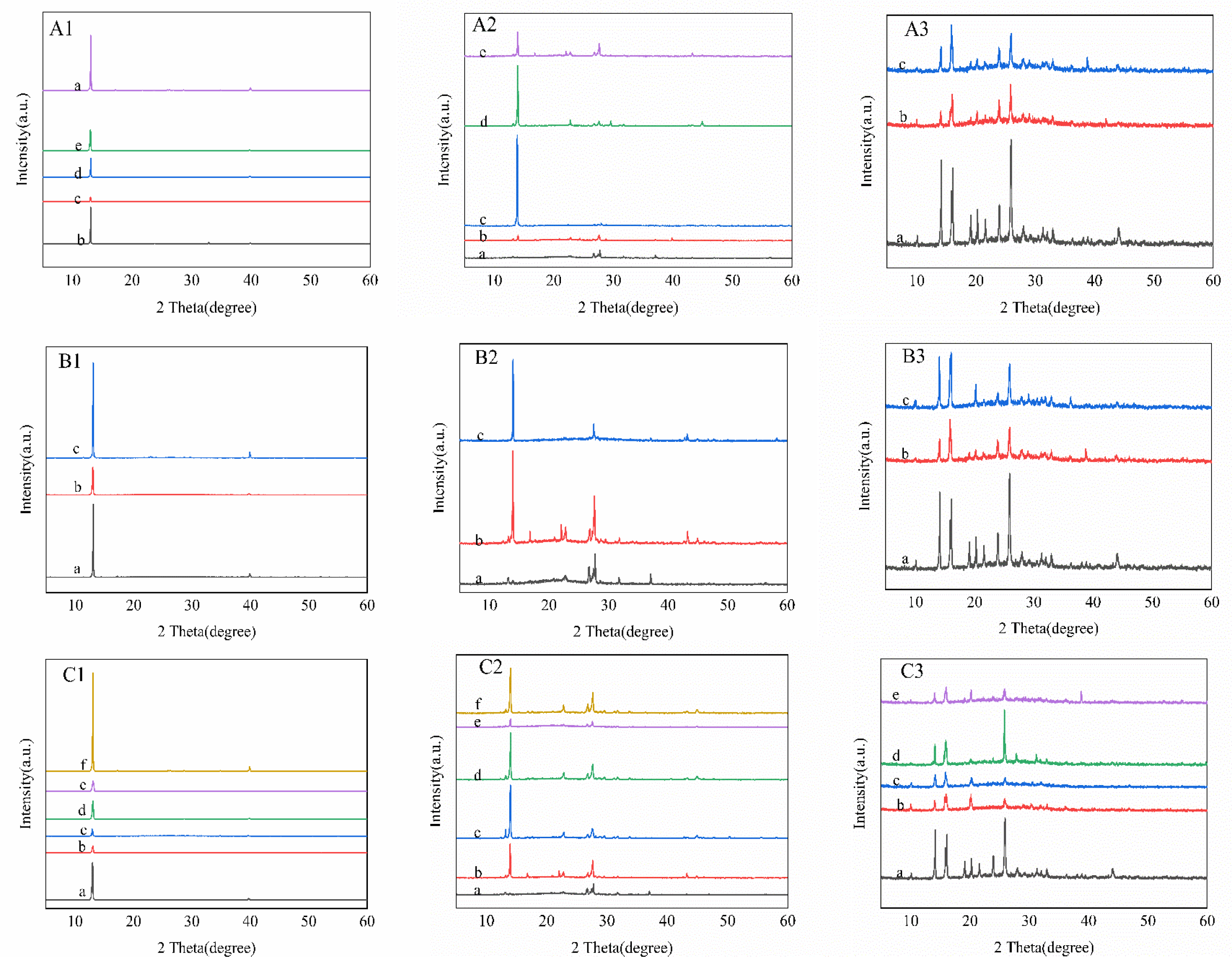
2.3. Crystallization of Vanillin Isomers in CMCS Gels
2.4. Crystal Recovery and Characterization
3. Conclusions
4. Experimental
4.1. Instruments and Reagents
4.1.1. Instruments
4.1.2. Reagents
4.2. Molecular Simulation
4.3. Preparation of CMCS Blank Gels and CMCS/Van Isomer Blend Gels
4.4. Determination of Sol Viscosity
4.5. Measurement of Rheological Properties
4.6. Measurement of Tensile Strength
- TS—tensile strength, MPA;
- Maximum tensile force, N;
- a—width, mm;
- b—thickness, mm.
4.7. Gel Hardness Test
4.8. DSC Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCrone, W.C. Crystallographic data 28. Vanillin I (3-Methoxy-4-hydroxybenzaldehyde. Anal. Chem. 1950, 22, 500. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Meng, X.; Li, L.; Zhou, X.; Li, J.; Bai, S. Environmental profile of natural biological vanillin production via life cycle assessment. J. Clean. Prod. 2021, 308, 127399. [Google Scholar] [CrossRef]
- Swaggerty, L.C.; He, H.; Genovese, J.K.; Callaway, T.R.; Kogut, M.H.; Piva, A.; Grilli, E. A microencapsulated feed additive containing organic acids, thymol, and vanillin increases in vitro functional activity of peripheral blood leukocytes from broiler chicks. Poult. Sci. 2020, 99, 3428–3436. [Google Scholar] [CrossRef]
- Sharma, S.; Pal, R.; Hameed, S.; Fatima, Z. Antimycobacterial mechanism of vanillin involves disruption of cell-surface integrity, virulence attributes, and iron homeostasis. Int. J. Mycobacteriol. 2016, 5, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, Y.; Sun, J.; Li, H.; Huang, M.; Sun, X.; Zhao, M. Elucidation of The Anti-Inflammatory Effect of Vanillin In Lps-Activated THP-1 Cells. J. Food Sci. 2019, 84, 1920–1928. [Google Scholar] [CrossRef]
- Li, G.; Kong, B.; Tong, Q.; LI, Y.; Chen, L.; Zeng, J.; Yu, H.; Xie, X.; Zhang, J. Vanillin downregulates NNMT and attenuates NNMT-related resistance to 5-fluorouracil via ROS-induced cell apoptosis in colorectal cancer cells. Oncol. Rep. 2021, 45, 110. [Google Scholar] [CrossRef]
- Ceballos, A.F.T. Antimicrobial and antioxidant activities of amines derived from vanillin as potential preservatives: Impact of the substituent chain length and polarity. Sustain. Chem. Pharm. 2021, 22, 100471. [Google Scholar]
- Vadivel, M.K.S.K. Pharmacokinetic properties and anti-proliferative mechanisms of vanillin against acute lymphoblastic leukemia (Jurkat) cells. S. Afr. J. Bot. 2021, 142, 82–87. [Google Scholar]
- Cocinero, E.J.; Lesarri, A.; Ecija, P.; Grabow, J.-U.; Fernandeza, J.A.; Castanoa, F. Conformational equilibria in vanillin and ethylvanillin. Phys. Chem. Chem. Phys. 2010, 12, 12486–12493. [Google Scholar] [CrossRef]
- Egawa, T.; Kameyama, A.; Takeuchi, H. Structural determination of vanillin, isovanillin and ethylvanillin by means of gas electron diffraction and theoretical calculations. J. Mol. Struct. 2006, 794, 92–102. [Google Scholar] [CrossRef]
- Furlanetto, M.P.; Sinigaglia, M.; Amaral, V.S.D.; Dihl, R.R.; de Andrade, H.H.R. Effect of Vanillin on Toxicant-Induced Lethality in the Drosophila melanogaster DNA Repair Test. Environ. Mol. Mutagen. 2007, 48, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, V.T.; Mourtzinos, I. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Beaudry, F.; Ross, A.; Lema, P.P.; Vachon, P. Pharmacokinetics of vanillin and its effects on mechanical hypersensitivity in a rat model of neuropathic pain. Phytother. Res. 2010, 24, 525–530. [Google Scholar] [CrossRef]
- Chen, X.-M.; Wei, M.; Zhang, H.-M.; Luo, C.-H.; Chen, Y.-K.; Chen, Y. Effect of vanillin and ethyl vanillin on cytochrome P450 activity in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 1897–1901. [Google Scholar] [CrossRef]
- Siqueira, J.D.; de Pellegrin, S.F.; Fioravanço, L.P.; Fontana, L.A.; Iglesias, B.A.; Chaves, O.A.; Back, D.F. Self-association synthesis with ortho-vanillin to promote mono- and heptanuclear complexes and their evaluation as antioxidant agents. J. Mol. Struct. 2022, 1256, 132480. [Google Scholar] [CrossRef]
- Fadli, K.; Bouchama, A.; Tabbiche, A.; Chiter, C.; Cornia, A.; Kumar, N.; Yahiaoui, M.; Zaidi, F. Anticancer potential of isovanillin-based symmetrical azine: Synthesis, structure, molecular modeling, in silico leukemia inhibition and MD simulation. J. Mol. Struct. 2024, 1312, 138580. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Tol, J.J.B.v.d.; Su, L.; Wang, Y.; Dey, S.; Zijlstra, P.; Fytas, G.; Vantomme, G.; Dankers, P.Y.W.; et al. Supramolecular polymers form tactoids through liquid–liquid phase separation. Nature 2024, 626, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.O.; Steed, J.W. Anion-tuning of supramolecular gel properties. Nat. Chem. 2009, 1, 437–442. [Google Scholar] [CrossRef]
- Singh, N.; Henningsen, T.; Metz, E.; Hamacher, R.; Cumberledge, E.; Hopkins, R.; Mazelsky, R. Solution growth of vanillin single crystals. Cryst. Mater. Lett. 1991, 12, 270–275. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Karuppannan, S. Nucleation control and separation of vanillin polymorphs I and II through the swift cooling crystallization process. CrystEngComm 2021, 23, 1634–1642. [Google Scholar] [CrossRef]
- Parimaladevi, P.; Kavitha, C.; Srinivasan, K. Investigation of the effect of liquid-liquid phase separation (LLPS) on nucleation and different growth stages of vanillin and bulk growth of defect-free single crystals from aqueous solution—A new approach. CrystEngComm 2014, 16, 2565–2569. [Google Scholar] [CrossRef]
- Ouyang, J.; Xing, X.; Chen, J.; Zhou, L.; Liu, Z.; Hen, J.Y.Y. Effects of solvent, supersaturation ratio and silica template on morphology and polymorph evolution of vanillin during swift cooling crystallization. Particuology 2022, 65, 93–104. [Google Scholar] [CrossRef]
- Supriya, S.; Sushmitha, S.; Srinivasan, K. Effective Control of Liquid–Liquid Phase Separation and Nucleation of Vanillin Single Crystals through a Vapor Diffusion Crystallization Process in Selected Solvent Environments. Cryst. Growth Des. 2019, 19, 6315–6323. [Google Scholar] [CrossRef]
- Parker, A.S.; Taylor, L.S.; Beaudoin, S.P. Polymer effects on crystallization at the amorphous atazanavir-water interface. J. Cryst. Growth 2021, 571, 126254. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, A.; Rodrigues, A. Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 1996, 35, 28–36. [Google Scholar] [CrossRef]
- Wu, G.; Heitz, M.; Chornet, E. Improved Alkaline Oxidation Process for the Production of Aldehydes Vanillin and Syringaldehyde) from Steam-Explosion Hardwood Lignin. Ind. Eng. Chern. Res. 1994, 33, 718–723. [Google Scholar] [CrossRef]
- Gomes, E.D.; Rodrigues, A.E. Lignin biorefinery Separation of vanillin, vanillic acid and acetovanillone by adsorption. Sep. Purif. Technol. 2019, 216, 92–101. [Google Scholar] [CrossRef]
- Foster, J.A.; Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.; Steed, J.W. Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef]
- Diao, Y.; Whaley, K.E.; Helgeson, M.E.; Woldeyes, M.A.; Doyle, P.S.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Gel-induced selective crystallization of polymorphs. J. Am. Chem. Soc. 2012, 134, 673–684. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.-J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef]
- Huang, C.; Tang, H.; Huang, X.; Chen, H.; Yang, K.; Yin, Q.; Zhang, L.; Li, X.; Mou, X.; Chen, S.; et al. Ethyl Vanillin Rapid Crystallization from Carboxymethyl Chitosan Ion-Switchable Hydrogels. Gels 2023, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 78, 118993. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.Z.; Wu, Y.-X.; Tao, L.; Zhang, Y.D.; Wang, S.R.; Zhang, G.C.; Zhang, J. Inhibitory effects and mechanisms of vanillin on gray mold and black rot of cherry tomatoes. Pestic. Biochem. Physiol. 2021, 175, 104859. [Google Scholar] [CrossRef]
- Ji, X.; Luo, Y.; Shen, M.; Yang, J.; Ha, X.; Xie, J. Effects of carboxymethyl chitosan on physicochemical, rheological properties and in vitro digestibility of yam starch. Int. J. Biol. Macromol. 2021, 192, 537–545. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Wang, Y.; Meng, Z.; Zhao, Z. A self-healing carboxymethyl chitosan/oxidized carboxymethyl cellulose hydrogel with fluorescent bioprobes for glucose detection. Carbohydr. Polym. 2021, 274, 118642. [Google Scholar] [CrossRef]
- Hu, W.; Cai, T.; Ma, Y.; Hobbs, J.K.; Farrance, O.; Reiter, G. Polymer crystallization under nano-confinement of droplets studied by molecular simulations. Faraday Discuss 2009, 143, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yi, J.; Ma, Y.; Bi, J. The role of amide groups in the mechanism of acid-induced pectin gelation: A potential pH-sensitive hydrogel based on hydrogen bond interactions. Food Hydrocoll. 2023, 141, 108741. [Google Scholar] [CrossRef]
- Ladeira, N.M.B.; Donnici, C.L.; de Mesquita, J.P.; Pereira, F.V. Preparation and characterization of hydrogels obtained from chitosan and carboxymethyl chitosan. J. Polym. Res. 2021, 28, 335. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256, 11759. [Google Scholar] [CrossRef]
- Majumder, S.; Busch, H.; Poudel, P.; Mecking, S.; Reiter, G. Growth Kinetics of Stacks of Lamellar Polymer Crystals. Macromolecules 2018, 51, 8738–8745. [Google Scholar] [CrossRef]
- Robinson, R.A.; Kiang, A.K. The ionization constants of vanillin and two of its isomers. J. Am. Chem. Soc. 1955, 51, 1398–1402. [Google Scholar] [CrossRef]
- Maor, I.; Koifman, N.; Kesselman, E.; Matsanov, P.; Shumilin, I.; Harries, D.; Weitz, I.S. Molecular self-assembly under nanoconfinement: Indigo carmine scroll structures entrapped within polymeric capsules. Nanoscale 2021, 13, 20462–20470. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, S.S. Second harmonic generation ability of vanillin polymorphs I and II. Opt. Laser Technol. 2021, 134, 106667. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lu, X.; Li, H.; Chen, H.; Yin, Q.; Hu, X.; Yang, K.; Yang, F.; Chen, L.; Yang, Z.; et al. Crystallization of Vanillin Isomers in Carboxymethyl Chitosan Gels. Gels 2025, 11, 285. https://doi.org/10.3390/gels11040285
Zhang L, Lu X, Li H, Chen H, Yin Q, Hu X, Yang K, Yang F, Chen L, Yang Z, et al. Crystallization of Vanillin Isomers in Carboxymethyl Chitosan Gels. Gels. 2025; 11(4):285. https://doi.org/10.3390/gels11040285
Chicago/Turabian StyleZhang, Lin, Xiaoling Lu, Hao Li, Hongjie Chen, Qi Yin, Xuehan Hu, Kang Yang, Fang Yang, Liya Chen, Zeng Yang, and et al. 2025. "Crystallization of Vanillin Isomers in Carboxymethyl Chitosan Gels" Gels 11, no. 4: 285. https://doi.org/10.3390/gels11040285
APA StyleZhang, L., Lu, X., Li, H., Chen, H., Yin, Q., Hu, X., Yang, K., Yang, F., Chen, L., Yang, Z., Long, Y., Shen, C., Yao, B., & Huang, C. (2025). Crystallization of Vanillin Isomers in Carboxymethyl Chitosan Gels. Gels, 11(4), 285. https://doi.org/10.3390/gels11040285




