Analysis, Properties, and Applications of Insect-Derived Chitosan: A Sustainable Path to Functional Polysaccharide Materials
Abstract
1. Introduction
2. Chitin and Chitosan—Sources, Properties and Extraction Methods
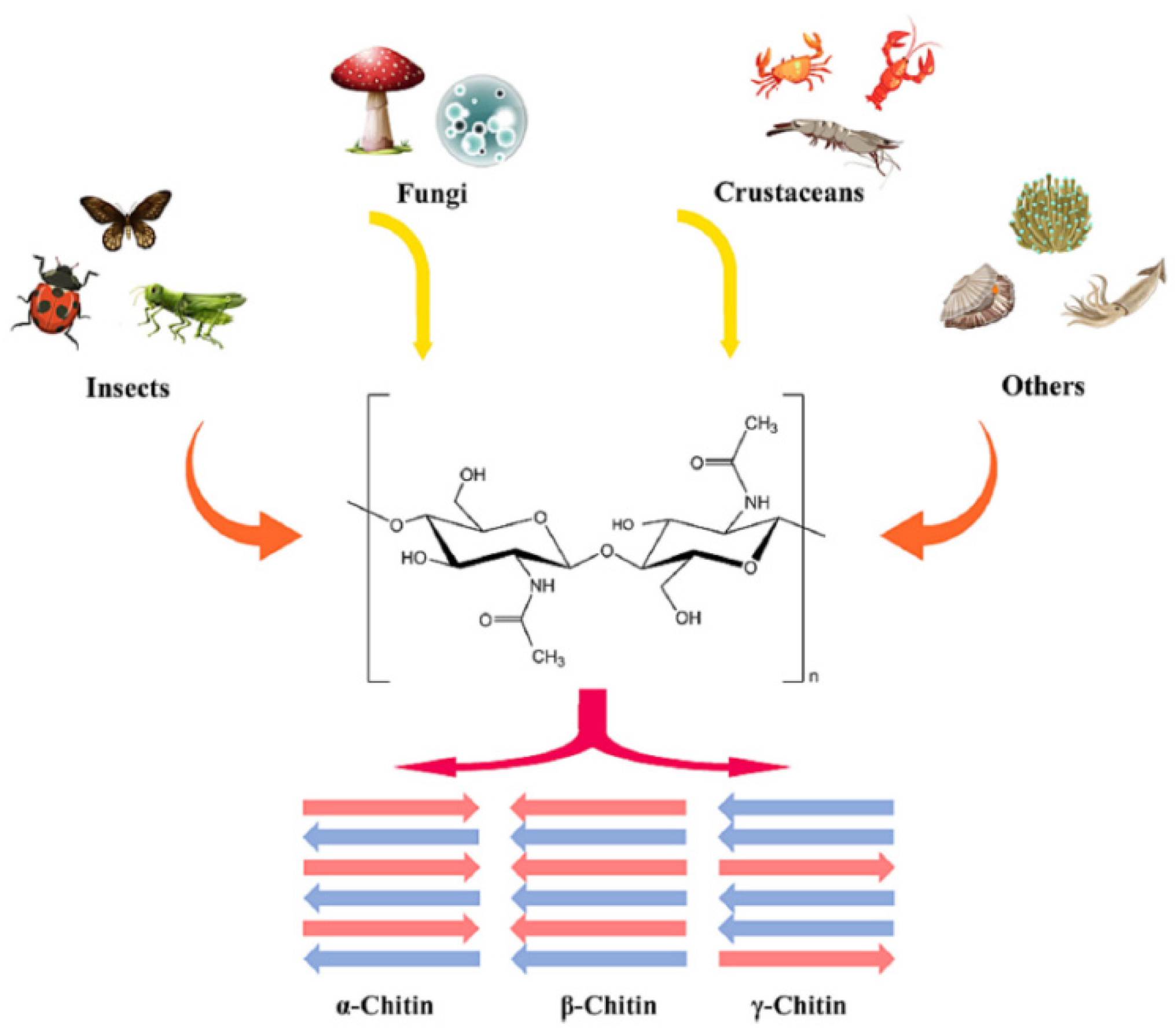
2.1. Occurrence and Structure of Chitin
2.2. Sources and Composition of Chitin
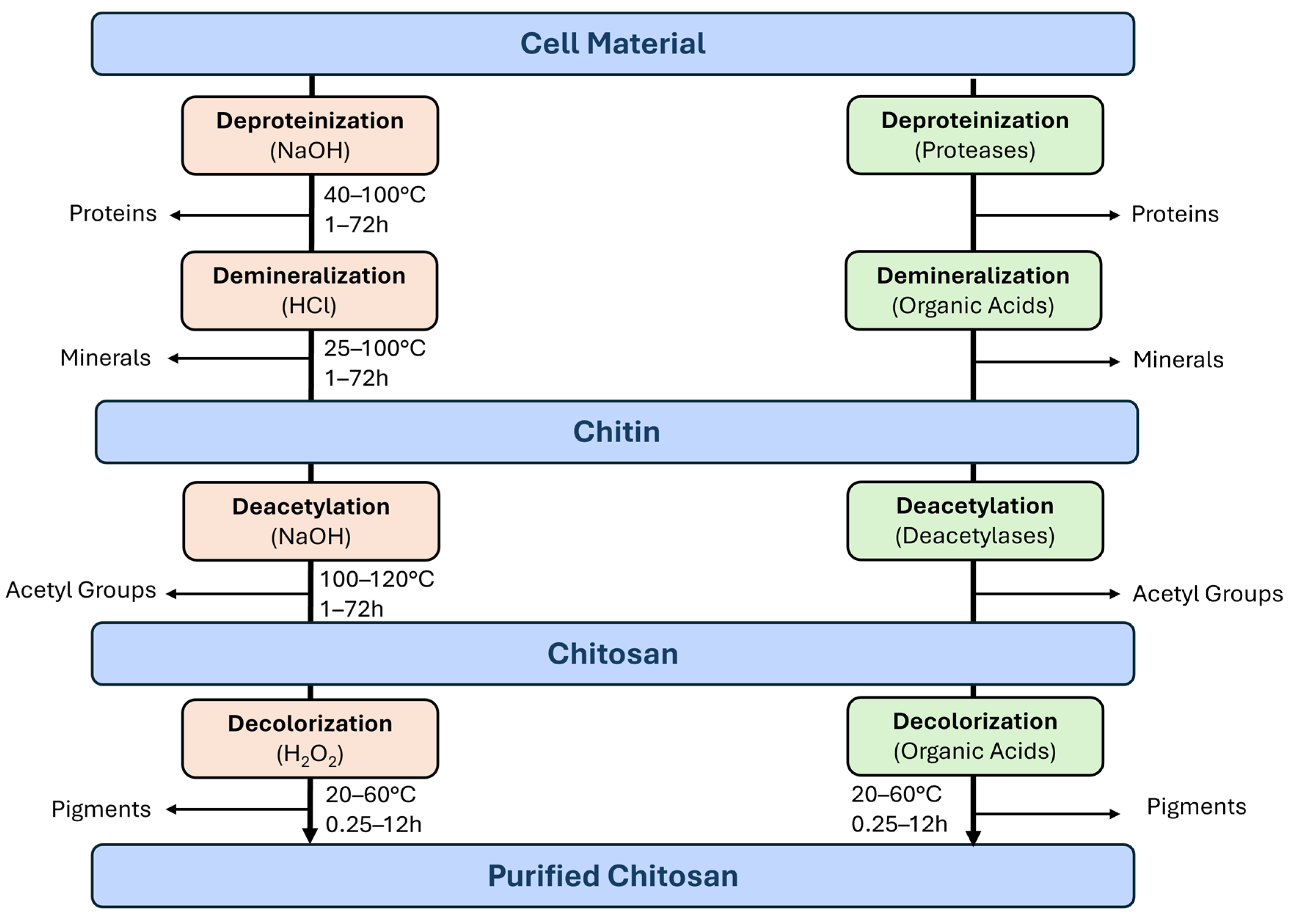
2.3. Purification of Chitin
2.4. Conversion of Chitin to Chitosan: Chemical and Biotechnological Paths
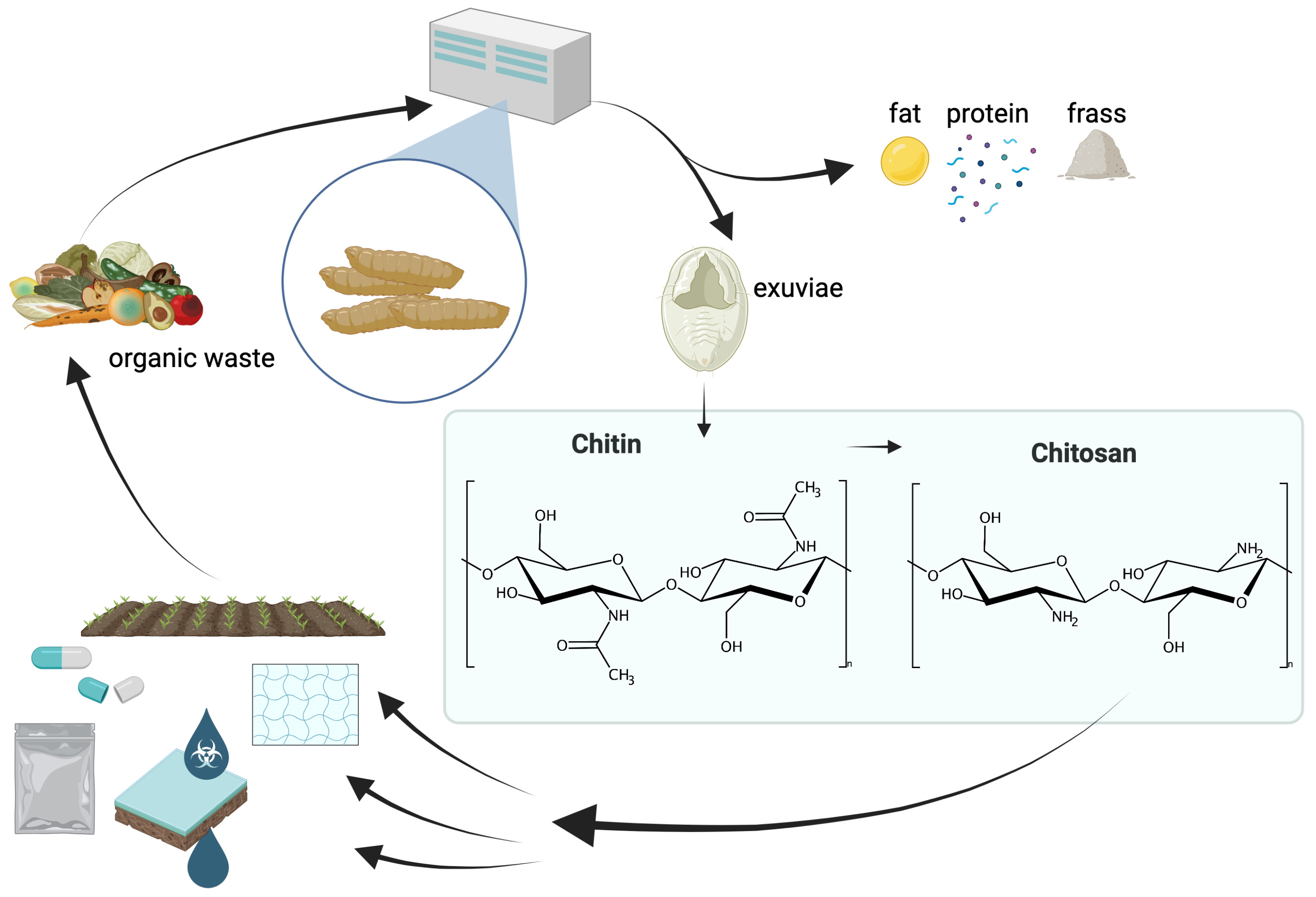
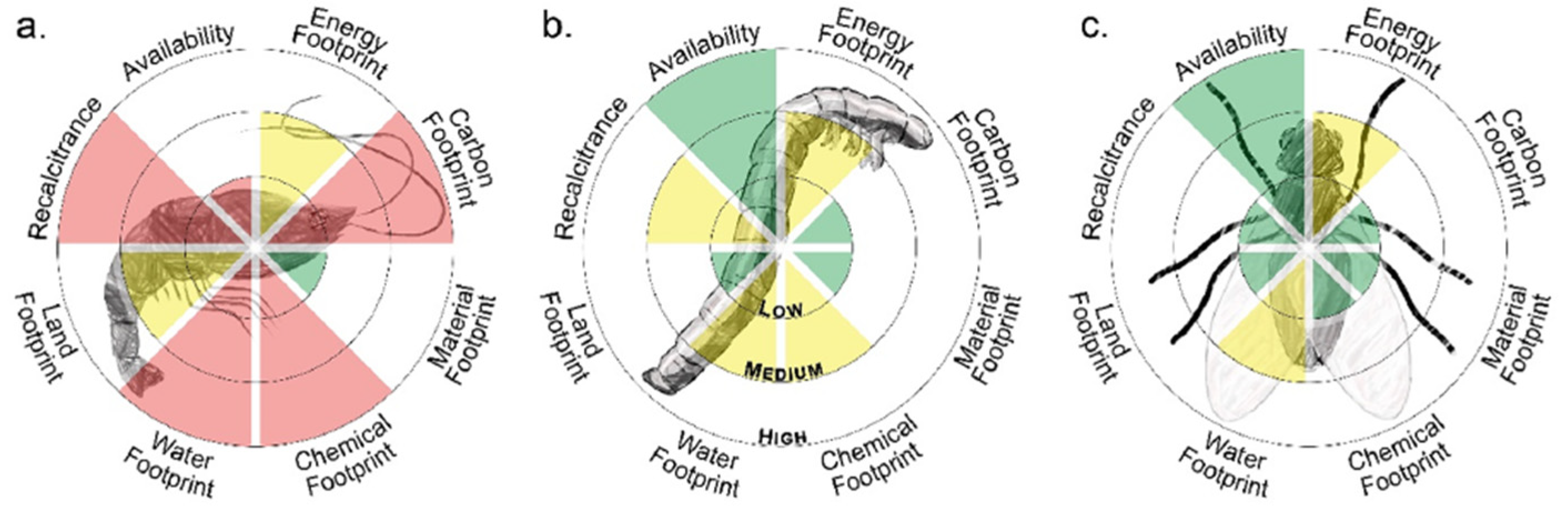
2.5. Insect-Based Chitin as a Sustainable Alternative
3. Analysis of Chitin and Chitosan
3.1. Methods for Quantification
3.1.1. Photometric Methods
3.1.2. Methods Based on Fluorescence
3.1.3. Chromatographic Methods
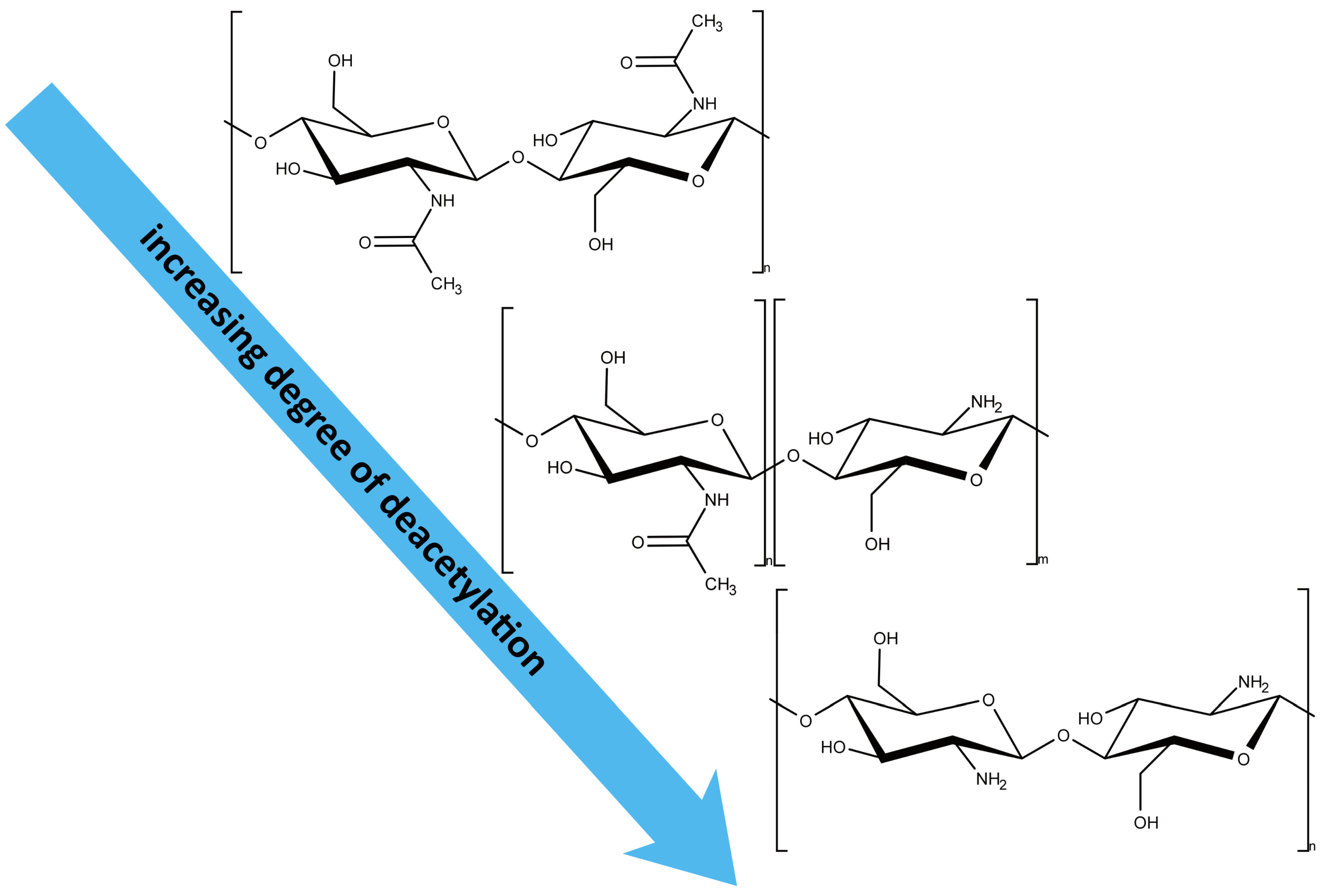
3.2. Determination of Degree of Deacetylation
3.3. Determination of Molecular Weight
4. Application of Chitin and Chitosan Derived from Insects
4.1. Packaging
4.2. Agriculture
4.3. Water Treatment and Absorption
4.4. Pharmaceuticals and Cosmetics
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Hamer, S.N.; Cord-Landwehr, S.; Biarnes, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.M.; Kolkenbrock, S. Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 2015, 5, 8716. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcantara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.u.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.u.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture–Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Naser El Deen, S.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; Fodor, I.; van der Weide, R.; Hoek-van den Hil, E.F.; Rezaei Far, A.; Veldkamp, T. Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae). Insects 2023, 14, 204. [Google Scholar] [CrossRef]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Kuzhir, P.; Godeau, G. Update on Chitin and Chitosan from Insects: Sources, Production, Characterization, and Biomedical Applications. Biomimetics 2024, 9, 297. [Google Scholar] [CrossRef]
- Tepper, K.; Edwards, O.; Sunna, A.; Paulsen, I.T.; Maselko, M. Diverting organic waste from landfills via insect biomanufacturing using engineered black soldier flies (Hermetia illucens). Commun. Biol. 2024, 7, 862. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Schneider, L.; Kisinga, B.; Stoehr, N.; Cord-Landwehr, S.; Schulte-Geldermann, E.; Moerschbacher, B.M.; Eder, K.; Jha, R.; Dusel, G. Dietary Protein Levels in Isoenergetic Diets Affect the Performance, Nutrient Utilization and Retention of Nitrogen and Amino Acids of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae. Insects 2025, 16, 240. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed. Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass-Valoriza. 2020, 12, 4777–4804. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the antimicrobial activity of chitosan. Polímeros Ciênc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Pasquier, E.; Beaumont, M.; Mattos, B.D.; Otoni, C.G.; Winter, A.; Rosenau, T.; Belgacem, M.N.; Rojas, O.J.; Bras, J. Upcycling Byproducts from Insect (Fly Larvae and Mealworm) Farming into Chitin Nanofibers and Films. ACS Sustain. Chem. Eng. 2021, 9, 13618–13629. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Berezina, N. Production and application of chitin. Phys. Sci. Rev. 2016, 1, 20160048. [Google Scholar] [CrossRef]
- Khajavian, M.; Vatanpour, V.; Castro-Munoz, R.; Boczkaj, G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2022, 275, 118702. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Delezuk, J.A.d.M.; Pavinatto, A.; Campana-Filho, S.P. Influence of the process parameters on β-chitin and α-chitin extraction: Probing about the grinding and particles size. Mater. Today Proc. 2019, 14, 722–732. [Google Scholar] [CrossRef]
- Ma, X.; Gozaydin, G.; Yang, H.; Ning, W.; Han, X.; Poon, N.Y.; Liang, H.; Yan, N.; Zhou, K. Upcycling chitin-containing waste into organonitrogen chemicals via an integrated process. Proc. Natl. Acad. Sci. USA 2020, 117, 7719–7728. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J. Facile production of chitin from crab shells using ionic liquid and citric acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of Chitin Hydrogel Under Mild Conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride-malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Bajaj, M.; Freiberg, A.; Winter, J.; Xu, Y.; Gallert, C. Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl. Microbiol. Biotechnol. 2015, 99, 9835–9846. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Varum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Ghormade, V.; Deshpande, M.V. Chitinolytic enzymes: An exploration. Enzym. Microb. Technol. 2000, 26, 473–483. [Google Scholar] [CrossRef]
- Pechsrichuang, P.; Lorentzen, S.B.; Aam, B.B.; Tuveng, T.R.; Hamre, A.G.; Eijsink, V.G.H.; Yamabhai, M. Bioconversion of chitosan into chito-oligosaccharides (CHOS) using family 46 chitosanase from Bacillus subtilis (BsCsn46A). Carbohydr. Polym. 2018, 186, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 45, 72–81. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- van Wisselingh, C. Mikrochemische Untersuchungen über die Zellwände der Fungi. In Jahrbücher Für Wissenschaftliche Botanik; Pfeffer, W., Strasburger, E., Eds.; Verlag von Gebrüder Borntraeger: Berlin, Germany, 1898; Volume 31. [Google Scholar]
- Campbell, F.L. The Detection and Estimation of Insect Chitin; and the Irrelation of “Chitinization” to Hardness and Pigmentation of the Cuticula of the American Cockroach, Periplaneta americana L. Ann. Entomol. Soc. Am. 1929, 22, 401–426. [Google Scholar] [CrossRef]
- Elson, L.A.; Morgan, W.T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem. J. 1933, 27, 1824–1828. [Google Scholar] [CrossRef]
- Morgan, W.T.; Elson, L.A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem. J. 1934, 28, 988–995. [Google Scholar] [CrossRef]
- Zuckerkandl, F.; Messiner-Klebermass, L. Über die Einwirkung von Imingruppen bildender Substanzen auf den Zuckerabbau durch Hefe. Biochem. Zeitrschift 1938, 293, 172–181. [Google Scholar]
- Dische, Z.; Borenfreund, E. A Spectrophotometric Method for the Microdetermination of Hexosamines. J. Biol. Chem. 1950, 184, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Boas, N.F. Method for the Determination of Hexosamines in Tissues. J. Biol. Chem. 1953, 204, 553–563. [Google Scholar] [CrossRef]
- Reissig, J.L.; Strominger, J.L.; Leloir, L.F. A Modified Colorimetric Method for the Estimation of N-Acetylamino Sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [CrossRef]
- Roseman, S.; Daffner, I. Colorimetric Method for Determination of Glucosamine and Galactosamine. Anal. Chem. 1956, 28, 1743–1746. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Exley, D. The Determination of 10–100 μmg. Quantities of Hexosamine. Biochem. J. 1957, 67, 52–60. [Google Scholar] [CrossRef]
- Svennerholm, L. The determination of hexosamines with special reference to nervous tissue. Acta Soc. Med. Ups. 1957, 61, 287–306. [Google Scholar]
- Sawicki, E.; Hauser, T.R.; Stanley, T.W.; Elbert, W. The 3-Methyl-2-benzothiazolone Hydrazone Test. Sensitive New Methods for the Detection, Rapid Estimation, and Determination of Aliphatic Aldehydes. Anal. Chem. 1961, 33, 93–96. [Google Scholar] [CrossRef]
- Ashwell, G.; Brown, N.C.; Volk, W.A. A Colorimetric Procedure for the Determination of N-Acetylated-3-Amino Hexoses. Arch. Biochem. Biophys. 1965, 112, 648–652. [Google Scholar] [CrossRef]
- Galambos, J.T.; Shapira, R. Spectrophotometric assay for hexosamines. Anal. Biochem. 1966, 15, 334–340. [Google Scholar] [CrossRef]
- Ride, J.P.; Drysdale, R.B. A chemical method for estimating Fusarium oxysporum f. lycopersici in infected tomato plants. Physiol. Plant Pathol. 1971, 1, 409–420. [Google Scholar] [CrossRef]
- Ride, J.P.; Drysdale, R.B. A rapid method for the chemical estimation of filamentous fungi in plant tissue. Physiol. Plant Pathol. 1972, 2, 7–15. [Google Scholar] [CrossRef]
- Lehmann, P.F.; White, L.O. Chitin Assay Used to Demonstrate Renal Localization and Cortisone-Enhanced Growth of Aspergillus fumigatus Mycelium in Mice. Infect. Immun. 1975, 12, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Kinoshita, T.; Hoshino, M. Analytical chemical studies on amino sugars. II. Determination of hexosamines using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Chem. Pharm. Bull. 1969, 17, 1505–1510. [Google Scholar] [CrossRef]
- Honda, S.; Nishimura, Y.; Chiba, H.; Kakehi, K. Determination Of Carbohydrates By Condensation With 3-Methyl-2-Benzothiazolinonehydrazone. Anal. Chim. Acta 1981, 131, 293–296. [Google Scholar] [CrossRef]
- Smith, R.L.; Gilkerson, E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal. Biochem. 1979, 98, 478–480. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Pacovsky, R.S.; Brown, M.S. Measurement of Mycorrhizal Infection in Soybeans. Soil Scl. Soc. Am. J. 1981, 45, 871–875. [Google Scholar] [CrossRef]
- Gurusiddaiah, S. A modified technique for the determination of fungal mass in decayed wood. Can. J. For. Res. 1978, 8, 486–490. [Google Scholar] [CrossRef]
- Wu, L.-C.; Stahmann, M.A. Chromatographic Estimation of Fungal Mass in Plant Materials. Phytopathology 1975, 65, 1032–1034. [Google Scholar] [CrossRef]
- Hubbard, J.D.; Seitz, L.M.; Mohr, H.E. Determination of Hexosamines in Chitin by Ion-Exchange Chromatography. J. Food Sci. 1979, 44, 1552–1553. [Google Scholar] [CrossRef]
- Zamani, A.; Jeihanipour, A.; Edebo, L.; Niklasson, C.; Taherzadeh, M.J. Determination of Glucosamine and N-Acetyl Glucosamine in Fungal Cell Walls. J. Agric. Food Chem. 2008, 56, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Vilariro, A.; Schoepp, H.; Arines, J. Chitin and Ergosterol Content of Extraradical and Intraradical Mycelium of the Vesicular-Arbuscular Mycorrhizal Fungus Glomus intraradices. Soil Biol. Biochem. 1994, 26, 711–717. [Google Scholar] [CrossRef]
- Matcham, S.E.; Jordan, B.R.; Wood, D.A. Estimation of fungal biomass in a solid substrate by three independent methods. Appl. Microbiol. Biotechnol. 1985, 21, 108–112. [Google Scholar] [CrossRef]
- Hepper, C.M. A Colorimetric Method Vesicular-Arbuscular for Estimating Mycorrhizal Infection in Roots. Soil Biol. Biochem. 1977, 9, 15–18. [Google Scholar] [CrossRef]
- Plassard, C.S.; Mousain, D.G.; Salsac, L.E. Estimation of mycelial growth of basidiomycetes by means of chitin determination. Phytochemistry 1982, 21, 345–348. [Google Scholar] [CrossRef]
- Hackman, R.H.; Goldberg, M. A Method for Determinations of Microgram Amounts of Chitin in Athropod Cuticles. Anal. Biochem. 1981, 110, 277–280. [Google Scholar] [CrossRef]
- Chen, G.C.; Johnson, B.R. Improved Colorimetric Determination of Cell Wall Chitin in Wood Decay Fungi. Appl. Environ. Microbiol. 1983, 46, 13–16. [Google Scholar] [CrossRef]
- Nitschke, J.; Altenbach, H.J.; Malolepszy, T.; Molleken, H. A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr. Res. 2011, 346, 1307–1310. [Google Scholar] [CrossRef]
- Francois, J.M. A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat. Protoc. 2006, 1, 2995–3000. [Google Scholar] [CrossRef]
- Sujeetha, M.; Sharmila, S.; Jayanthi, J.; Ragunathan, M. Quantitative and Qualitative Analysis of Chitin and Chitosan from the Shell of the Mud Crab, Scylla Serrata (Forskal, 1775). Int. J. Pharm. Ther. 2015, 6, 69–72. [Google Scholar]
- Katano, H.; Takakuwa, M.; Hayakawa, H.; Kimoto, H. Determination of Chitin Based on the Colorimetric Assay of Glucosamine in Acidic Hydrolysate. Anal. Sci. 2016, 32, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.M.; Renner, C.; Strigl, M.G.; Fritz, M. A Simple and Reliable Method for the Determination and Localization of Chitin in Abalone Nacre. Chem. Mater. 2002, 14, 3252–3259. [Google Scholar] [CrossRef]
- Nakata, R.; Yoshinaga, N.; Teraishi, M.; Okumoto, Y.; Mori, N. An easy, inexpensive, and sensitive method for the quantification of chitin in insect peritrophic membrane by image processing. Biosci. Biotechnol. Biochem. 2019, 83, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, K.Y. Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem. Mol. Biol. 2006, 36, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Rezende, G.L.; Martins, A.J.; Gentile, C.; Farnesi, L.C.; Pelajo-Machado, M.; Peixoto, A.A.; Valle, D. Embryonic desiccation resistance in Aedes aegypti: Presumptive role of the chitinized serosal cuticle. BMC Dev. Biol. 2008, 8, 82. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Brito, J.M.; Linss, J.G.; Pelajo-Machado, M.; Valle, D.; Rezende, G.L. Physiological and morphological aspects of Aedes aegypti developing larvae: Effects of the chitin synthesis inhibitor novaluron. PLoS ONE 2012, 7, e30363. [Google Scholar] [CrossRef]
- Bahmed, K.; Quiles, F.; Bonaly, R.; Coulon, J. Fluorescence and Infrared Spectrometric Study of Cell Walls from Candida, Kluyveromyces, Rhodotorula and Schizosaccharomyces Yeasts in Relation with Their Chemical Composition. Biomacromolecules 2003, 4, 1763–1772. [Google Scholar] [CrossRef]
- Flaven-Pouchon, J.; Moussian, B. Fluorescent Microscopy-Based Detection of Chitin in Intact Drosophila melanogaster. Front Physiol. 2022, 13, 856369. [Google Scholar] [CrossRef]
- Henriques, B.S.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Determination of Chitin Content in Insects: An Alternate Method Based on Calcofluor Staining. Front Physiol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Maeda, M.; Kinoshita, T.; Tsuji, A. A Novel Fluorophotometric Method for Determiation of Hexosamines Using Pyridoxal and Zinc(II) Ion. Anal. Biochem. 1970, 38, 121–129. [Google Scholar] [CrossRef]
- Radhakrishnamurthy, B.; Dalferes, E.R., Jr.; Berenson, G.S. Determination of hexosamines by gas-liquid chromatography. Anal. Biochem. 1966, 17, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hase, S.; Matsushima, Y. Amino Sugar Analysis by Gas-liquid Chromatography. J. Biochem. 1969, 66, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bierstedt, A.; Stankiewicz, A.; Briggs, D.E.G.; Evershed, R.P. Quantitative and qualitative analysis of chitin in fossil arthropods using a combination of colorimetric assay and pyrolysis–gas chromatography–mass spectrometry. Analyst 1998, 123, 139–145. [Google Scholar] [CrossRef]
- Ekblad, A.; Näsholm, T. Determination of chitin in fungi and mycorrhizal roots by an improved HPLC analysis of glucosamine. Plant Soil 1996, 178, 29–35. [Google Scholar] [CrossRef]
- Zhu, X.; Cai, J.; Yang, J.; Su, Q. Determination of glucosamine in impure chitin samples by high-performance liquid chromatography. Carbohydr. Res. 2005, 340, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.O.; Martinez, M.V.; Hernandez, J.L.; Lage Yusty, M.A. High-performance liquid chromatographic determination of chitin in the snow crab, Chionoecetes opilio. J. Chromatogr. A 2006, 1116, 189–192. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Delgado-Rosas, K.E. Quantitation of Glucosamine From Shrimp Waste Using HPLC. J. Chromatogr. Sci. 2007, 45, 195–199. [Google Scholar] [CrossRef]
- Sulc, M. Chitin quantitation (as glucosamine) in food raw materials by HPLC-C18-DAD with off-line derivatization. MethodsX 2024, 12, 102729. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Waszkuc, T.; Mohammed, F. Determination of Glucosamine in Raw Materials and Dietary Supplements Containing Glucosamine Sulfate and/or Glucosamine Hydrochloride by High-Performance Liquid Chromatography with FMOC-Su Derivatization: Collaborative Study. J AOAC Int. 2005, 88, 1048–1058. [Google Scholar] [CrossRef]
- Han, X.; Heinonen, M. Development of ultra-high performance liquid chromatographic and fluorescent method for the analysis of insect chitin. Food Chem. 2021, 334, 127577. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical Physicochemical Behaviors of Chitosan in Aqueous Solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bo, S.; Li, S.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbriéres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1980, 2, 115–116. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Taylor, K.D.A.; Roberts, G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of Degree Of Deacetylation of Chitosan—Comparision of Methods. Prog. Chem. Appl. Chitin Deriv. 2012, 27, 5–20. [Google Scholar]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Kadak, A.E. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 1144–1148. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrières, J.; Rhazi, M.; Alagui, A.; Vincendon, M.; Vottero, P. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer 2000, 41, 2463–2469. [Google Scholar] [CrossRef]
- Amamou, O.; Kefil, S.; Denis, J.P.; Boubaker, T.; Cardinal, S. Revisiting the Determination of the Degree of Deacetylation Using Potentiometric Titration: A New Equation for Modified Chitosan. Molecules 2024, 29, 2962. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; Escarcega-Galaz, A.A.; Campas-Baypoli, O.N.; Martinez-Ibarra, D.M.; Rascon-Leon, S. Measurement of the degree of deacetylation in chitosan films by FTIR, (1)H NMR and UV spectrophotometry. MethodsX 2024, 12, 102583. [Google Scholar] [CrossRef]
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field n.m.r. spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Desbrières, J.; Heux, L.; Mazeau, K.; Rinaudo, M. Overview on structural characterization of chitosan molecules in relation with their behavior in solution. Macromol. Symp. 2001, 168, 1–20. [Google Scholar] [CrossRef]
- Heux, L.; Brugnerotto, J.; Desbrieres, J.; Versali, M.F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef]
- Kohlhoff, M.; Niehues, A.; Wattjes, J.; Beneteau, J.; Cord-Landwehr, S.; El Gueddari, N.E.; Bernard, F.; Rivera-Rodriguez, G.R.; Moerschbacher, B.M. Chitinosanase: A fungal chitosan hydrolyzing enzyme with a new and unusually specific cleavage pattern. Carbohydr. Polym. 2017, 174, 1121–1128. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Ihmor, P.; Niehues, A.; Luftmann, H.; Moerschbacher, B.M.; Mormann, M. Quantitative Mass-Spectrometric Sequencing of Chitosan Oligomers Revealing Cleavage Sites of Chitosan Hydrolases. Anal. Chem. 2017, 89, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, E. Studien zur Praxis der Bestimmung des Molekulargewichts aus Dampfdruckerniedrigungen. Z. Phys. Chem. 1889, 4U, 532–552. [Google Scholar] [CrossRef]
- Klages, F.; Möhler, K. Molekulargewichts-bestimmung aus der Dampfdruckerniedrigung) (IV, Mitteil. über das anomale osmotische Verhalten von Kettenmolekeln). Chem. Berichte 1951, 84, 56–67. [Google Scholar] [CrossRef]
- Rast, K. Eine Verbesserung der Bargerschen Methode der Molekulargewichts-Bestimmung. Berichte Dtsch. Chem. Ges. 1921, 54, 1979–1987. [Google Scholar] [CrossRef]
- Elias, H.G. Bestimmung des Molekulargewichts in der Ultrazentrifuge nach dem Archibald-Verfahren. Angew. Chem. 1961, 73, 209–215. [Google Scholar] [CrossRef]
- Wu, A.C.M. Determination of Molecular-Weight Distribution of Chitosan by High-Performance Liquid Chromatography. Methods Enzymol. 1988, 128, 87–99. [Google Scholar]
- Lavertu, M.; Methot, S.; Tran-Khanh, N.; Buschmann, M.D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 2006, 27, 4815–4824. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Chen, R.H.; Hwa, H.-D. Effect of molecular weight of chitosan with the same degree of deacetylation on the thermal, mechanical, and permeability properties of the prepared membrane. Carbohydr. Polym. 1996, 29, 353–358. [Google Scholar] [CrossRef]
- Arcidiacono, S.; Kaplan, D.L. Molecular weight distribution of chitosan isolated from Mucor rouxii under different culture and processing conditions. Biotechnol. Bioeng. 1992, 39, 281–286. [Google Scholar] [CrossRef]
- Chang, K.L.; Tai, M.C.; Cheng, F.H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food. Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Miura, Y.; Kaneko, H.; Nishimura, S.-I.; Nishi, N.; Iwase, M.; Tokura, S. Molecular Weight Dependency of Antimicrobial Activity By Chitosan Oligomers; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Prasertsung, I.; Damrongsakkul, S.; Terashima, C.; Saito, N.; Takai, O. Preparation of low molecular weight chitosan using solution plasma system. Carbohydr. Polym. 2012, 87, 2745–2749. [Google Scholar] [CrossRef]
- Xuan Du, D.; Xuan Vuong, B. Study on Preparation of Water-Soluble Chitosan with Varying Molecular Weights and Its Antioxidant Activity. Adv. Mater. Sci. Eng. 2019, 2019, 8781013. [Google Scholar] [CrossRef]
- Chou, C.M.; Mi, F.L.; Horng, J.L.; Lin, L.Y.; Tsai, M.L.; Liu, C.L.; Lu, K.Y.; Chu, C.Y.; Chen, Y.T.; Lee, Y.A.; et al. Characterization and toxicology evaluation of low molecular weight chitosan on zebrafish. Carbohydr. Polym. 2020, 240, 116164. [Google Scholar] [CrossRef]
- Draczynski, Z. Honeybee corpses as an available source of chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Yomota, C.; Miyazaki, T.; Okada, S. Determination of the viscometric constants for chitosan and the application of universal calibration procedure in its gel permeation chromatography. Colloid Polym. Sci. 1993, 271, 76–82. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic viscosity-molecular weight relationship for chitosan. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Lalitha, R.G.; Tharanathan, R.N. Low molecular weight chitosans: Preparation with the aid of papain and characterization. Biochim. Biophys. Acta 2004, 1670, 137–146. [Google Scholar] [CrossRef]
- Tajik, H.; Moradi, M.; Rohani, S.M.; Erfani, A.M.; Jalali, F.S. Preparation of chitosan from brine shrimp (Artemia urmiana) cyst shells and effects of different chemical processing sequences on the physicochemical and functional properties of the product. Molecules 2008, 13, 1263–1274. [Google Scholar] [CrossRef]
- Erdogan, S.; Kaya, M. High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. Int. J. Biol. Macromol. 2016, 89, 118–126. [Google Scholar] [CrossRef]
- Di Nardo, T.; Hadad, C.; Nguyen Van Nhien, A.; Moores, A. Synthesis of high molecular weight chitosan from chitin by mechanochemistry and aging. Green Chem. 2019, 21, 3276–3285. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, J.; Wei, Y.; Hong, X. Preparation of chitooligosaccharides by the enzymatic hydrolysis of chitosan. Polym. Degrad. Stab. 2009, 94, 1895–1899. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Chen, Y.; Xiong, S.; Wang, G.; Chen, J.; Yang, G. A novel strategy for MALDI-TOF MS analysis of small molecules. J. Am. Soc. Mass Spectrom. 2010, 21, 154–160. [Google Scholar] [CrossRef]
- Chae, K.S.; Shin, C.S.; Shin, W.S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci. Biotechnol. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Madhuprakash, J.; El Gueddari, N.E.; Moerschbacher, B.M.; Podile, A.R. Production of bioactive chitosan oligosaccharides using the hypertransglycosylating chitinase-D from Serratia proteamaculans. Bioresour. Technol. 2015, 198, 503–509. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential oils encapsulated in chitosan microparticles against Candida albicans biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef]
- Chaves, A.V.; Freire, R.M.; Feitosa, V.P.; Ricardo, N.M.P.S.; Denardin, J.C.; Andrade Neto, D.M.; Fechine, P.B.A. Hydroxyapatite-Based Magnetic Bionanocomposite as Pharmaceuticals Carriers in Chitosan Scaffolds. J. Compos. Sci. 2021, 5, 37. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Kaczor, M.; Bulak, P.; Proc-Pietrycha, K.; Kirichenko-Babko, M.; Bieganowski, A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas-Review. Biology 2022, 12, 25. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2023, 20, 6667. [Google Scholar] [CrossRef]
- Witono, J.R.; Setyadi, F.F.; Deandra, P.P.; Wanta, K.C.; Miryanti, A.; Santoso, H.; Astuti, D.A.; Bulin, C.D.Q.M. A Comprehensive Analysis of Chitin Extraction from the Black Soldier Fly for Chitosan Production. Period. Polytech. Chem. Eng. 2024, 68, 507–522. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Greunen, L.v.; Zeiri, A.; Yudhistira, B.; Ahmad, A.; Monnye, M. The potential of chitin and chitosan from dead black soldier fly (BSF) (Hermetia illucens) for biodegradable packaging material—A critical review. Process Saf. Environ. Prot. 2024, 189, 1342–1367. [Google Scholar] [CrossRef]
- Le, T.M.; Tran, C.L.; Nguyen, T.X.; Duong, Y.H.P.; Le, P.K.; Tran, V.T. Green Preparation of Chitin and Nanochitin from Black Soldier Fly for Production of Biodegradable Packaging Material. J. Polym. Environ. 2023, 31, 3094–3105. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Ianniciello, D.; Scieuzo, C.; Salvia, R.; Hahn, T.; Zibek, S.; Falabella, P. Usage of chitosan from Hermetia illucens as a preservative for fresh Prunus species fruits: A preliminary analysis. Chem. Biol. Technol. Agric. 2023, 10, 101. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275, 112924. [Google Scholar] [CrossRef]
- Tafi, E.; Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Salvia, R.; Scieuzo, C.; Ranieri, A.; Castagna, A.; Lepuri, S.; Hahn, T.; et al. Preliminary investigation on the effect of insect-based chitosan on preservation of coated fresh cherry tomatoes. Sci. Rep. 2023, 13, 7030. [Google Scholar] [CrossRef]
- Bulak, P.; Proc-Pietrycha, K.; Kaczor, M.; Zlotko, K.; Polakowski, C.; Wiacek, D.; Waniak-Nowicka, H.; Zieba, E.; Wasko, A.; Oleszczuk, P.; et al. A novel type of biochar from chitinous Hermetia illucens waste with a built-in stimulating effect on plants and soil arthropods. Sci. Rep. 2023, 13, 8306. [Google Scholar] [CrossRef]
- Kemboi, V.J.; Kipkoech, C.; Njire, M.; Were, S.; Lagat, M.K.; Ndwiga, F.; Wesonga, J.M.; Tanga, C.M. Biocontrol Potential of Chitin and Chitosan Extracted from Black Soldier Fly Pupal Exuviae against Bacterial Wilt of Tomato. Microorganisms 2022, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Zlotko, K.; Wasko, A.; Kaminski, D.M.; Budziak-Wieczorek, I.; Bulak, P.; Bieganowski, A. Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption. Polymers 2021, 13, 818. [Google Scholar] [CrossRef] [PubMed]
- Elouali, S.; Dahbane, O.; Ait Hamdan, Y.; Eladlani, N.; Rhazi, M. Exploring the potential use of chitosan derived from Hermetia illucens waste for olive oil mill wastewater treatment. Euro-Mediterr. J. Environ. Integr. 2024, 9, 2095–2107. [Google Scholar] [CrossRef]
- Ben Amor, I.; Hemmami, H.; Laouini, S.E.; Zeghoud, S.; Benzina, M.; Achour, S.; Naseef, A.; Alsalme, A.; Barhoum, A. Use of Insect-Derived Chitosan for the Removal of Methylene Blue Dye from Wastewater: Process Optimization Using a Central Composite Design. Materials 2023, 16, 5049. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, K.; Jamieson, W.D.; Droge, M.; Mailander, V.; Jenkins, A.T.A.; Weiss, C.K.; Landfester, K. Advanced dextran based nanogels for fighting Staphylococcus aureus infections by sustained zinc release. J. Mater. Chem. B 2014, 2, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, K.; Marsico, F.; Koynov, K.; Landfester, K.; Weiss, C.K.; Wurm, F.R. Selective Interfacial Olefin Cross Metathesis for the Preparation of Hollow Nanocapsules. ACS Macro Lett 2014, 3, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Aschenbrenner, E.M.; Weiss, C.K.; Landfester, K. Enzymatically degradable nanogels by inverse miniemulsion copolymerization of acrylamide with dextran methacrylates as crosslinkers. Polym. Chem. 2012, 3, 204–216. [Google Scholar] [CrossRef]
- Aschenbrenner, E.; Bley, K.; Koynov, K.; Makowski, M.; Kappl, M.; Landfester, K.; Weiss, C.K. Using the polymeric ouzo effect for the preparation of polysaccharide-based nanoparticles. Langmuir 2013, 29, 8845–8855. [Google Scholar] [CrossRef]
- Wurm, F.R.; Weiss, C.K. Nanoparticles from renewable polymers. Front Chem. 2014, 2, 49. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Shagdarova, B.; Sinitsyna, O.; Sinitsyn, A.; Varlamov, V. Evaluation of Antibacterial and Antifungal Properties of Low Molecular Weight Chitosan Extracted from Hermetia illucens Relative to Crab Chitosan. Molecules 2022, 27, 577. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liang, S.-H.; Lai, W.-L.; Lee, J.-X.; Wang, Y.-P.; Liu, Y.-T.; Wang, S.-H.; Lee, M.-H. Sustainable Extraction of Chitin from Spent Pupal Shell of Black Soldier Fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A. Antibacterial action of insect chitosan/gum Arabic nanocomposites encapsulating eugenol and selenium nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 102219. [Google Scholar] [CrossRef]
- Al-Saggaf, M.S.; Saravanan, R. Formulation of Insect Chitosan Stabilized Silver Nanoparticles with Propolis Extract as Potent Antimicrobial and Wound Healing Composites. Int. J. Polym. Sci. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Flores-Espinoza, A.I.; Garcia-Contreras, R.; Guzman-Rocha, D.A.; Aranda-Herrera, B.; Chavez-Granados, P.A.; Jurado, C.A.; Alfawaz, Y.F.; Alshabib, A. Gelatin-Chitosan Hydrogel Biological, Antimicrobial and Mechanical Properties for Dental Applications. Biomimetics 2023, 8, 575. [Google Scholar] [CrossRef]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef]
- Zong, C.; Yu, Y.; Song, G.; Luo, T.; Li, L.; Wang, X.; Qin, S. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. 2012, 237, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Chen, L.; Wu, Q.; Yu, C. Chitosan oligosaccharides enhance lipid droplets via down-regulation of PCSK9 gene expression in HepG2 cells. Exp. Cell Res. 2018, 366, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Rajaram, R.; Kaleshkumar, K.; Gayathri, N.; Sivasudha, T.; Kandasamy, S. Synergistic effect of novel chitosan combined metformin drug on streptozotocin-induced diabetes mellitus rat. Int. J. Biol. Macromol. 2020, 153, 1335–1349. [Google Scholar] [CrossRef]
- Bhumika, P.; Parth, R.; Prasant Kumar, J.; Sriram, S. Investigation of Chitosan for Prevention of Diabetic Progression Through Gut Microbiota Alteration in Sugar Rich Diet Induced Diabetic Rats. Curr. Pharm. Biotechnol. 2016, 17, 173–184. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, M.; Li, Y.; Qian, J.; Li, Y.; Han, X.; Tan, J.; Luo, Y.; Wang, Q.; Qin, C. Chitosan oligosaccharide combined with running benefited the immune status of rats. Int. Immunopharmacol. 2020, 88, 106915. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef]
- Omer, A.M.; Sadik, W.A.-A.; El-Demerdash, A.-G.M.; Hassan, H.S. Formulation of pH-sensitive aminated chitosan–gelatin crosslinked hydrogel for oral drug delivery. J. Saudi Chem. Soc. 2021, 25, 101384. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef]
- Simons, R.E.; Zevy, D.L.; Jafferany, M. Psychodermatology of vitiligo: Psychological impact and consequences. Dermatol. Ther. 2020, 33, e13418. [Google Scholar] [CrossRef] [PubMed]
- Aflakseir, A.; Jamali, S.; Mollazadeh, J. Prevalence of Body Dysmorphic Disorder Among a Group of College Students in Shiraz. Zahedan J. Res. Med. Sci. 2021, 23, e95247. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandia, M.L.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Yamada, H.; Saito, Y.; Nawa, T.; Isobe, M.; Yamamoto, T.; Aoki, D.; Matsushita, Y.; Fukushima, K. Investigation of dyeing behavior of oxidative dye in fine structures of the human hair cuticle by nanoscale secondary ion mass spectrometry. Ski. Res. Technol. 2015, 21, 295–301. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chiu, Y.F.; Wu, M.H.; Li, M.H.; Wu, C.N.; Chen, W.S.; Huang, C.H. Gelatin/Chitosan Bilayer Patches Loaded with Cortex Phellodendron amurense/Centella asiatica Extracts for Anti-Acne Application. Polymers 2021, 13, 579. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin/Chitosan-Melanin Complexes from Black Soldier Fly Hermetia Illucens. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2019 the 5th International Conference on Architecture, Materials and Construction (ICAMC 2019); Lisbon, Portugal, 2–4 December 2019, IOP Publishing Ltd.: Bristol, UK, 2020; Volume 809. [Google Scholar] [CrossRef]
- Khayrova, A.S.; Sazhnev, N.A.; Korobovskaya, D.V.; Lopatin, S.A.; Kildeeva, N.R.; Varlamov, V.P. Isolation of Chitosan-Melanin Complex from Black Soldier Fly Adults and Obtaining Nanofibrous Materials Based on It. Appl. Biochem. Microbiol. 2024, 60, 201–206. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, H.L.; Gandras, L.; Schneider, L.; Witthohn, M.; Troidl, K.; Muffler, K.; Weiss, C.K. Analysis, Properties, and Applications of Insect-Derived Chitosan: A Sustainable Path to Functional Polysaccharide Materials. Gels 2025, 11, 291. https://doi.org/10.3390/gels11040291
Schäfer HL, Gandras L, Schneider L, Witthohn M, Troidl K, Muffler K, Weiss CK. Analysis, Properties, and Applications of Insect-Derived Chitosan: A Sustainable Path to Functional Polysaccharide Materials. Gels. 2025; 11(4):291. https://doi.org/10.3390/gels11040291
Chicago/Turabian StyleSchäfer, Hanna L., Lars Gandras, Laura Schneider, Marco Witthohn, Kerstin Troidl, Kai Muffler, and Clemens K. Weiss. 2025. "Analysis, Properties, and Applications of Insect-Derived Chitosan: A Sustainable Path to Functional Polysaccharide Materials" Gels 11, no. 4: 291. https://doi.org/10.3390/gels11040291
APA StyleSchäfer, H. L., Gandras, L., Schneider, L., Witthohn, M., Troidl, K., Muffler, K., & Weiss, C. K. (2025). Analysis, Properties, and Applications of Insect-Derived Chitosan: A Sustainable Path to Functional Polysaccharide Materials. Gels, 11(4), 291. https://doi.org/10.3390/gels11040291








