Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications
Abstract
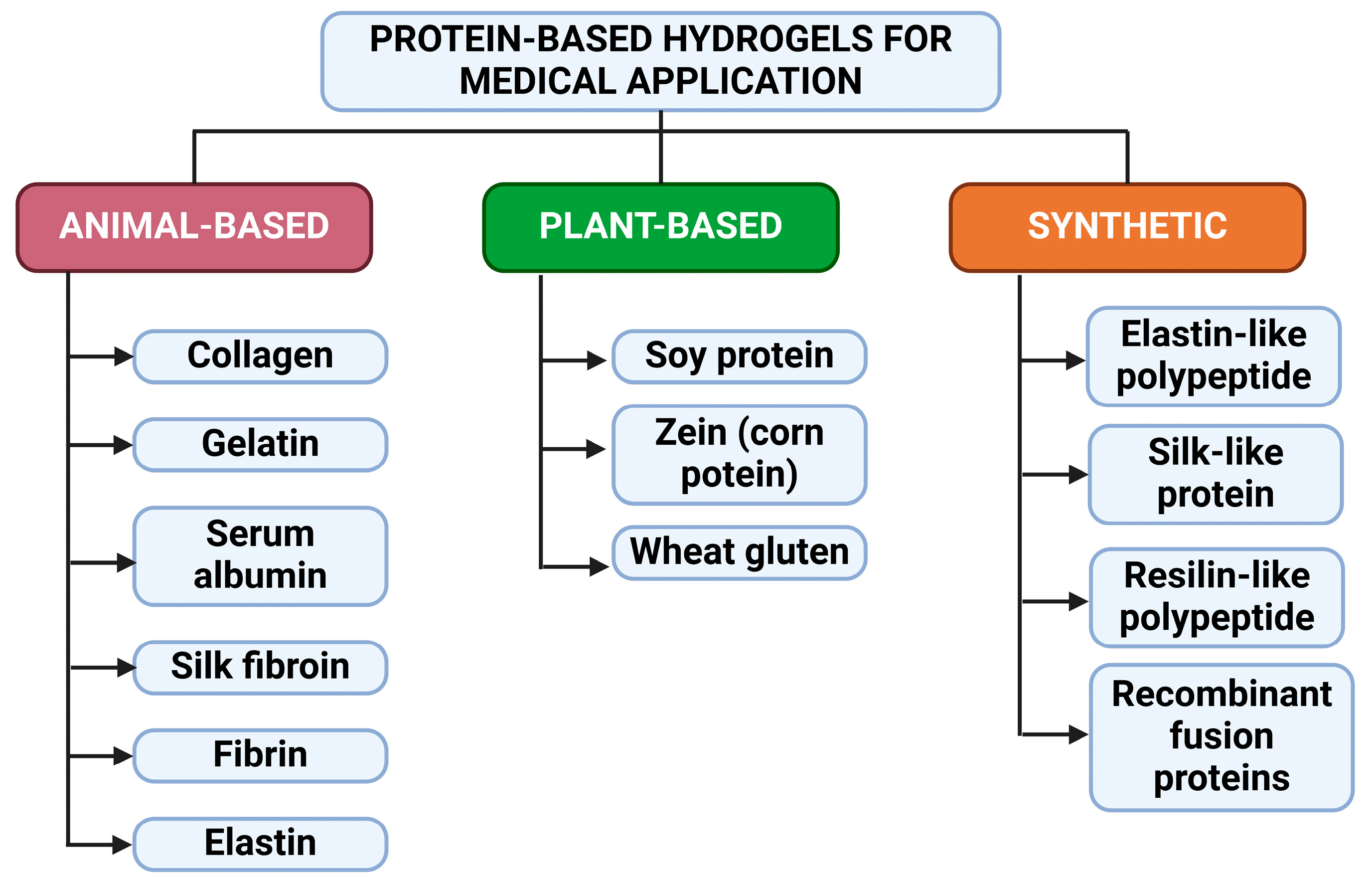
:1. Introduction
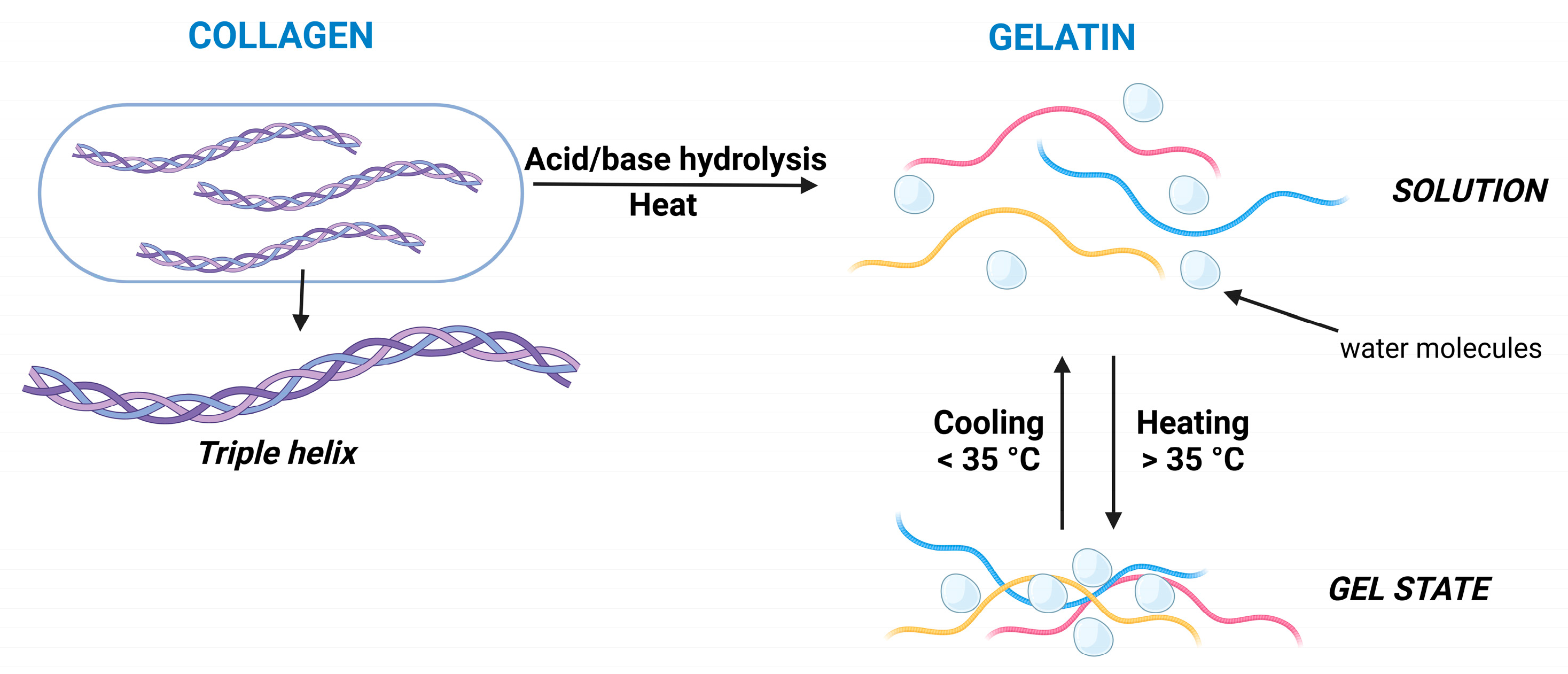
2. Collagen-Based Hydrogels for Medicinal Applications
2.1. General Structure, Gelling Mechanism, and Properties of Collagen
2.2. Recent Advancements in Collagen-Based Drug/Cell-Delivery Systems
3. Gelatin-Based Hydrogels for Medicinal Applications
3.1. General Structure, Gelling Mechanism, and Properties of Gelatin
3.2. Recent Advancements in Gelatin-Based Hydrogels as Drug Delivery Systems
4. Albumin-Based Hydrogels for Medicinal Applications
4.1. General Structure, Gelation Mechanism, and Properties of Albumin Hydrogels
4.2. Application of Albumin-Based Hydrogels for Therapy
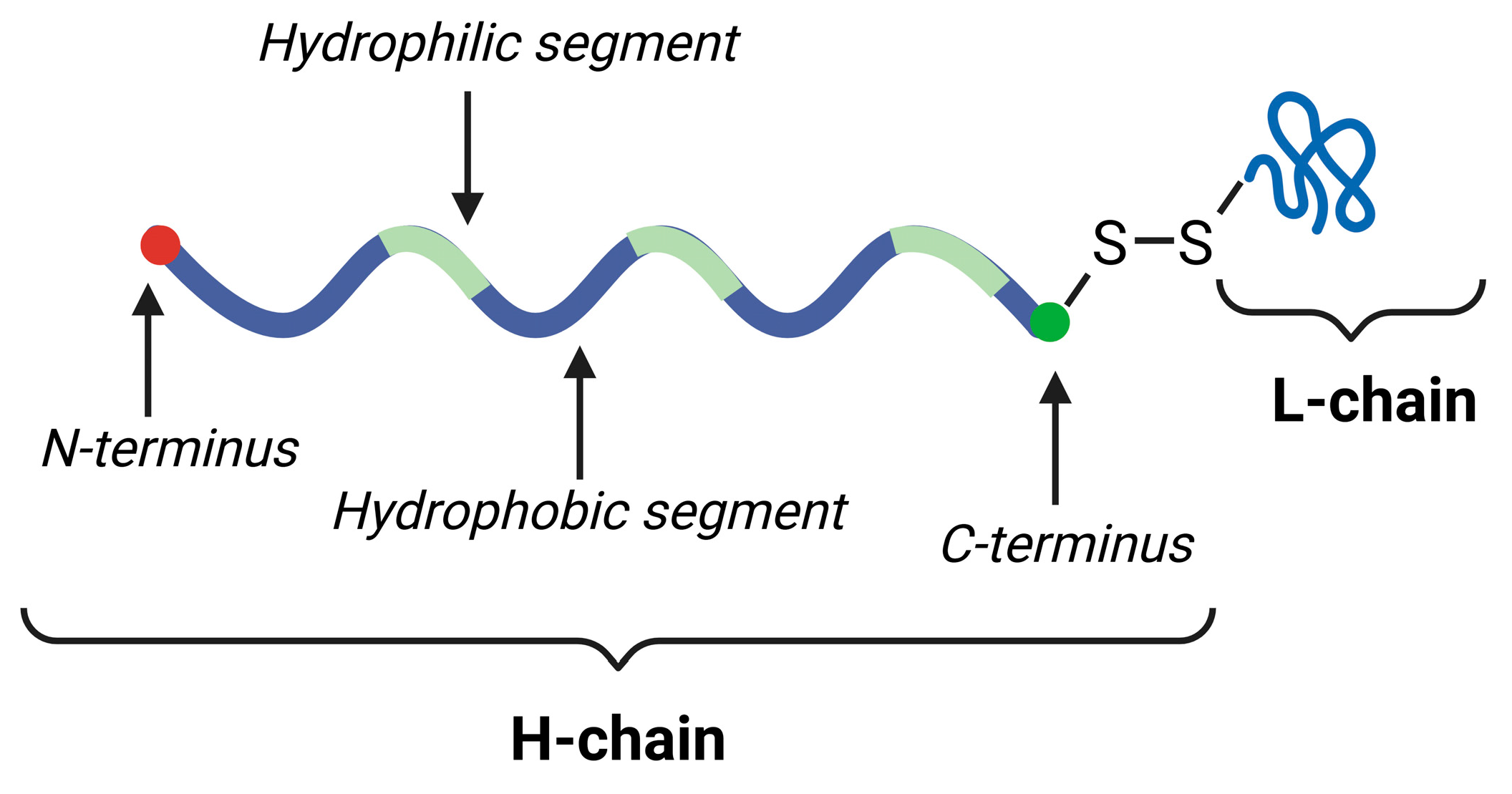
5. Silk Fibroin-Based Hydrogels for Medicinal Applications
5.1. Structure, Gelation Mechanism, and Properties of Silk Fibroin
5.2. Application of Silk Fibroin-Based Hydrogels in Medicine
6. Fibrin-Based Hydrogels for Medicinal Applications
Recent Advancements in Fibrin-Based Gel Systems for Medical Applications
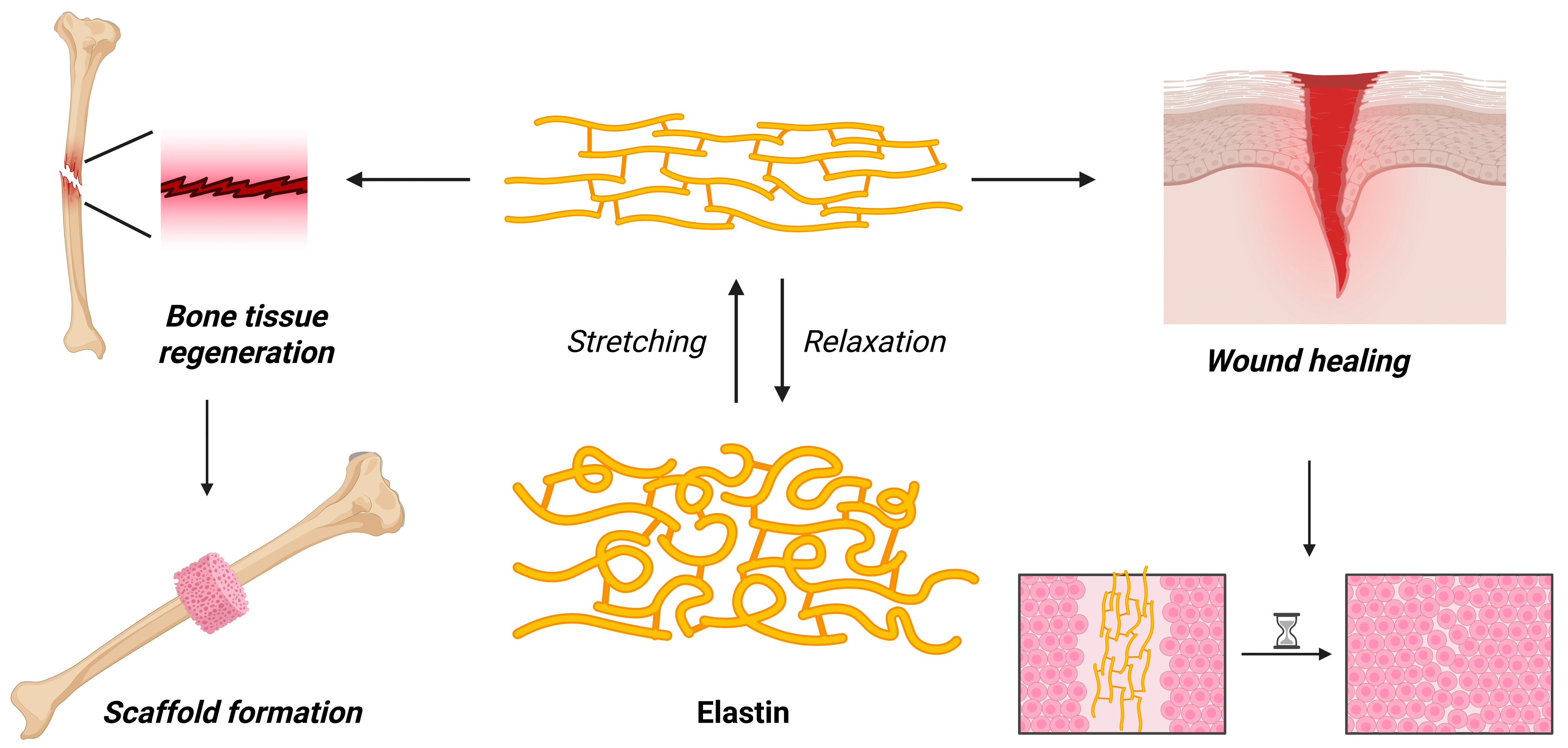
7. Elastin-Based Hydrogels for Medicinal Applications
7.1. General Structure, Gelation Mechanism, and Properties
7.2. Recent Advancements in the Application of Elastin-Based Hydrogels for Medical Applications
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | 3-dimensional |
| 4T1 | stage IV human breast cancer cell line |
| ALP | alkaline phosphatase |
| BDEE | 1,4-butanedioldiglycidyl ether |
| CT-26 | undifferentiated colon carcinoma cell line |
| DNA | deoxyribonucleic acid |
| E. coli | Escherichia coli |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| HLC | human-like collagen |
| kDa | kilodalton |
| PNIPAM | poly(N-isopropylacrylamide) |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| S. aureus | Staphylococcus aureus |
References
- Khan, F.; Mohd, A.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, Classification and Properties of Hydrogels: Their Applications in Drug Delivery and Agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; He, B.; Wang, S.; Kong, F. Synthesis of Cellulose Aerogels as Promising Carriers for Drug Delivery: A Review. Cellulose 2021, 28, 2697–2714. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, L.; Parakhonskiy, B.V.; Skirtach, A.G. Hard, Soft, and Hard-and-Soft Drug Delivery Carriers Based on CaCO3 and Alginate Biomaterials: Synthesis, Properties, Pharmaceutical Applications. Pharmaceutics 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Syed Azhar, S.N.A.; Ashari, S.E.; Zainuddin, N.; Hassan, M. Nanostructured Lipid Carriers-Hydrogels System for Drug Delivery: Nanohybrid Technology Perspective. Molecules 2022, 27, 289. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, X.; Ma, C.; Chu, X.; Wang, L.; Xu, W. A Review on Recent Advances of Protein-Polymer Hydrogels. Eur. Polym. J. 2022, 162, 110881. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.-Y.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Research Progress on Polysaccharide/Protein Hydrogels: Preparation Method, Functional Property, and Application as Delivery Systems for Bioactive Ingredients. Food Res. Int. 2021, 147, 110542. [Google Scholar] [CrossRef]
- Fu, L.; Li, L.; Bian, Q.; Xue, B.; Jin, J.; Li, J.; Cao, Y.; Jiang, Q.; Li, H. Cartilage-like Protein Hydrogels Engineered via Entanglement. Nature 2023, 618, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, H.; Zhu, L.; Liu, Z.; Yang, J.; Qin, G.; Wu, J.; Tang, Y.; Zhang, D.; Chen, Q.; et al. A General Protein Unfolding-Chemical Coupling Strategy for Pure Protein Hydrogels with Mechanically Strong and Multifunctional Properties. Adv. Sci. 2022, 9, 2102557. [Google Scholar] [CrossRef]
- Bian, Q.; Fu, L.; Li, H. Engineering Shape Memory and Morphing Protein Hydrogels Based on Protein Unfolding and Folding. Nat. Commun. 2022, 13, 137. [Google Scholar] [CrossRef]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA Delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.; Nguyen, M.K.; Huynh, C.T.; Sarett, S.M.; Ge, P.; Chetverikova, M.; Nguyen, K.; Grosh, D.; Duvall, C.L.; Alsberg, E. Hydrogel Microspheres for Spatiotemporally Controlled Delivery of RNA and Silencing Gene Expression within Scaffold-Free Tissue Engineered Constructs. Acta Biomater. 2021, 124, 315–326. [Google Scholar] [CrossRef]
- Fan, B.; Jia, H.; Yu, H.; Xu, Y.; Li, R.; Qiu, J.; Wang, J.; Wan, X.; Liu, J. Hydrogel-Based Intranasal Delivery Systems for The Treatment of Allergic Rhinitis. Adv. Ther. 2023, 6, 2300035. [Google Scholar] [CrossRef]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical Application of Mesenchymal Stem Cell in Regenerative Medicine: A Narrative Review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.-M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy-a New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan Films and Scaffolds for Regenerative Medicine Applications: A Review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Mardikasari, S.A.; Katona, G.; Sipos, B.; Ambrus, R.; Csóka, I. Preparation and Optimization of Bovine Serum Albumin Nanoparticles as a Promising Gelling System for Enhanced Nasal Drug Administration. Gels 2023, 9, 896. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, J.; Dai, T.; McClements, D.J.; Liu, C. The Effect of Whey Protein-Puerarin Interactions on the Formation and Performance of Protein Hydrogels. Food Hydrocoll. 2021, 113, 106444. [Google Scholar] [CrossRef]
- Zhu, P.; Huang, W.; Guo, X.; Chen, L. Strong and Elastic Pea Protein Hydrogels Formed through pH-Shifting Method. Food Hydrocoll. 2021, 117, 106705. [Google Scholar] [CrossRef]
- Hong, L.; Chen, G.; Cai, Z.; Liu, H.; Zhang, C.; Wang, F.; Xiao, Z.; Zhong, J.; Wang, L.; Wang, Z.; et al. Balancing Microthrombosis and Inflammation via Injectable Protein Hydrogel for Inflammatory Bowel Disease. Adv. Sci. 2022, 9, 2200281. [Google Scholar] [CrossRef]
- Ouyang, J.; Bu, Q.; Tao, N.; Chen, M.; Liu, H.; Zhou, J.; Liu, J.; Deng, B.; Kong, N.; Zhang, X.; et al. A Facile and General Method for Synthesis of Antibiotic-Free Protein-Based Hydrogel: Wound Dressing for the Eradication of Drug-Resistant Bacteria and Biofilms. Bioact. Mater. 2022, 18, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Gherghescu, I.; Delgado-Charro, M.B. The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA. Pharmaceutics 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, T.; Blind, E.; Janssen, H. Regulatory Aspects of the Development of Drugs for Metabolic Bone Diseases—FDA and EMA Perspective. Br. J. Clin. Pharmacol. 2019, 85, 1208–1212. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and Its Derivatives: From Structure and Properties to Their Applications in Food Industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Huang, Y.; Zhang, J.; Qin, Z.; Wei, B.; Xu, C.; Zhu, L.; Wang, H. The Impact of Spatial Structures of Collagen on the Hemostatic Properties of Collagen/Calcium Alginate Composite Membranes. Int. J. Biol. Macromol. 2025, 288, 138753. [Google Scholar] [CrossRef]
- Moeinzadeh, S.; Park, Y.; Lin, S.; Yang, Y.P. In-Situ Stable Injectable Collagen-Based Hydrogels for Cell and Growth Factor Delivery. Materialia 2021, 15, 100954. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Chen, Y.; Yue, O.; Bai, Z.; Cui, B.; Jiang, H.; Liu, X. A Review of Recent Progress on Collagen-Based Biomaterials. Adv. Healthc. Mater. 2023, 12, 2202042. [Google Scholar] [CrossRef]
- Gommes, C.J.; Louis, T.; Bourgot, I.; Noël, A.; Blacher, S.; Maquoi, E. Remodelling of the Fibre-Aggregate Structure of Collagen Gels by Cancer-Associated Fibroblasts: A Time-Resolved Grey-Tone Image Analysis Based on Stochastic Modelling. Front. Immunol. 2023, 13, 988502. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Fang, X.; Lin, F.; Fang, J.; Xiong, C. Collagen Gel Contraction Assays: From Modelling Wound Healing to Quantifying Cellular Interactions with Three-Dimensional Extracellular Matrices. Eur. J. Cell Biol. 2022, 101, 151253. [Google Scholar] [CrossRef]
- Yang, X.; Ahmad, K.; Yang, T.; Fan, Y.; Zhao, F.; Jiang, S.; Chen, P.; Hou, H. Collagen-Based Hydrogel Sol-Gel Phase Transition Mechanism and Their Applications. Adv. Colloid Interface Sci. 2025, 340, 103456. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2021, 21, 2000280. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q. Preparation and Characterization of Polyvinyl Alcohol/Secondary Collagen Fiber Gel Membrane with Excellent Mechanical Property. J. Vinyl Addit. Technol. 2024, 30, 130–141. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Sukumaran, H.G.; Dara, P.K.; Ganesan, B.; Ashraf, M.; Anandan, R.; Mathew, S.; Nagarajarao, R.C. Nano-Encapsulation of Curcumin in Fish Collagen Grafted Succinyl Chitosan Hydrogel Accelerates Wound Healing Process in Experimental Rats. Food Hydrocoll. Health 2022, 2, 100061. [Google Scholar] [CrossRef]
- Hwang, J.; An, E.-K.; Zhang, W.; Kim, H.J.; Eom, Y.; Jin, J.-O. Dual-Functional Alginate and Collagen–Based Injectable Hydrogel for the Treatment of Cancer and Its Metastasis. J. Nanobiotechnol. 2022, 20, 245. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A Novel Smart Injectable Hydrogel Prepared by Microbial Transglutaminase and Human-like Collagen: Its Characterization and Biocompatibility. Mater. Sci. Eng. C 2016, 68, 317–326. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, W.; Li, B.; Ni, Y.; Yuan, T.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Fabrication and Characterization of Collagen-Based Injectable and Self-Crosslinkable Hydrogels for Cell Encapsulation. Colloids Surf. B Biointerfaces 2018, 167, 448–456. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid—Based Injectable Hydrogels for Tissue Engineering Applications—Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. Novel Hyaluronic Acid-Tyrosine/Collagen-Based Injectable Hydrogels as Soft Filler for Tissue Engineering. Int. J. Biol. Macromol. 2019, 141, 700–712. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Wei, M. Synthesis and Characterization of a Novel Injectable Alginate–Collagen–Hydroxyapatite Hydrogel for Bone Tissue Regeneration. J. Mater. Chem. B 2015, 3, 3081–3090. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.; Li, X.; Xu, Y.; Lu, G.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A Di-Self-Crosslinking Hyaluronan-Based Hydrogel Combined with Type I Collagen to Construct a Biomimetic Injectable Cartilage-Filling Scaffold. Acta Biomater. 2020, 111, 197–207. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An Injectable Collagen-Genipin-Carbon Dot Hydrogel Combined with Photodynamic Therapy to Enhance Chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Luo, Z.; Li, D.; Lu, J.; Wang, Q.; Xiao, Y.; Zhang, X. Development of an Injectable Thiolated Icariin Functionalized Collagen/Hyaluronic Hydrogel to Promote Cartilage Formation in Vitro and in Vivo. J. Mater. Chem. B 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Guzdek-Zając, K.; Karewicz, A.; Horak, W.; Lach, R.; Wójcik, K.; Nowakowska, M. Bioactive yet Antimicrobial Structurally Stable Collagen/Chitosan/Lysine Functionalized Hyaluronic Acid—Based Injectable Hydrogels for Potential Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 155, 938–950. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, F.; Tang, L.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; Hu, X.; Lin, S.; Ding, C. Super-Ductile, Injectable, Fast Self-Healing Collagen-Based Hydrogels with Multi-Responsive and Accelerated Wound-Repair Properties. Chem. Eng. J. 2021, 405, 126756. [Google Scholar] [CrossRef]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid Gelatin Hydrogels in Nanomedicine Applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Xiang, L.; Cui, W. Biomedical Application of Photo-Crosslinked Gelatin Hydrogels. J. Leather Sci. Eng. 2021, 3, 3. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, Z.; Xia, P.; Wang, Z.; Zhao, X.; Jiang, X.; Wang, T.; Gao, Q.; Xu, J.; Shan, D.; et al. Tough Gelatin Hydrogel for Tissue Engineering. Adv. Sci. 2023, 10, 2301665. [Google Scholar] [CrossRef]
- Ullah, K.; Ali Khan, S.; Murtaza, G.; Sohail, M.; Azizullah; Manan, A.; Afzal, A. Gelatin-Based Hydrogels as Potential Biomaterials for Colonic Delivery of Oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef]
- Zeinali Kalkhoran, A.H.; Naghib, S.M.; Vahidi, O.; Rahmanian, M. Synthesis and Characterization of Graphene-Grafted Gelatin Nanocomposite Hydrogels as Emerging Drug Delivery Systems. Biomed. Phys. Eng. Express 2018, 4, 055017. [Google Scholar] [CrossRef]
- Changez, M. The Effect of Composition of Poly(Acrylic Acid)–Gelatin Hydrogel on Gentamicin Sulphate Release: In Vitro. Biomaterials 2003, 24, 527–536. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and Characterization of Bacterial Cellulose and Gelatin-Based Hydrogel Composites for Drug-Delivery Systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of Gelatin-based Biodegradable Hydrogel Nanocomposite and Their Application as Drug Delivery Agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Kobayashi, T.; Mizuta, M.; Hiwatashi, N.; Kishimoto, Y.; Nakamura, T.; Kanemaru, S.; Hirano, S. Drug Delivery System of Basic Fibroblast Growth Factor Using Gelatin Hydrogel for Restoration of Acute Vocal Fold Scar. Auris Nasus Larynx 2017, 44, 86–92. [Google Scholar] [CrossRef]
- Kobayashi, H.; Minatoguchi, S.; Yasuda, S.; Bao, N.; Kawamura, I.; Iwasa, M.; Yamaki, T.; Sumi, S.; Misao, Y.; Ushikoshi, H.; et al. Post-Infarct Treatment with an Erythropoietin-Gelatin Hydrogel Drug Delivery System for Cardiac Repair. Cardiovasc. Res. 2008, 79, 611–620. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Liu, H.; Li, S.; An, Z.; Feng, Z. Construction of Bupivacaine-loaded Gelatin-based Hydrogel Delivery System for Sciatic Nerve Block in Mice. J. Biomed. Mater. Res. 2024, 112, 1975–1984. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive Nanocomposite Hydrogels Based on Gelatin/Poly (N–Isopropylacrylamide) (PNIPAM) for Controlled Drug Delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl Chitosan Microspheres Loaded Hyaluronic Acid/Gelatin Hydrogels for Controlled Drug Delivery and the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J. Thermosensitive Chitosan-Gelatin-Based Hydrogel Containing Curcumin-Loaded Nanoparticles and Latanoprost as a Dual-Drug Delivery System for Glaucoma Treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, S.; Hou, Y. Insight on Serum Albumin: From Structure and Biological Properties to Functional Biomaterials for Bone Repair. ACS Biomater. Sci. Eng. 2023, 9, 2235–2250. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ameen, F.; Ur Rehman, S.; Sarwar, T.; Tabish, M. Studying the Interaction of Drug/Ligand with Serum Albumin. J. Mol. Liq. 2021, 336, 116200. [Google Scholar] [CrossRef]
- Maciążek-Jurczyk, M.; Morak-Młodawska, B.; Jeleń, M.; Kopeć, W.; Szkudlarek, A.; Owczarzy, A.; Kulig, K.; Rogóż, W.; Pożycka, J. The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity. Pharmaceuticals 2021, 14, 285. [Google Scholar] [CrossRef]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; et al. Development of Meloxicam-Human Serum Albumin Nanoparticles for Nose-to-Brain Delivery via Application of a Quality by Design Approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Tanidjaja, I.; Damodaran, S. Influence of Amino Acids on Thermal Stability and Heat-Set Gelation of Bovine Serum Albumin. Food Chem. 2021, 337, 127670. [Google Scholar] [CrossRef]
- Nnyigide, T.O.; Nnyigide, O.S.; Hyun, K. Rheological and Molecular Dynamics Simulation Studies of the Gelation of Human Serum Albumin in Anionic and Cationic Surfactants. Korean J. Chem. Eng. 2023, 40, 1871–1881. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release. Int. J. Mol. Sci. 2022, 23, 7352. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, H.; Shin, S.Y.; Jodat, Y.A.; Jhun, H.; Lim, W.; Seo, J.W.; Kim, G.; Mun, J.Y.; Zhang, K.; et al. Photo-Cross-Linkable Human Albumin Colloidal Gels Facilitate In Vivo Vascular Integration for Regenerative Medicine. ACS Omega 2021, 6, 33511–33522. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Qian, K.; Cai, J.; Yang, Y.; Zhu, L.; Liu, B. Therapy for Gastric Cancer with Peritoneal Metastasis Using Injectable Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 1100–1112. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Wu, L.; Zhou, Z.; Wang, Y.; Li, W.; Yuan, H.; Lu, Z.; Yan, D.; Chen, S.; et al. A Novel Hydrogel Orthotopic Injection Model in Moderately Hypofractionated Radiation Therapy for Prostate Cancer: Adaptive Degradation and Durable Imaging. Front. Oncol. 2023, 12, 1077900. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Levy, G.K.; Macdonald, J.; Justin, A.W.; Markaki, A.E. Functionalisation of a Heat-Derived and Bio-Inert Albumin Hydrogel with Extracellular Matrix by Air Plasma Treatment. Sci. Rep. 2020, 10, 12429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and Fast Self-Healing Protein Hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef]
- Kenawy, E.R.S.; Kamoun, E.A.; Ghaly, Z.S.; Abdel-baset, M.S.; Mahmoud, A.E.-M.; Yehia, A.-G.M. Novel Physically Cross-Linked Curcumin-Loaded PVA/Aloe Vera Hydrogel Membranes for Acceleration of Topical Wound Healing: In Vitro and In Vivo Experiments. Arab. J. Sci. Eng. 2023, 48, 497–514. [Google Scholar] [CrossRef]
- Deng, L.; Xia, T.; Cheng, W.; Yang, M.; Zhu, W.; Chen, X. Injectable Redox Albumin-Based Hydrogel with in-Situ Loaded Dihydromyricetin. Colloids Surf. B Biointerfaces 2022, 220, 112871. [Google Scholar] [CrossRef]
- Zhou, C.-Z. Fine Organization of Bombyx Mori Fibroin Heavy Chain Gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, X.; Liu, L.; Xu, W.; Shao, R. Challenges and Opportunities of Silk Protein Hydrogels in Biomedical Applications. Mater. Adv. 2022, 3, 2291–2308. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, P.; Cui, L.; Yu, Y.; Deng, C.; Wang, Q.; Fan, X. Self-Crosslinking of Silk Fibroin Using H2O2-Horseradish Peroxidase System and the Characteristics of the Resulting Fibroin Membranes. Appl. Biochem. Biotechnol. 2017, 182, 1548–1563. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel Silk Fibroin/Elastin Wound Dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Farokhi, M.; Aleemardani, M.; Solouk, A.; Mirzadeh, H.; Teuschl, A.H.; Redl, H. Crosslinking Strategies for Silk Fibroin Hydrogels: Promising Biomedical Materials. Biomed. Mater. 2021, 16, 022004. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Y.; Wen, S.; Hu, Y.; Min, Y. Distinctive Stress-Stiffening Responses of Regenerated Silk Fibroin Protein Polymers under Nanoscale Gap Geometries: Effect of Shear on Silk Fibroin-Based Materials. Biomacromolecules 2018, 19, 1223–1233. [Google Scholar] [CrossRef]
- Kojic, N.; Pritchard, E.M.; Tao, H.; Brenckle, M.A.; Mondia, J.P.; Panilaitis, B.; Omenetto, F.; Kaplan, D.L. Focal Infection Treatment Using Laser-Mediated Heating of Injectable Silk Hydrogels with Gold Nanoparticles. Adv. Funct. Mater. 2012, 22, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Goczkowski, M.; Gobin, M.; Hindié, M.; Agniel, R.; Larreta-Garde, V. Properties of Interpenetrating Polymer Networks Associating Fibrin and Silk Fibroin Networks Obtained by a Double Enzymatic Method. Mater. Sci. Eng. C 2019, 104, 109931. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Xu, Y.; Liu, G.; Liu, Y.; Li, X.; Li, M. Regulating the Degradation Rate of Silk Fibroin Films through Changing the Genipin Crosslinking Degree. Polym. Degrad. Stab. 2014, 109, 226–232. [Google Scholar] [CrossRef]
- Tsukada, M.; Nagura, M.; Ishikawa, H.; Shiozaki, H. Structural Characteristics of Silk Fibers Treated with Epoxides. J. Appl. Polym. Sci. 1991, 43, 643–649. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An Innovative Bi-Layered Wound Dressing Made of Silk and Gelatin for Accelerated Wound Healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, S.; Xin, W.; Chen, Y.; Fu, Q.; Li, L.; Jiao, Y. Synergic Adhesive Chemistry-Based Fabrication of BMP-2 Immobilized Silk Fibroin Hydrogel Functionalized with Hybrid Nanomaterial to Augment Osteogenic Differentiation of rBMSCs for Bone Defect Repair. Int. J. Biol. Macromol. 2021, 192, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Cao, L.; Liu, Y.; Wu, J.; Zeng, D.; Hu, L.; Zhang, X.; Jiang, X. Biocompatible Silk/Calcium Silicate/Sodium Alginate Composite Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2018, 199, 244–255. [Google Scholar] [CrossRef]
- Shen, K.; Duan, A.; Cheng, J.; Yuan, T.; Zhou, J.; Song, H.; Chen, Z.; Wan, B.; Liu, J.; Zhang, X.; et al. Exosomes Derived from Hypoxia Preconditioned Mesenchymal Stem Cells Laden in a Silk Hydrogel Promote Cartilage Regeneration via the miR-205–5p/PTEN/AKT Pathway. Acta Biomater. 2022, 143, 173–188. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Deng, Z.; Nie, X.; Pan, Y.; Cheng, G. Degradation Profiles of the Poly(ε-Caprolactone)/Silk Fibroin Electrospinning Membranes and Their Potential Applications in Tissue Engineering. Int. J. Biol. Macromol. 2024, 266, 131124. [Google Scholar] [CrossRef]
- Jiang, L.; Su, D.; Ding, S.; Zhang, Q.; Li, Z.; Chen, F.; Ding, W.; Zhang, S.; Dong, J. Salt-Assisted Toughening of Protein Hydrogel with Controlled Degradation for Bone Regeneration. Adv. Funct. Mater. 2019, 29, 1901314. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The Use of Injectable Sonication-Induced Silk Hydrogel for VEGF165 and BMP-2 Delivery for Elevation of the Maxillary Sinus Floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk Fibroin/Hydroxyapatite Composite Hydrogel Induced by Gamma-Ray Irradiation for Bone Tissue Engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk Implants for the Healing of Critical Size Bone Defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef]
- Chao, P.G.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk Hydrogel for Cartilage Tissue Engineering. J. Biomed. Mater. Res. 2010, 95B, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Li, X.; Cao, Z.; Mo, Q.; Sheng, R.; Ling, C.; Chi, J.; Yao, Q.; Chen, J.; et al. Multifunctional Polyphenol-Based Silk Hydrogel Alleviates Oxidative Stress and Enhances Endogenous Regeneration of Osteochondral Defects. Mater. Today Bio 2022, 14, 100251. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Yu, Y.; Zhao, Y. In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing. Research 2022, 2022, 9794745. [Google Scholar] [CrossRef]
- Li, C.; Cui, W. 3D Bioprinting of Cell-Laden Constructs for Regenerative Medicine. Eng. Regen. 2021, 2, 195–205. [Google Scholar] [CrossRef]
- Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Wang, H.; Zhao, Y.; Chai, R. Natural Proteins-Derived Asymmetric Porous Conduit for Peripheral Nerve Regeneration. Appl. Mater. Today 2022, 27, 101431. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Chen, D.; Lin, J.; Li, W.; Guo, S.; Wu, R.; Zhao, X.; Lin, T.; Chen, G.; et al. An Injectable and Photocurable Methacrylate-Silk Fibroin Hydrogel Loaded with bFGF for Spinal Cord Regeneration. Mater. Des. 2022, 217, 110670. [Google Scholar] [CrossRef]
- Tang, X.; Gu, X.; Huang, T.; Chen, X.; Zhou, Z.; Yang, Y.; Ling, J. Anisotropic Silk-Inspired Nerve Conduit with Peptides Improved the Microenvironment for Long-Distance Peripheral Nerve Regeneration. ACS Macro Lett. 2021, 10, 1501–1509. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, W.; Zhang, X.; Zhou, Z.; Ding, Z.; Zhou, X.; Lu, Q.; Kaplan, D.L. Nerve Growth Factor-Laden Anisotropic Silk Nanofiber Hydrogels to Regulate Neuronal/Astroglial Differentiation for Scarless Spinal Cord Repair. ACS Appl. Mater. Interfaces 2022, 14, 3701–3715. [Google Scholar] [CrossRef]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.-C. Design and Characterization of a Chitosan-Enriched Fibrin Hydrogel for Human Dental Pulp Regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef]
- Nazari, B.; Kazemi, M.; Kamyab, A.; Nazari, B.; Ebrahimi-Barough, S.; Hadjighassem, M.; Norouzi-Javidan, A.; Ai, A.; Ahmadi, A.; Ai, J. Fibrin Hydrogel as a Scaffold for Differentiation of Induced Pluripotent Stem Cells into Oligodendrocytes. J. Biomed. Mater. Res. 2020, 108, 192–200. [Google Scholar] [CrossRef]
- Pereira, R.V.S.; EzEldeen, M.; Ugarte-Berzal, E.; Martens, E.; Malengier-Devlies, B.; Vandooren, J.; Vranckx, J.J.; Matthys, P.; Opdenakker, G. Physiological Fibrin Hydrogel Modulates Immune Cells and Molecules and Accelerates Mouse Skin Wound Healing. Front. Immunol. 2023, 14, 1170153. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological Advances in Fibrin for Tissue Engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Tanaka, R.; Saito, Y.; Fujiwara, Y.; Jo, J.; Tabata, Y. Preparation of Fibrin Hydrogels to Promote the Recruitment of Anti-Inflammatory Macrophages. Acta Biomater. 2019, 89, 152–165. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Yang, H.; Lu, K.; Zhang, H.; Wang, Y.; Wang, J.; Ruan, L.; Shen, Z.; Yu, Q.; et al. An Injectable Alginate/Fibrin Hydrogel Encapsulated with Cardiomyocytes and VEGF for Myocardial Infarction Treatment. J. Mater. Sci. Technol. 2023, 143, 198–206. [Google Scholar] [CrossRef]
- Sudhadevi, T.; Vijayakumar, H.S.; Hariharan, E.V.; Sandhyamani, S.; Krishnan, L.K. Optimizing Fibrin Hydrogel toward Effective Neural Progenitor Cell Delivery in Spinal Cord Injury. Biomed. Mater. 2022, 17, 014102. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, Y.; Dong, L.; Desai, M.S.; Xia, J.; Liang, M.; Lee, S.-W.; Mi, S.; Sun, W. Design of Functional Hydrogels Using Smart Polymer Based on Elastin-like Polypeptides. Chem. Eng. J. 2022, 435, 135155. [Google Scholar] [CrossRef]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, 2101329. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Allegra, M.; Puleio, R.; Giammona, G. Hyaluronic Acid and α-Elastin Based Hydrogel for Three Dimensional Culture of Vascular Endothelial Cells. J. Drug Deliv. Sci. Technol. 2018, 46, 28–33. [Google Scholar] [CrossRef]
- Dragojevic, S.; Turner, L.; Pal, P.; Janorkar, A.V.; Raucher, D. Elastin-like Polypeptide Hydrogels for Tunable, Sustained Local Chemotherapy in Malignant Glioma. Pharmaceutics 2022, 14, 2072. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Huang, M.S.; Navarro, R.; Chiang, G.; Hu, C.; Oropeza, B.P.; Johansson, P.K.; Suhar, R.A.; Foster, A.A.; LeSavage, B.L.; et al. Elastin-like Protein Hydrogels with Controllable Stress Relaxation Rate and Stiffness Modulate Endothelial Cell Function. J. Biomed. Mater. Res. 2023, 111, 896–909. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Roy, S. Elastin-Inspired Supramolecular Hydrogels: A Multifaceted Extracellular Matrix Protein in Biomedical Engineering. Soft Matter 2021, 17, 3266–3290. [Google Scholar] [CrossRef]
- Tian, D.-M.; Wan, H.-H.; Chen, J.-R.; Ye, Y.-B.; He, Y.; Liu, Y.; Tang, L.-Y.; He, Z.-Y.; Liu, K.-Z.; Gao, C.-J.; et al. In-Situ Formed Elastin-Based Hydrogels Enhance Wound Healing via Promoting Innate Immune Cells Recruitment and Angiogenesis. Mater. Today Bio 2022, 15, 100300. [Google Scholar] [CrossRef]
- Stojic, M.; Ródenas-Rochina, J.; López-Donaire, M.L.; González De Torre, I.; González Pérez, M.; Rodríguez-Cabello, J.C.; Vojtová, L.; Jorcano, J.L.; Velasco, D. Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers 2021, 13, 2114. [Google Scholar] [CrossRef]
- Marsico, G.; Jin, C.; Abbah, S.A.; Brauchle, E.M.; Thomas, D.; Rebelo, A.L.; Orbanić, D.; Chantepie, S.; Contessotto, P.; Papy-Garcia, D.; et al. Elastin-like Hydrogel Stimulates Angiogenesis in a Severe Model of Critical Limb Ischemia (CLI): An Insight into the Glyco-Host Response. Biomaterials 2021, 269, 120641. [Google Scholar] [CrossRef]
- Contessotto, P.; Orbanić, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like Recombinamers-Based Hydrogel Modulates Post-Ischemic Remodeling in a Non-Transmural Myocardial Infarction in Sheep. Sci. Transl. Med. 2021, 13, eaaz5380. [Google Scholar] [CrossRef]
- Pal, P.; Nguyen, Q.C.; Benton, A.H.; Marquart, M.E.; Janorkar, A.V. Drug-Loaded Elastin-Like Polypeptide–Collagen Hydrogels with High Modulus for Bone Tissue Engineering. Macromol. Biosci. 2019, 19, 1900142. [Google Scholar] [CrossRef] [PubMed]



| Advantages | Disadvantages (Challenge) |
|---|---|
| Biocompatibility and biodegradability | Susceptibility to enzymatic degradation in vivo |
| Supports cell adhesion and proliferation | Alone, they usually provide poor mechanical strength, requiring crosslinking |
| Engineered to control drug release (sustained generally) | Batch-to-batch variability due to their natural origin |
| Injectable forms are available | Can be unstable against thermal or pH changes |
| Tunable physicochemical properties ensuring targetability | Limited shelf life due to biological instability |
| Can be modified to behave in a stimuli-responsive manner (pH, temperature, or enzymes) | Extraction and purification processes are difficult and complex |
| Excellent water-holding and swelling capacity mediates high drug loading capability | Difficult to sterilize them, increasing the risk of contamination with pathogens if they are derived from animal sources |
| Provides an extracellular matrix-like environment for cells | Without stabilization, they can degrade too easily, leading to potential burst drug release |
| Often non-toxic and non-immunogenic | The high water content can reduce loading capacity for hydrophobic drugs |
| Sustainable sourcing is possible with environmentally friendly production methods | If the mechanical strength is increased too much, the slow diffusion of large molecules may be hindered |
| Can be combined with polymers to enhance their physicochemical and applicability parameters further | Severe regulatory challenges due to their biological origin |
| Indication | Additional Agents | Main Result | Reference |
|---|---|---|---|
| Bone tissue regeneration | Sodium alginate |
| [32] |
| Bone tissue regeneration | Hyaluronic acid, chitosan |
| [45] |
| Bone tissue regeneration | Hyaluronic acid-tyrosine |
| [46] |
| Bone tissue regeneration | Sodium alginate, Hydroxyapatite |
| [47] |
| Bone tissue regeneration | Chitosan, lysine, hyaluronic acid |
| [51] |
| Lung carcinoma, breast tumor | Sodium alginate, immune stimulators |
| [42] |
| Wound repair | - |
| [43] |
| Wound repair | Guar gum, poly(N-isopropylacrylamide), graphene oxide, borax |
| [52] |
| Cartilage regeneration | Activated chondroitin sulphate, chondrocytes |
| [44] |
| Cartilage regeneration | Hyaluronic acid |
| [48] |
| Cartilage regeneration | Carbon dot nanoparticles |
| [49] |
| Cartilage regeneration | Icariin, hyaluronic acid |
| [50] |
| Indication | Additional Agents | Main Result | Reference |
|---|---|---|---|
| - | Bacterial cellulose |
| [60] |
| Wound healing | Cooper-oxide nanoparticles |
| [61] |
| - | Graphene |
| [58] |
| Cancer | Oxaliplatin, acrylic acid |
| [57] |
| Vocal fold scarring | Basic fibroblast growth factor |
| [62] |
| Post-myocardial infarct regeneration | Erythropoietin |
| [63] |
| Static nerve block | Bupivacaine |
| [64] |
| Infection control | Gentamicin sulphate |
| [59] |
| - | (model active substance), poly(N-isopropylacrylamide) |
| [65] |
| Inflammatory bowel disease | (model active substance), hyaluronic acid |
| [66] |
| Glaucoma | chitosan, curcumin, latanoprost |
| [67] |
| Indication | Additional Agents | Main Results | Reference |
|---|---|---|---|
| gastric cancer with peritoneal metastasis | Paclitaxel embedded in red blood cell membrane nanoparticles |
| [76] |
| prostate cancer | - |
| [77] |
| tissue regeneration | - |
| [79] |
| wound healing | - |
| [80] |
| tissue regeneration | - |
| [75] |
| bacterial infections | dihydromyricetin |
| [81] |
| Indication | Additional Agents | Main Results | Reference |
|---|---|---|---|
| bone regeneration | gelatin |
| [101] |
| bone regeneration | bone morphogenetic protein-2 and vascular endothelial growth factor |
| [102] |
| bone regeneration | hydroxyapatite nanoparticles |
| [103] |
| bone regeneration | - |
| [104] |
| cartilage regeneration | - |
| [99,106] |
| cartilage regeneration | polyvinyl alcohol |
| [109] |
| peripheral nerve injury | - |
| [110] |
| spinal cord regeneration | fibroblast growth factor |
| [111] |
| nerve regeneration | - |
| [112] |
| Indication | Additional Agents | Main Results | Reference |
|---|---|---|---|
| inflammation | - |
| [118] |
| dental pulp regeneration | chitosan |
| [114] |
| myocardial infarction | vascular endothelial growth factor, cardiomyocytes |
| [119] |
| spinal cord injury | neural progenitor cells |
| [120] |
| nerve tissue regeneration | basic fibroblast growth factor, epidermal growth factor, platelet-derived growth factor |
| [115] |
| Indication | Additional Agents | Main Results | Reference |
|---|---|---|---|
| wound healing | acryloyl-(polyethylene glycol)-N-hydroxysuccinimide ester |
| [127] |
| skin tissue regeneration | human plasma |
| [128] |
| vascular regeneration | hyaluronic acid |
| [123] |
| limb ischemia | - |
| [129] |
| ischemic heart disease | - |
| [130] |
| bone tissue regeneration | Recombinant human bone morphogenetic protein-2 |
| [131] |
| malignant glioblastoma | doxorubicin |
| [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katona, G.; Sipos, B.; Csóka, I. Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications. Gels 2025, 11, 306. https://doi.org/10.3390/gels11050306
Katona G, Sipos B, Csóka I. Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications. Gels. 2025; 11(5):306. https://doi.org/10.3390/gels11050306
Chicago/Turabian StyleKatona, Gábor, Bence Sipos, and Ildikó Csóka. 2025. "Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications" Gels 11, no. 5: 306. https://doi.org/10.3390/gels11050306
APA StyleKatona, G., Sipos, B., & Csóka, I. (2025). Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications. Gels, 11(5), 306. https://doi.org/10.3390/gels11050306








