Development and Application of Anthocyanin-Based Complex Polysaccharide Gels Based on Blueberry Pomace for Monitoring Beef Freshness
Abstract
:1. Introduction
2. Results and Discussion
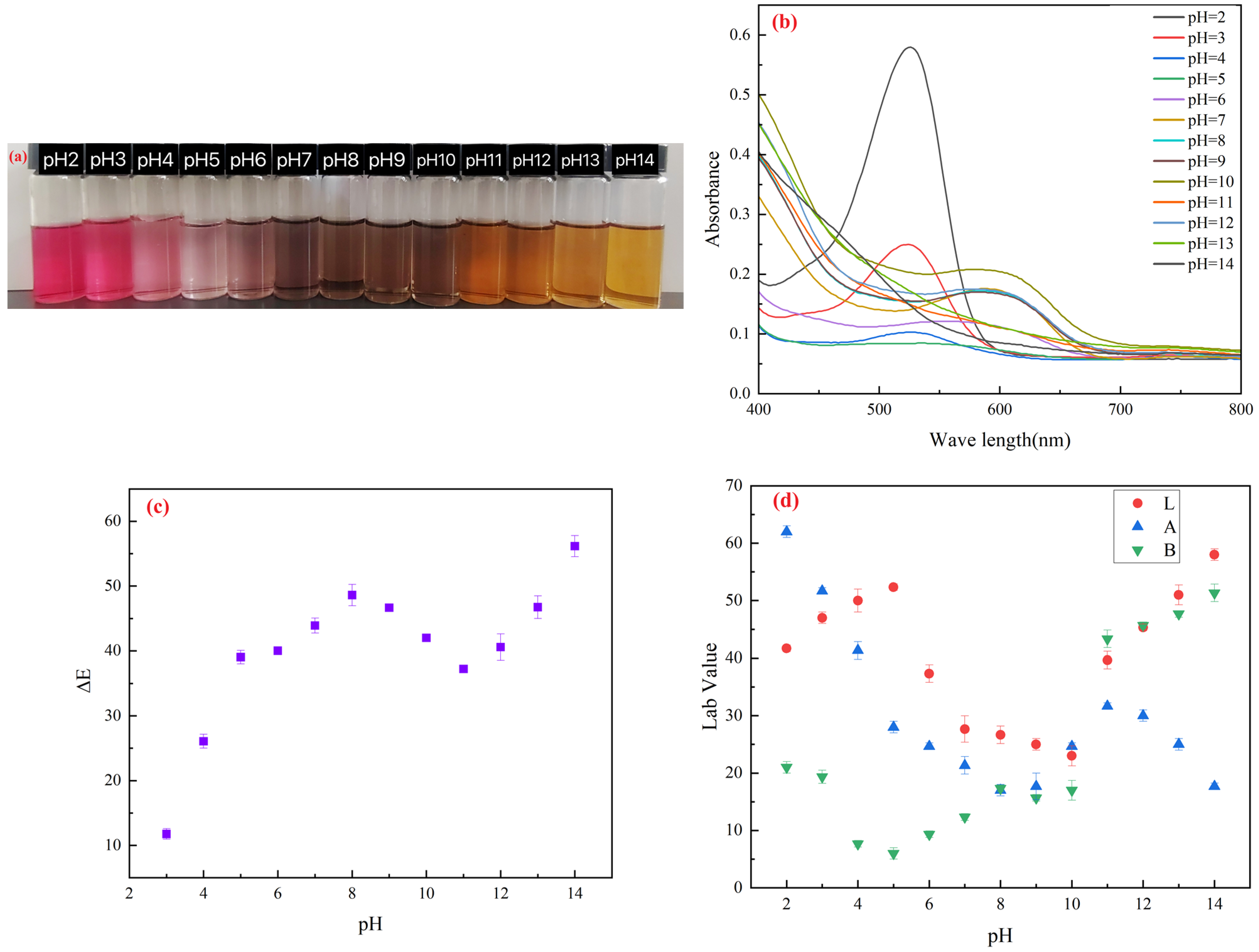
2.1. Quantification and Characterization of Anthocyanins from Blueberry Pomace
2.2. Characterization and Analysis of Complex Polysaccharide Gels
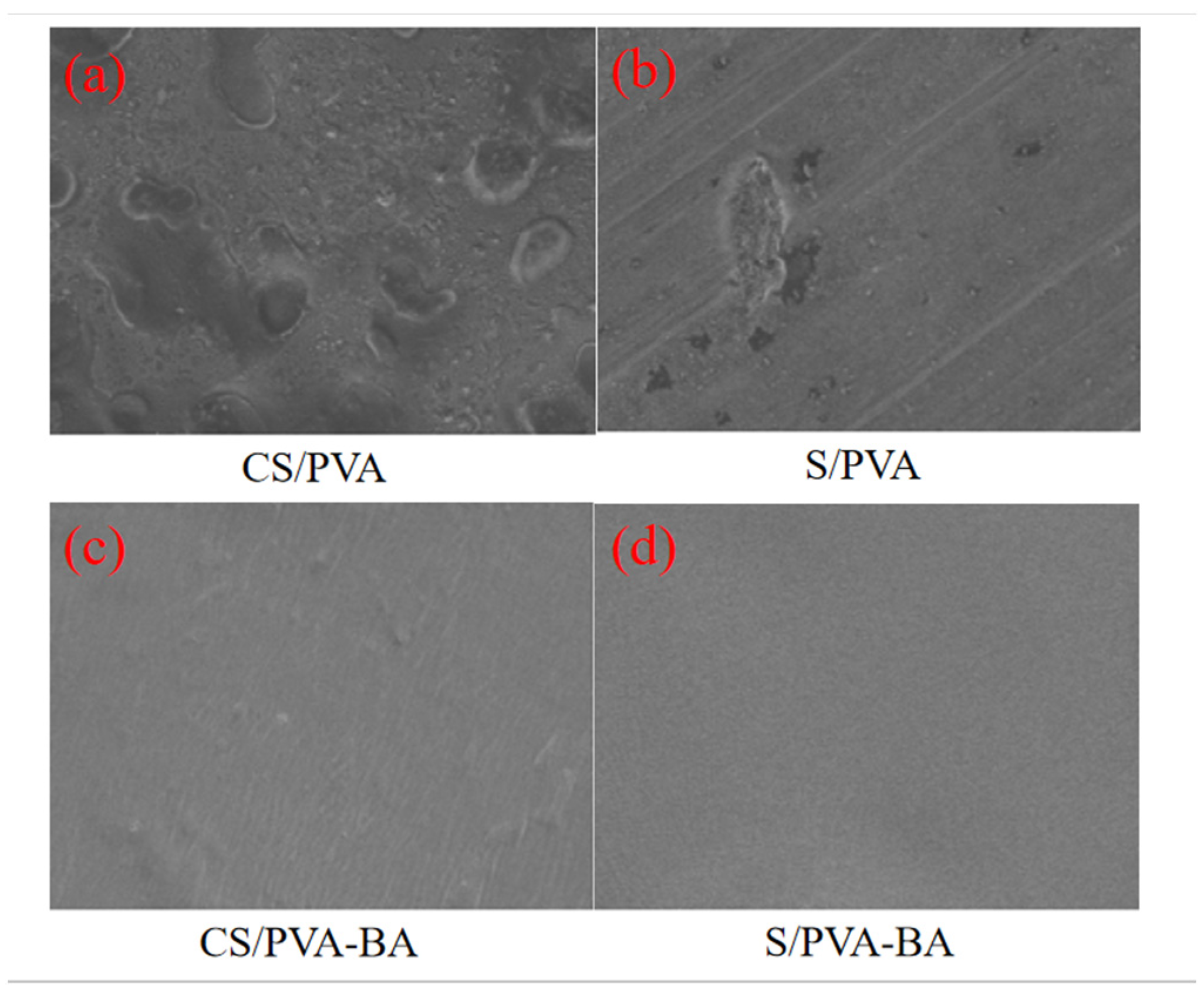
2.2.1. SEM Analysis of Gels
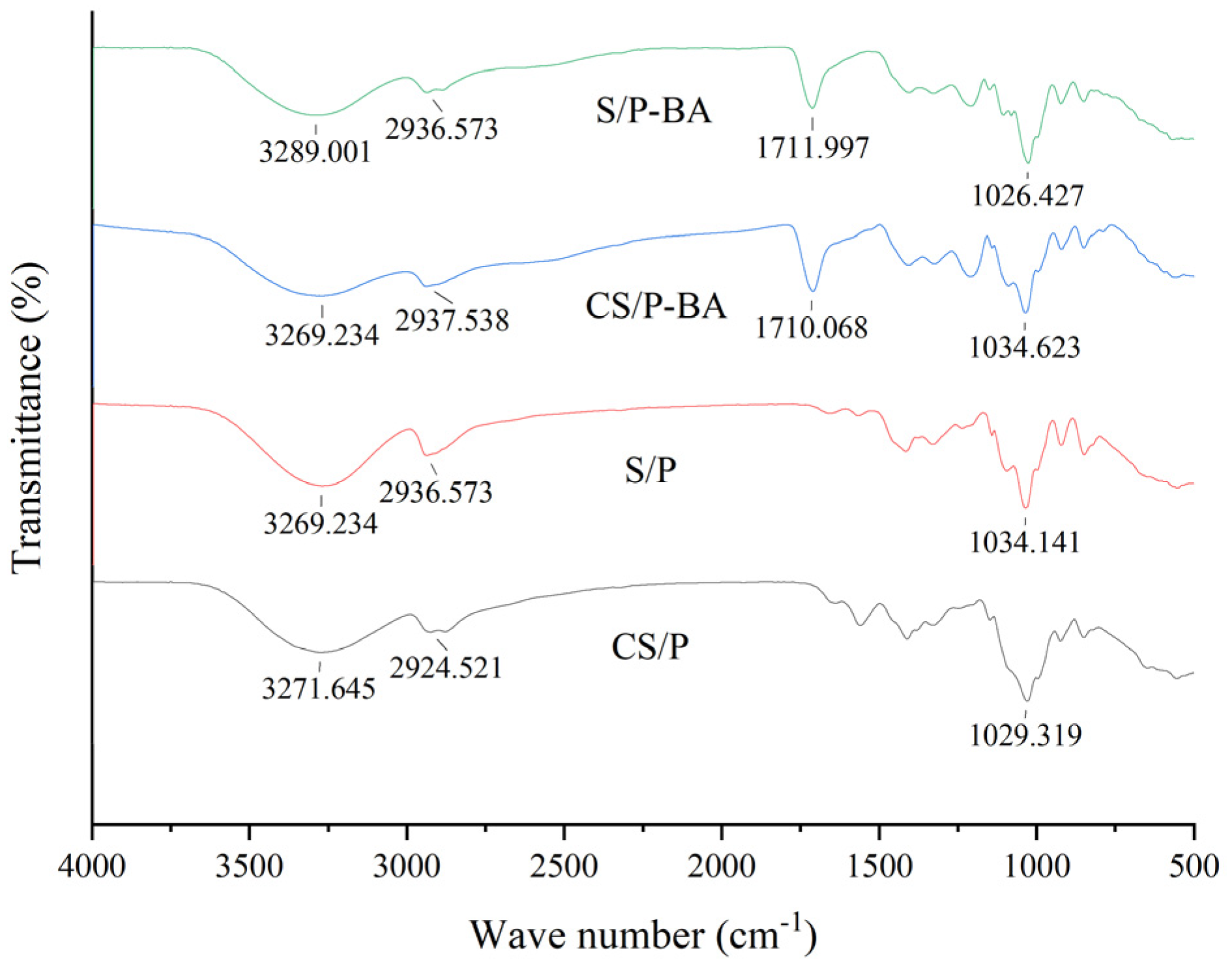
2.2.2. Infrared Spectrum Analysis of Gel
2.2.3. Analysis of Physical Properties of Gel
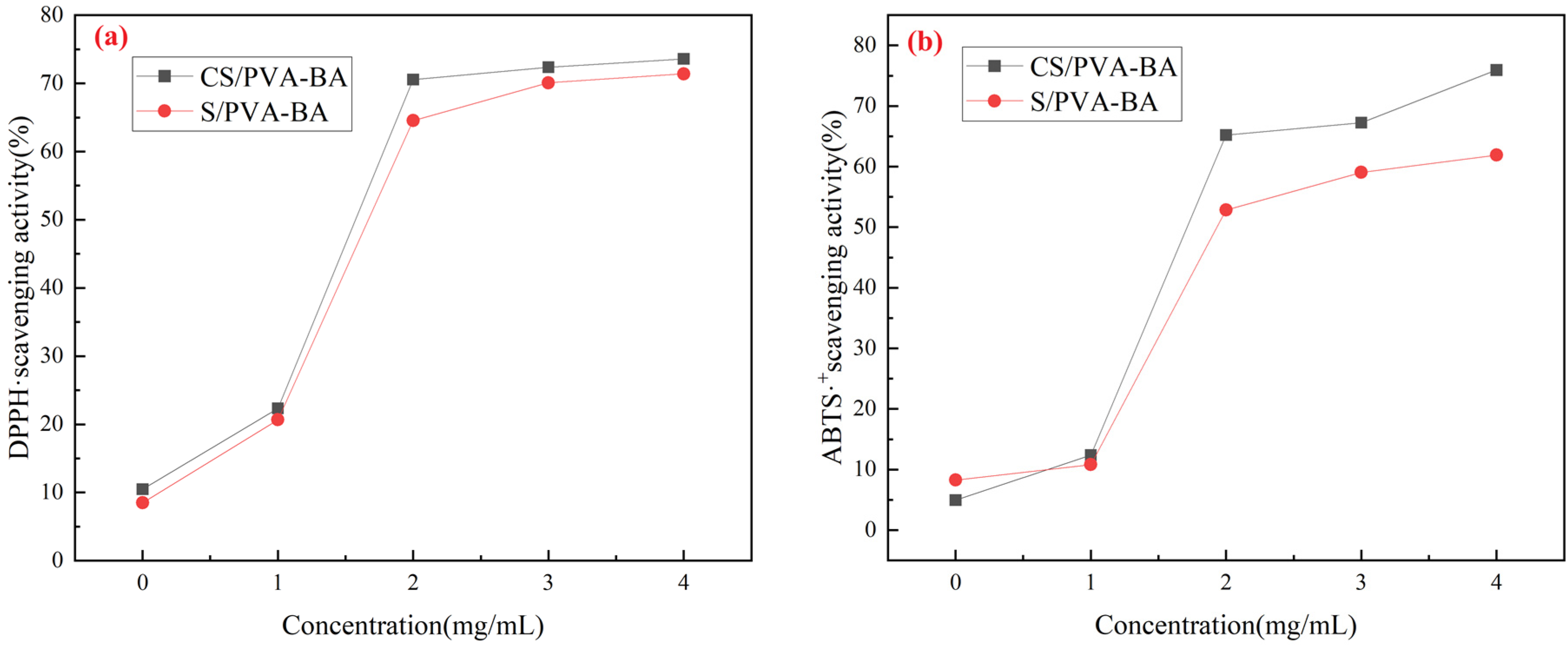
2.3. Antioxidant Properties of the Complex Polysaccharide Gels
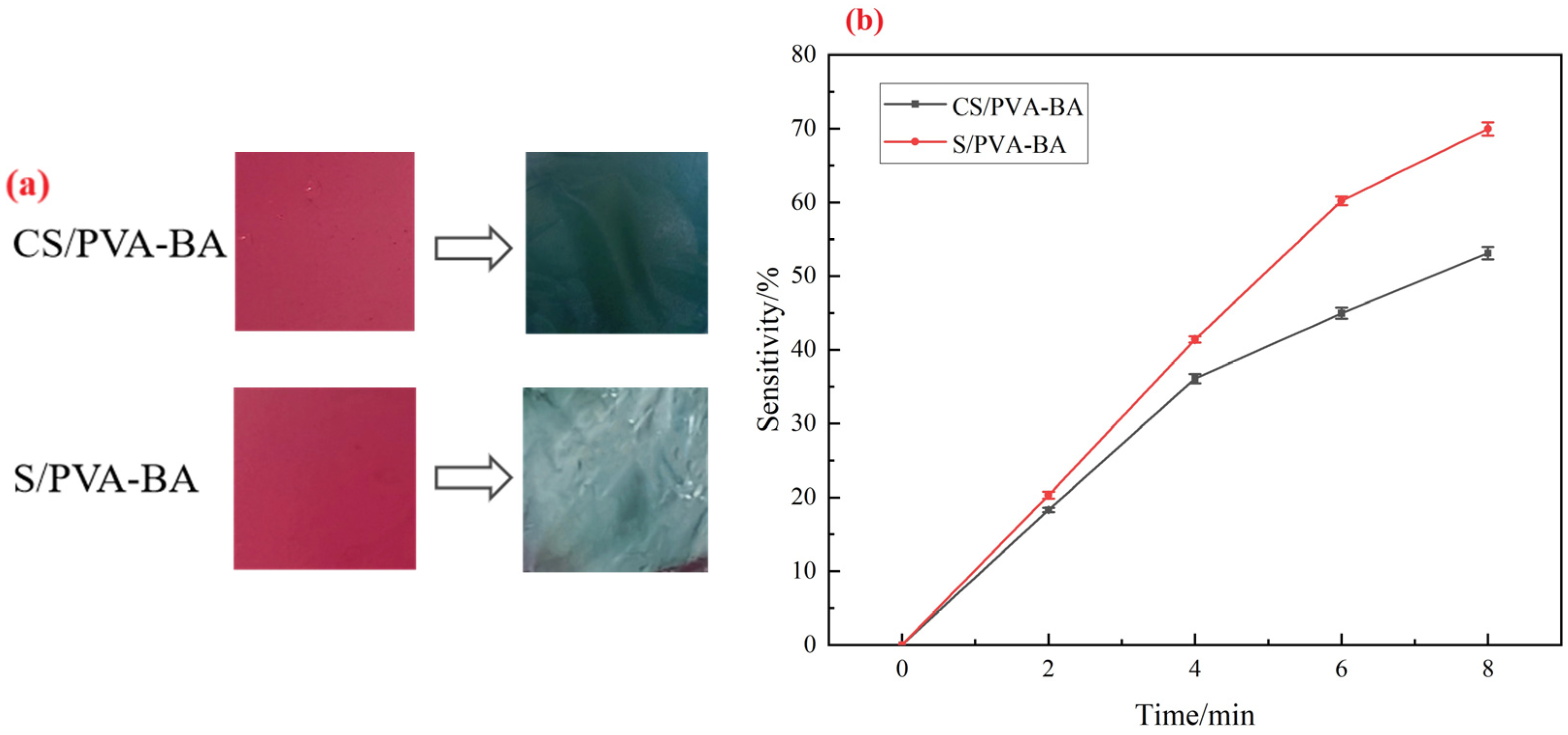
2.4. Analysis of Sensitivity of Complex Polysaccharide Gels to Ammonia
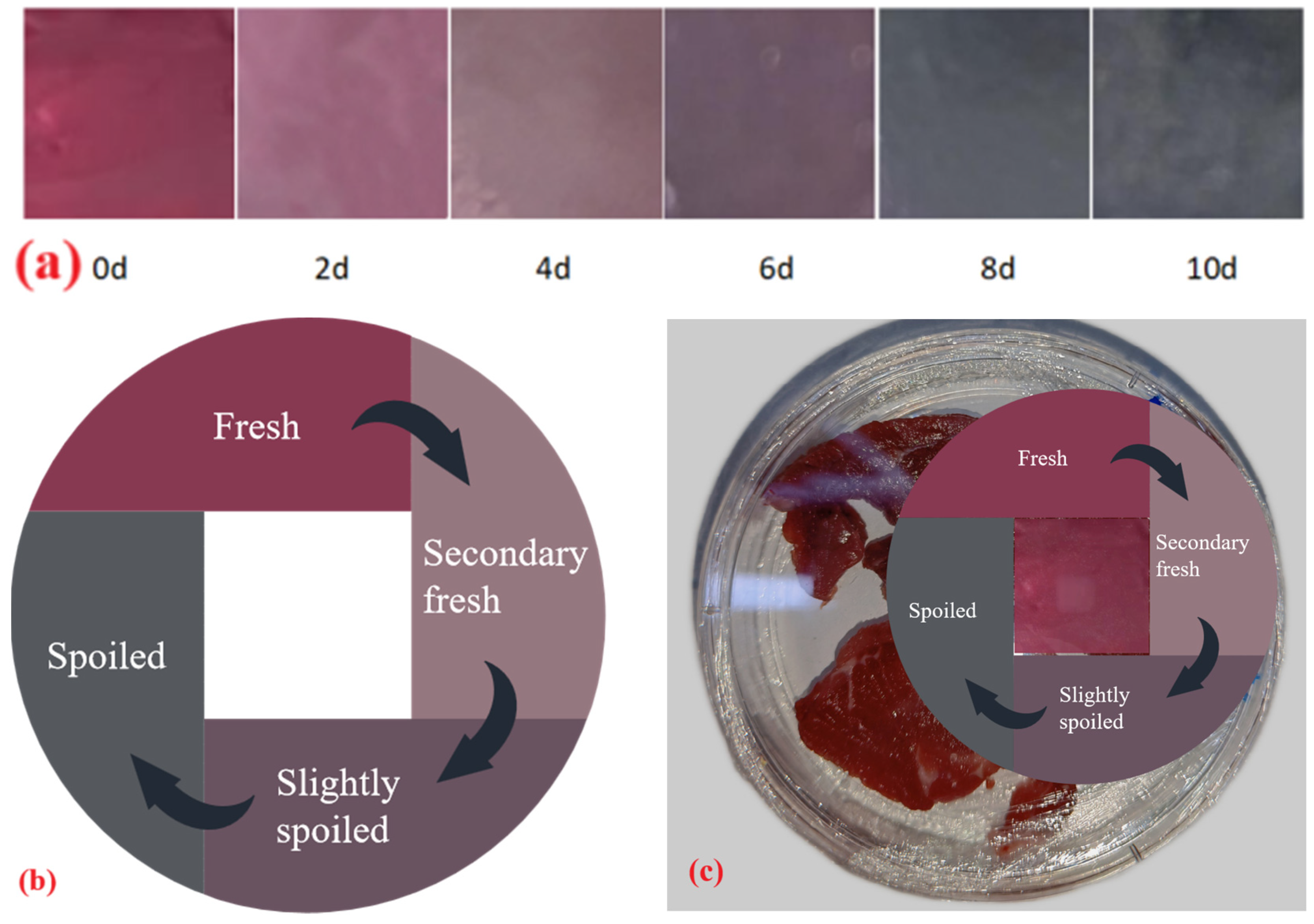
2.5. Application of Complex Polysaccharide Gels in Beef Freshness Monitoring
2.5.1. Analysis of Changes in Beef Physicochemical Properties
2.5.2. Freshness Monitoring Experiment
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Quantification of Anthocyanins from Blueberry Pomace
4.3. pH Response of Anthocyanin Solution
4.3.1. Color Response of Anthocyanins to pH
4.3.2. UV-VIS Spectra of Anthocyanins at Different pH Values
4.4. Preparation of Complex Polysaccharide Gels
4.5. Characterization of Gels
4.5.1. Microstructural Analysis
4.5.2. FT-IR Analysis
4.5.3. Thickness and Mechanical Properties
4.5.4. Moisture Content
4.5.5. Determination of Water Vapor Transmission Coefficient
4.6. Antioxidant Properties
4.6.1. DPPH Radical Scavenging Activity
4.6.2. ABTS Radical Scavenging Activity
4.7. Ammonia Sensitivity
4.8. Application in Beef Freshness Monitoring
4.8.1. Color Change
4.8.2. TVB-N Determination
4.8.3. pH Measurement
4.8.4. Total Bacterial Count
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Dai, L. Analysis of texture parameters and determination of freshness index of beef in low temperature storage. Trans. Chin. Soc. Agric. Eng. 2016, 32, 267–272. [Google Scholar]
- Zhang, R.; Xu, G.; Su, Y.; Rao, S. Potential Application of Ovalbumin Gel Nanoparticles Loaded with Carvacrol in the Preservation of Fresh Pork. Gels 2023, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Kang, Y.; Liu, L.J.; Wang, X.H. The effect of super-chilled preservation on shelf life and quality of beef during storage. Food Sci. Technol. 2022, 42, e73222. [Google Scholar] [CrossRef]
- Pitirollo, O.; Messinese, E.; Grimaldi, M.; Barbanti, D.; Cavazza, A. Effects of a Biobased Antioxidant Gel on Meat Shelf-Life: Oxidative Stability and Color as Quality Parameters. Gels 2025, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, Y.; Zhang, H.; Zhang, Q.Y.; Wu, X.D.; Wang, Y.; Sun, F.D.; Shi, S.; Xia, X.F. A review on improving the sensitivity and color stability of naturally sourced pH-sensitive indicator films. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13390. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Lee, K.; Baek, S.; Seo, J. Development of a pH indicator composed of high moisture-absorbing materials for real-time monitoring of chicken breast freshness. Food Sci. Biotechnol. 2017, 26, 37–42. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar] [CrossRef]
- Wang, P.K.; Liu, J.N.; Zhuang, Y.H.; Fei, P. Acylating blueberry anthocyanins with fatty acids: Improvement of their lipid solubility and antioxidant activities. Food Chem. X 2022, 15, 100420. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Agcam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef]
- Lei, L.; Zhou, J.; Huang, Y.; Zhao, H.; Zhao, M.; Chen, J. Optimization of Extraction Conditions of Anthocyanin from Blueberry Pomace and Its Antioxidant Activity. Sci. Technol. Food Ind. 2018, 39, 178–184. [Google Scholar]
- Akhtar, H.M.S.; Ahmed, S.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Brzezinska, M.S.; Dembinska, K.; Kalwasinska, A. Carboxymethyl cellulose based films enriched with polysaccharides from mulberry leaves (Morus alba L.) as new biodegradable packaging material. Int. J. Biol. Macromol. 2023, 253, 127633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef]
- Li, S.Z.; Ren, Y.; Hou, Y.J.; Zhan, Q.P.; Jin, P.; Zheng, Y.H.; Wu, Z.G. Polysaccharide-Based Composite Films: Promising Biodegradable Food Packaging Materials. Foods 2024, 13, 3674. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Fan, M.Z.; Luo, X.L.; Huang, L.L.; Chen, L.H. Acidic ionic liquid catalyzed crosslinking of oxycellulose with chitosan for advanced biocomposites. Carbohydr. Polym. 2014, 113, 108–114. [Google Scholar] [CrossRef]
- Liu, W.M.; Chen, L.; McClements, D.J.; Peng, X.W.; Xu, Z.L.; Jin, Z.Y. Development of starch film to realize the value-added utilization of starch in food and biomedicine. Food Biosci. 2024, 57, 103521. [Google Scholar] [CrossRef]
- Zong, Z.; Liu, M.; Chen, H.; Farag, M.A.; Wu, W.; Fang, X.; Niu, B.; Gao, H. Preparation and characterization of a novel intelligent starch/gelatin binary film containing purple sweet potato anthocyanins for Flammulina velutipes mushroom freshness monitoring. Food Chem. 2023, 405, 134839. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, L. Preparation and Application of Blueberry Anthocyanin Intelligent Indicator Films. J. Chin. Inst. Food Sci. Technol. 2022, 22, 281–290. [Google Scholar]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Chen, S.Y.; Qian, Y.F.; Wang, Y.; Lyu, L.; Zhou, X.H. EVOH encapsulating blueberry anthocyanin dispersed in PVA nanofibers for real-time shrimp freshness monitoring based on pH-responsive colorimetric. J. Text. Inst. 2024, 12, 1–16. [Google Scholar] [CrossRef]
- Prietto, L.; Pinto, V.Z.; El Halal, S.L.M.; de Morais, M.G.; Costa, J.A.V.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.D. Ultrafine fibers of zein and anthocyanins as natural pH indicator. J. Sci. Food Agric. 2018, 98, 2735–2741. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Zhang, P.; Zhang, B.; Qi, Y.; Xian, L.; Wang, W.; Chen, L. Advances in smart pH complex membranes based on anthocyanins from different extracts. Food Ferment. Ind. 2025, 51, 373–380. [Google Scholar]
- Yao, B.; Zhao, H.; Wu, W.; Li, W. Comparative Stability of Anthocyanins from Five Blueberry Cultivars. Food Sci. 2017, 38, 142–147. [Google Scholar]
- Zou, X.B.; Zhang, J.J.; Shi, J.Y.; Jiang, C.P.; Zhai, X.D.; Wang, S.; Zhao, H.; Liang, N.N. Intelligent Indicator Film Based on Roselle Anthocyanins for Monitoring Pork Freshness. Food Sci. 2017, 38, 243–248. [Google Scholar]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef]
- Dong, Y.; Li, W.; Sun, W.; Jia, L.; Li, L.; Jin, Z.; Sun, W. Porous poly-L-lactic acid nanofiber indicator membrane loaded with anthocyanins for non-destructive testing of mutton freshness. Food Ferment. Ind. 2024, 50, 278–283. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. International journal of biological macromolecules. Int. J. Biol. Macromol. 2024, 280, 135553. [Google Scholar]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Toro-Márquez, L.A.; Merino, D.; Mendieta, J.R. Hydrogen-bonding interactions and compostability of bionanocomposite films prepared from corn starch and nano-fillers with and without added Jamaica flower extract. Food Hydrocoll. 2019, 89, 283–293. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Kan, J.; Liu, J. Active/intelligent packaging films developed by immobilizing anthocyanins from purple sweetpotato and purple cabbage in locust bean gum, chitosan and κ-carrageenan-based matrices. Int. J. Biol. Macromol. 2022, 211, 238–248. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ooi, C.W.; Song, C.P.; Wang, C.Y.; Liu, B.L.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef]
- Liang, J.; Huang, F.; Yu, Y.; Ouyang, Y.; Hu, Y.; Xiang, D.; Shu, Y. Preparation and Properties of Starch/Polyvinyl Alcohol Composite. Plastics 2020, 49, 5. [Google Scholar]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Zou, J.H.; Zhong, H.M.; Jiang, C.; Zhu, G.C.; Lin, X.E.; Huang, Y.Y. Ginkgo biloba leaf polysaccharide-stabled selenium nanozyme as an efficient glutathione peroxidase mimic for the preservation of bananas and cherry tomatoes. Food Chem. 2024, 459, 140443. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.M.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Meda, N.R.; Stevanovic, T. Suitability of DPPH spiking for antioxidant screening in natural products: The example of galloyl derivatives from red maple bark extract. Anal. Bioanal. Chem. 2017, 409, 5225–5237. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Xiong, Z. Preparation of Complex Polysaccharide Gels with Zanthoxylum bungeanum Essential Oil and Their Application in Fish Preservation. Gels 2024, 10, 533. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioglu, A.; Altinkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Chen, R.; Li, H.; Wang, J.; He, Z. Preparation of antioxidant activity packaging film and its application in meat products. Food Ferment. Ind. 2021, 47, 287–294. [Google Scholar]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Vareltzis, K.; Koufidis, D.; Gavriilidou, E.; Papavergou, E.; Vasiliadou, S. Effectiveness of a natural rosemary (Rosmarinus officinalis) extract on the stability of filleted and minced fish during frozen storage. Z. Für Leb. Und-Forsch. A 1997, 205, 93–96. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Chéret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in postmortem aging in fish: Understanding of proteolysis and disorganization of the myofibrillar structure. Crit. Rev. Food Sci. Nutr. 2006, 46, 409–421. [Google Scholar] [CrossRef]
- Lin, Y.D.; Ma, J.; Cheng, J.H.; Sun, D.W. Visible detection of chilled beef freshness using a paper-based colourimetric sensor array combining with deep learning algorithms. Food Chem. 2024, 441, 138344. [Google Scholar] [CrossRef]
- Liu, X.X.; Song, X.S.; Gou, D.J.; Li, H.L.; Jiang, L.; Yuan, M.L.; Yuan, M.W. A polylactide based multifunctional hydrophobic film for tracking evaluation and maintaining beef freshness by an electrospinning technique. Food Chem. 2023, 428, 136784. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Ji, Z.T.; Zhang, M.; Yang, X.T. Porphyrin fluorescence imaging for real-time monitoring and visualization of the freshness of beef stored at different temperatures. Food Chem. 2024, 442, 138420. [Google Scholar] [CrossRef]
- Bhadury, D.; Nadeem, H.; Lin, M.; Dyson Jennifer, M.; Tuck Kellie, L.; Tanner, J. Application of on-pack pH indicators to monitor freshness of modified atmospheric packaged raw beef. Food Qual. Saf. 2024, 8, 710–719. [Google Scholar] [CrossRef]
- Hao, J.; Wang, J.; Wang, J.; Wang, S.; Zhao, C.; Fan, Z. A novel multifunctional bioactive film based on Gelidium amausli polysaccharide incorporated with lingonberry anthocyanin for fish freshness monitoring and preservation. LWT 2025, 215, 117173. [Google Scholar] [CrossRef]
- Xue, J. Anthocyanin-Based Intelligent Packaging Material for Monitoring Salmon Freshness. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2019. [Google Scholar]
- Golasz, L.B.; Silva, J.d.; Silva, S.B.d. Filme com antocianinas como indicador da deterioração de carne suína refrigerada. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- GB/T 1040.3-2006; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- Valencia, G.A.; Luciano, C.G.; Lourenço, R.V.; Bittante, A.; Sobral, P.J.D. Morphological and physical properties of nano-biocomposite films based on collagen loaded with laponite®. Food Packag. Shelf Life 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Huang, M.; Zhou, G. Antioxidant activity of peptides in postmortem aged duck meat as affected by cooking and in vitro digestion. Int. J. Food Prop. 2019, 22, 727–736. [Google Scholar] [CrossRef]
- Dodange, S.; Shekarchizadeh, H.; Kadivar, M. Real-time tracking of fish quality using a cellulose filter paper colorimetric indicator incorporated with prickly pear fruit betacyanins. LWT 2024, 205, 116523. [Google Scholar] [CrossRef]
- Kuswandi, B.; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- GB 5009.228-2016; National Food Safety Standard—Determination of Volatile Basic Nitrogen in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 4789.2-2016; National Food Safety Standard—Microbiological Examination of Food—Enumeration of Total Aerobic Bacterial Count. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.

| Complex Polysaccharide Gels Name | Thickness/μm | Tensile Strength/MPa | Elongation at Break/% | Moisture Content/% | Water Vapor Transmission Coefficient × 10−4/(g·mm)/(m2·h·Pa) |
|---|---|---|---|---|---|
| CS/PVA | 55.00 ± 2.00 c | 23.94 ± 1.08 c | 62.27 ± 1.26 b | 10.89 ± 0.23 b | 2.56 ± 0.12 b |
| S/PVA | 71.33 ± 1.51 b | 27.95 ± 1.69 a | 43.45 ± 1.09 c | 11.97 ± 0.57 a | 3.25 ± 0.31 ab |
| CS/PVA-BA | 84.48 ± 1.83 b | 25.42 ± 2.01 b | 87.84 ± 2.34 a | 8.33 ± 0.57 c | 2.68 ± 0.11 b |
| S/PVA-BA | 99.00 ± 1.55 a | 28.31 ± 1.28 a | 64.52 ± 2.33 b | 10.12 ± 0.15 b | 3.81 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, J.; Xu, F.; Yu, S.; Hao, J.; Wang, J.; Fan, Z. Development and Application of Anthocyanin-Based Complex Polysaccharide Gels Based on Blueberry Pomace for Monitoring Beef Freshness. Gels 2025, 11, 385. https://doi.org/10.3390/gels11060385
Zhi J, Xu F, Yu S, Hao J, Wang J, Fan Z. Development and Application of Anthocyanin-Based Complex Polysaccharide Gels Based on Blueberry Pomace for Monitoring Beef Freshness. Gels. 2025; 11(6):385. https://doi.org/10.3390/gels11060385
Chicago/Turabian StyleZhi, Jingxi, Fuqian Xu, Shuhuan Yu, Jiahui Hao, Jie Wang, and Ziluan Fan. 2025. "Development and Application of Anthocyanin-Based Complex Polysaccharide Gels Based on Blueberry Pomace for Monitoring Beef Freshness" Gels 11, no. 6: 385. https://doi.org/10.3390/gels11060385
APA StyleZhi, J., Xu, F., Yu, S., Hao, J., Wang, J., & Fan, Z. (2025). Development and Application of Anthocyanin-Based Complex Polysaccharide Gels Based on Blueberry Pomace for Monitoring Beef Freshness. Gels, 11(6), 385. https://doi.org/10.3390/gels11060385






