Caffeine as a Gelator
Abstract
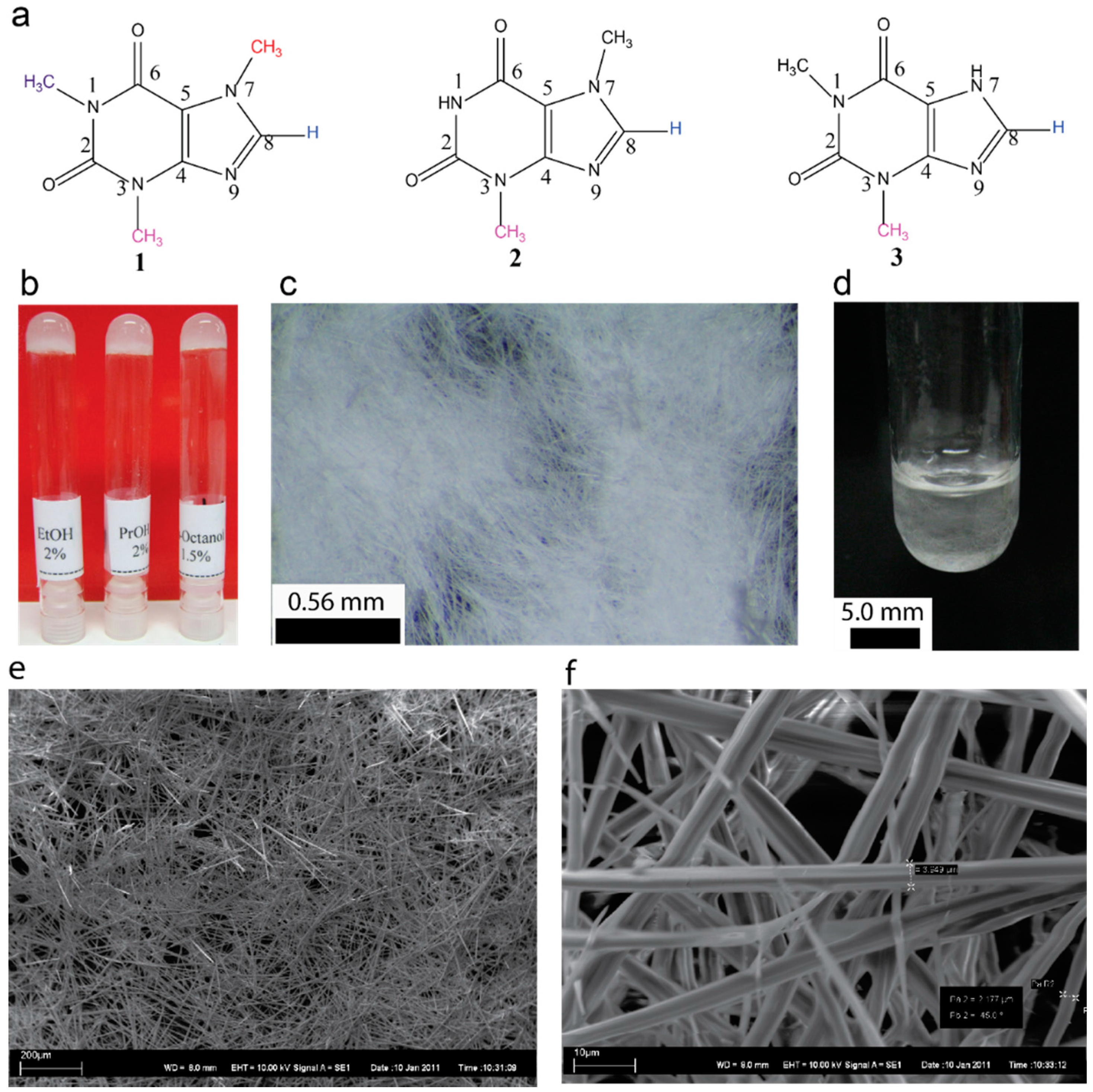
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Recrystallization of Caffeine
4.3. Gelation Tests
4.4. Scanning Electron Microscopy (SEM) Studies
4.5. Solid-State NMR Studies
4.6. Variable Temperature NMR of Toulene-d8 Gel
4.7. Variable Temperature NMR of 1-Octanol Gel
4.8. Elemental Analysis
- Elemental analysis of commercial caffeine: C, 49.31; H, 5.20; N, 28.62.
- Elemental analysis of recrystallized form of caffeine: C, 49.66; H, 5.26; N, 28.78.
- Theoretical composition of caffeine (C8H10N4O2): C, 49.48; H, 5.19; N, 28.85.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, A.R.F. A History of Coffee in Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Springer-Verlag: New York, NY, US, 1985; pp. 1–12. [Google Scholar]
- Weinberg, B.A.; Bealer, B.K. The World of Caffeine: The Science and Culture of the World’s Most Popular Drug; Routledge: New York, NY, USA, 2001. [Google Scholar]
- Suzuki, T.; Ashihara, H.; Waller, G.R. Purine and purine alkaloid metabolism in Camellia and Coffea plants. Phytochemistry 1992, 31, 2575–2584. [Google Scholar] [CrossRef]
- Alikaridis, F. Natural constituents of Ilex species. J. Ethnopharmacol. 1987, 20, 121–144. [Google Scholar] [CrossRef]
- Clifford, M.N.; Ramirez, J.R. Phenols and caffeine in wet-processed coffee beans and coffee pulp. Food Chem. 1991, 40, 35–42. [Google Scholar] [CrossRef]
- Mehr, C.B.; Biswal, R.N.; Collins, J.L.; Cochran, H.D. Supercritical carbon dioxide extraction of caffeine from guarana. J. Supercrit. Fluids 1996, 9, 185–191. [Google Scholar] [CrossRef]
- Li, S.; Hartland, S. A new industrial process for extracting cocoa butter and xanthines with supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1996, 73, 423–429. [Google Scholar] [CrossRef]
- Nathanson, J. Caffeine and related methylxanthines: Possible naturally occurring pesticides. Science 1984, 226, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.G.; Armstrong, J.W.; Campbell, E. Pest control: Caffeine as a repellent for slugs and snails. Nature 2002, 417, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, D.E.; Rimm, E.B.; Giovannucci, E.; Colditz, G.; Stampfer, M.; Willett, W. Coffee, caffeine, and cardiovascular disease in men. N. Engl. J. Med. 1990, 323, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345, 1181–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S167–S174. [Google Scholar]
- Song, F.; Qureshi, A.A.; Han, J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012, 72, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Kerzendorfer, C.; O’Driscoll, M. UVB and Caffeine: Inhibiting the DNA Damage Response to Protect Against the Adverse Effects of UVB. J. Invest. Dermatol. 2009, 129, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Sabisz, M.; Skladanowski, A. Modulation of cellular response to anticancer treatment by caffeine: Inhibition of cell-cycle checkpoints, DNA repair and more. Curr. Pharm. Biotechnol. 2008, 9, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.K.; Henry, R.F.; Lou, X.; Duers, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of caffeine and hydroxybenzoic acids composed of multiple supramolecular heterosynthons: Screening via solution-mediated phase transformation and structural characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]
- Leyssens, T.; Tumanova, N.; Robeyns, K.; Candoni, N.; Veesler, S. Solution cocrystallization, an effective tool to explore the variety of cocrystal systems: Caffeine/dicarboxylic acid cocrystals. CrystEngComm 2014, 16, 9603–9611. [Google Scholar] [CrossRef]
- Carlucci, L.; Gavezzotti, A. Molecular recognition and crystal energy landscapes: An X-ray and computational study of caffeine and other methylxanthines. Chem. Eur. J. 2004, 11, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Simo, A.; Fenger, R.; Christen, W.; Rademann, K.; Panne, U.; Emmerling, F. Morphological diversity of caffeine on surfaces: needles and hexagons. Cryst. Growth Des. 2012, 12, 583–588. [Google Scholar] [CrossRef]
- Schultheiss, N.; Roe, M.; Boerrigter, S.X.M. Cocrystals of nutraceutical p-coumaric acid with caffeine and theophylline: Polymorphism and solid-state stability explored in detail using their crystal graphs. CrystEngComm 2011, 13, 611–619. [Google Scholar] [CrossRef]
- Hedoux, A.; Guinet, Y.; Paccou, L.; Danede, F.; Derollez, P. Polymorphic transformation of anhydrous caffeine upon grinding and hydrostatic pressurizing analyzed by low-frequency raman spectroscopy. J. Pharm. Sci. 2013, 102, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Sutor, J. The structures of the pyrimidines and purines. VII. The crystal structure of caffeine. Acta Cryst. 1958, 11, 453–458. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Lawson, E.; de Matas, M.; Shields, L.; York, P. Metamorphosis of caffeine hydrate and anhydrous caffeine. J. Chem. Soc., Perkin Trans. 1997, 2, 1985–1990. [Google Scholar] [CrossRef]
- Lehmann, C.W.; Stowasser, F. The crystal structure of anhydrous β-caffeine as determined from X-ray powder-diffraction data. Chem. Eur. J. 2007, 13, 2908–2911. [Google Scholar] [CrossRef] [PubMed]
- Enright, G.D.; Terskikh, V.V.; Brouwer, D.H.; Ripmeester, J.A. The Structure of Two Anhydrous Polymorphs of Caffeine from single-crystal diffraction and ultrahigh-field solid-state 13C NMR Spectroscopy. Cryst. Growth Des. 2007, 7, 1406–1410. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Jones, W. Formation of tubular crystals of pharmaceutical compounds. Cryst. Growth Des. 2010, 10, 365–370. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials With Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Nonappa; Maitra, U. Unlocking the potential of bile acids in synthesis, supramolecular/materials chemistry and nanoscience. Org. Biomol. Chem. 2008, 6, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zinic, M.; Vögtle, F.; Fages, F. Cholesterol-based gelators. Top. Curr. Chem. 2005, 256, 39–76. [Google Scholar] [PubMed]
- Smith, D.K. Organic Nanostructures; Atwood, J.L., Steed, J.W., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 111–154. [Google Scholar]
- Weiss, R.G. Preface to the molecular and polymer gels; materials with self-assembled fibrillar networks special issue. Langmuir 2009, 25, 8369. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Lana, Y.; Corradinia, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Llansola, F.; Miravet, J.F.; Escuder, B. Aldehyde responsive supramolecular hydrogels: Towards biomarker-specific delivery system. Chem. Commun. 2011, 47, 4706–4708. [Google Scholar] [CrossRef] [PubMed]
- Bunzen, H.; Nonappa; Kalenius, E.; Hietala, S.; Kolehmainen, E. Subcomponent self-assembly: A quick way to novel metallogels. Chem. Eur. J. 2013, 19, 12978–12981. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, H.; Nonappa; Lahtinen, M.; Wimmer, Z.; Kolehmainen, E. A steroid-based gelator of A(LS)2 type: Tuning gel properties by metal coordination. Soft Matter 2012, 8, 7840–7847. [Google Scholar] [CrossRef]
- Svobodová, H.; Nonappa; Wimmer, Z.; Kolehmainen, E. Design, synthesis and stimuli responsive gelation of novel stigmasterol-amino acid conjugates. J. Colloid Interface Sci. 2011, 361, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Leiserowitz, L.; Addadi, L.; Weiner, S.; Hamilton, A.D. Characterization of an organic hydrogel: A cryo-TEM and X- ray diffraction study. Adv. Mater. 2003, 15, 38–42. [Google Scholar] [CrossRef]
- Wang, R.; Geiger, C.; Chen, L.; Swanson, B.; Whitten, D.G. Direct Observation of sol−gel conversion: The role of the solvent in organogel formation. J. Am. Chem. Soc. 2000, 122, 2399–2400. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.-Y.; Xiong, J.; Li, J. Real-time observation of fiber network formation in molecular organogel: Supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 2006, 110, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Nonappa; Maitra, U. Simple esters of cholic acid as potent organogelators: Direct imaging of the collapse of SAFINs. Soft Matter 2007, 3, 1428–1433. [Google Scholar] [CrossRef]
- Iqbal, S.; Rodríguez-Lansola, F.; Escuder, B.; Miravet, J.F.; Verbruggen, I.; Willem, R. HRMAS 1H NMR as a tool for the study of supramolecular gels. Soft Matter 2010, 6, 1875–1878. [Google Scholar] [CrossRef]
- Nonappa; Lahtinen, M.; Behera, B.; Kolehmainen, E.; Maitra, U. Unraveling the packing pattern leading to gelation using SS NMR and X-ray diffraction: Direct observation of the evolution of self-assembled fibers. Soft Matter 2010, 6, 1748–1757. [Google Scholar] [CrossRef]
- Nonappa; Šaman, D.; Kolehmainen, E. Studies on supramolecular gel formation using DOSY NMR. Mag. Res. Chem. 2015, 53, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Noponen, V.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E.; Sievänen, E. Bile acid–amino acid ester conjugates: Gelation, structural properties, and thermoreversible solid to solid phase transition. Soft Matter 2010, 6, 3789–3796. [Google Scholar] [CrossRef]
- Ikonen, S.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E. Bile acid-derived mono- and diketals—Synthesis, structural characterization and self-assembling properties. Org. Biomol. Chem. 2010, 8, 2784–2794. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonappa; Kolehmainen, E. Caffeine as a Gelator. Gels 2016, 2, 9. https://doi.org/10.3390/gels2010009
Nonappa, Kolehmainen E. Caffeine as a Gelator. Gels. 2016; 2(1):9. https://doi.org/10.3390/gels2010009
Chicago/Turabian StyleNonappa, and Erkki Kolehmainen. 2016. "Caffeine as a Gelator" Gels 2, no. 1: 9. https://doi.org/10.3390/gels2010009
APA StyleNonappa, & Kolehmainen, E. (2016). Caffeine as a Gelator. Gels, 2(1), 9. https://doi.org/10.3390/gels2010009







