Hydrogel Microparticles as Sensors for Specific Adhesion: Case Studies on Antibody Detection and Soil Release Polymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Functionalization of SCPs with Adhesion Molecules
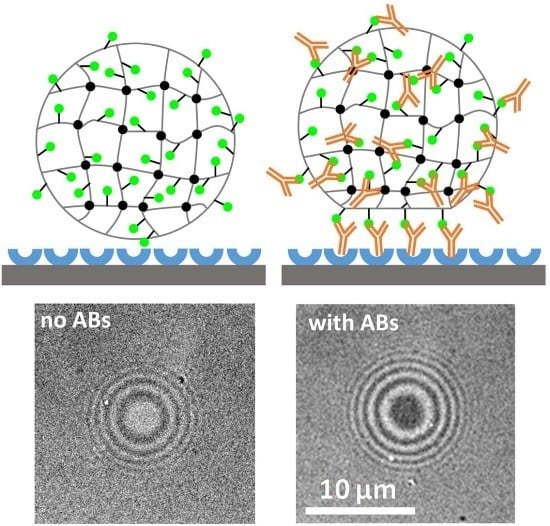
2.2. Detection of Antibodies with a Combined SCP Pull-Down and Adhesion Assay
2.3. Characterization of Soil Release Polymers by SCP Adhesion Assays
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Soft Colloidal Probe Preparation
4.3. SCP Characterization
4.4. Surface Preparation
4.5. Determination of Functionalization Degree via TBO Titration
4.6. Reflection Interference Contrast (RICM) Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [PubMed]
- Max, E.; Häfner, W.; Wilco Bartels, F.; Sugiharto, A.; Wood, C.; Fery, A. A novel AFM based method for force measurements between individual hair strands. Ultramicroscopy 2010, 110, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.N.M.; da-Silva, A.-C.; Tomé, B. Acoustic wave biosensors: Physical models and biological applications of quartz crystal microbalance. Trends Biotechnol. 2009, 27, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Dufrene, Y.F.; Pelling, A.E. Force nanoscopy of cell mechanics and cell adhesion. Nanoscale 2013, 5, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Wang, Y.M.; Huang, L.S.; Lin, S.M. Atomic force microscopy: Determination of unbinding force, off rate and energy barrier for protein-ligand interaction. Micron 2007, 38, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.A.; Ulbricht, M.; Belfort, G. Intermolecular forces between a protein and a hydrophilic modified polysulfone film with relevance to filtration. Langmuir 2000, 16, 10419–10427. [Google Scholar] [CrossRef]
- Pussak, D.; Behra, M.; Schmidt, S.; Hartmann, L. Synthesis and functionalization of poly(ethylene glycol) microparticles as soft colloidal probes for adhesion energy measurements. Soft Matter 2012, 8, 1664–1672. [Google Scholar] [CrossRef]
- Moy, V.T.; Jiao, Y.K.; Hillmann, T.; Lehmann, H.; Sano, T. Adhesion energy of receptor-mediated interaction measured by elastic deformation. Biophys. J. 1999, 76, 1632–1638. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. surface energy and contact of elastic solids. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1971, 324, 301. [Google Scholar] [CrossRef]
- Limozin, L.; Sengupta, K. Quantitative Reflection Interference Contrast Microscopy (RICM) in Soft Matter and Cell Adhesion. Chem. Phys. Chem. 2009, 10, 2752–2768. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Wang, H.; Hartmann, L.; Pompe, T.; Schmidt, S. Quantification of protein-materials interaction by soft colloidal probe spectroscopy. Phys. Chem. Chem. Phys. PCCP 2015, 17, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Reinecke, A.; Wojcik, F.; Pussak, D.; Hartmann, L.; Harrington, M.J. Metal-mediated molecular self-healing in histidine-rich mussel peptides. Biomacromolecules 2014, 15, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Pussak, D.; Ponader, D.; Mosca, S.; Ruiz, S.V.; Hartmann, L.; Schmidt, S. Mechanical Carbohydrate Sensors Based on Soft Hydrogel Particles. Angew. Chem. Int. Ed. 2013, 52, 6084–6087. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Wang, H.; Rathke, T.; Anderegg, U.; Möller, S.; Schnabelrauch, M.; Pompe, T.; Schmidt, S. Polymer hydrogel particles as biocompatible AFM probes to study CD44/hyaluronic acid interactions on cells. Polymer 2016, 102, 342–349. [Google Scholar] [CrossRef]
- Pussak, D.; Ponader, D.; Mosca, S.; Pompe, T.; Hartmann, L.; Schmidt, S. Specific adhesion of carbohydrate hydrogel particles in competition with multivalent inhibitors evaluated by AFM. Langmuir 2014, 30, 6142–6150. [Google Scholar] [CrossRef] [PubMed]
- Helfricht, N.; Doblhofer, E.; Bieber, V.; Lommes, P.; Sieber, V.; Scheibel, T.; Papastavrou, G. Probing the adhesion properties of alginate hydrogels: A new approach towards the preparation of soft colloidal probes for direct force measurements. Soft Matter 2017, 13, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman: New York, NJ, USA, 2010. [Google Scholar]
- Lei, Q.Q.; Liu, J.W.; Zheng, H. Potential role of anti-p53 antibody in diagnosis of lung cancer: Evidence from a bivariate meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3012–3018. [Google Scholar] [PubMed]
- Simon, J.A.; Cabiedes, J.; Ortiz, E.; Alcocer-Varela, J.; Sanchez-Guerrero, J. Anti-nucleosome antibodies in patients with systemic lupus erythematosus of recent onset. Potential utility as a diagnostic tool and disease activity marker. Rheumatology 2004, 43, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Wang, H.; Pussak, D.; Mosca, S.; Hartmann, L. Probing multivalency in ligand-receptor-mediated adhesion of soft, biomimetic interfaces. Beilstein J. Org. Chem. 2015, 11, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Dorman, G.; Nakannura, H.; Pulsipher, A.; Prestwich, G.D. The Life of Pi Star: Exploring the Exciting and Forbidden Worlds of the Benzophenone Photophore. Chem. Rev. 2016, 116, 15284–15398. [Google Scholar] [CrossRef] [PubMed]
- Glaubitz, M.; Medvedev, N.; Pussak, D.; Hartmann, L.; Schmidt, S.; Helm, C.A.; Delcea, M. A novel contact model for AFM indentation experiments on soft spherical cell-like particles. Soft Matter 2014, 10, 6732–6741. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.M.; Voss, E.W. Mechanism of quenching of fluorescein by anti-fluorescein igg antibodies. Immunochemistry 1977, 14, 533–541. [Google Scholar] [CrossRef]
- Liu, M.Y.; Jia, C.P.; Huang, Y.Y.; Lou, X.H.; Yao, S.H.; Jin, Q.H.; Zhao, J.L.; Xiang, J.Q. Highly sensitive protein detection using enzyme-labeled gold nanoparticle probes. Analyst 2010, 135, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.P.; Zhong, X.Q.; Hua, B.; Liu, M.Y.; Jing, F.X.; Lou, X.H.; Yao, S.H.; Xiang, J.Q.; Jin, Q.H.; Zhao, J.L. Nano-ELISA for highly sensitive protein detection. Biosens. Bioelectron. 2009, 24, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, M.M.; Yuan, L.; Cheng, Z.P.; Wu, Z.Q.; Chen, H. Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. Analyst 2012, 137, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Garcia-D’Angeli, A.; Brennan, J.P.; Huo, Q. Predicting detection limits of enzyme-linked immunosorbent assay (ELISA) and bioanalytical techniques in general. Analyst 2014, 139, 439–445. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NJ, USA, 1979. [Google Scholar]
- Erath, J.; Schmidt, S.; Fery, A. Characterization of adhesion phenomena and contact of surfaces by soft colloidal probe AFM. Soft Matter 2010, 6, 1432–1437. [Google Scholar] [CrossRef]
- O’Lenick, A.J. Soil release polymers. J. Surfactants Deterg. 1999, 2, 553–557. [Google Scholar] [CrossRef]
- Sheth, S.R.; Leckband, D. Measurements of attractive forces between proteins and end-grafted poly(ethylene glycol) chains. Proc. Natl. Acad. Sci. USA 1997, 94, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Sedeva, I.G.; Fornasiero, D.; Ralston, J.; Beattie, D.A. The Influence of Surface Hydrophobicity on Polyacrylamide Adsorption. Langmuir 2009, 25, 4514–4521. [Google Scholar] [CrossRef] [PubMed]
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)-and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J. The different faces of poly(ethylene glycol). Proc. Natl. Acad. Sci. USA 1997, 94, 8378–8379. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Choi, S.Y.; Shim, B.; Lee, J.; Cuddihy, M.; Kotov, N.A. Molecularly engineered nanocomposites: Layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules 2005, 6, 2914–2918. [Google Scholar] [CrossRef] [PubMed]







| Polymer | Adhesion Energies [µJ m−2] | ||
|---|---|---|---|
| Antiredeposition | Antiadhesive Coating | Direct Binding | |
| none/reference | 1700 a | ||
| Poly(propylene terephthalate)-co-Poly(ethylene glycol) (nonionic) PPT-co-PEG | 41 | 1038 | 38 b |
| Copolymer A: cationic/neutral hydrophilic ratio 22:78 | 163 | 480 | 91 |
| Copolymer B: cationic/neutral hydrophilic ratio 70:30 | 306 | 1020 | 126 |
| Poly(acrylamide) | 1142 | 1429 | 243 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelczyk, A.K.; Wang, H.; Lindhorst, A.; Waschke, J.; Pompe, T.; Kropf, C.; Luneau, B.; Schmidt, S. Hydrogel Microparticles as Sensors for Specific Adhesion: Case Studies on Antibody Detection and Soil Release Polymers. Gels 2017, 3, 31. https://doi.org/10.3390/gels3030031
Strzelczyk AK, Wang H, Lindhorst A, Waschke J, Pompe T, Kropf C, Luneau B, Schmidt S. Hydrogel Microparticles as Sensors for Specific Adhesion: Case Studies on Antibody Detection and Soil Release Polymers. Gels. 2017; 3(3):31. https://doi.org/10.3390/gels3030031
Chicago/Turabian StyleStrzelczyk, Alexander Klaus, Hanqing Wang, Andreas Lindhorst, Johannes Waschke, Tilo Pompe, Christian Kropf, Benoit Luneau, and Stephan Schmidt. 2017. "Hydrogel Microparticles as Sensors for Specific Adhesion: Case Studies on Antibody Detection and Soil Release Polymers" Gels 3, no. 3: 31. https://doi.org/10.3390/gels3030031
APA StyleStrzelczyk, A. K., Wang, H., Lindhorst, A., Waschke, J., Pompe, T., Kropf, C., Luneau, B., & Schmidt, S. (2017). Hydrogel Microparticles as Sensors for Specific Adhesion: Case Studies on Antibody Detection and Soil Release Polymers. Gels, 3(3), 31. https://doi.org/10.3390/gels3030031