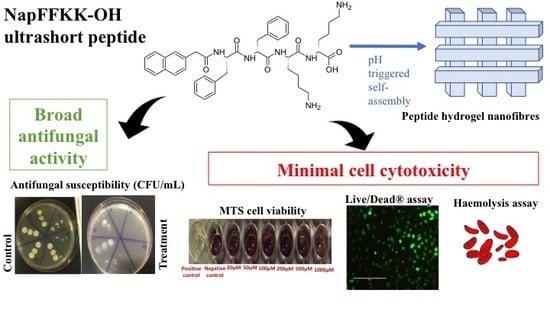
Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogel Formation and Characterisation
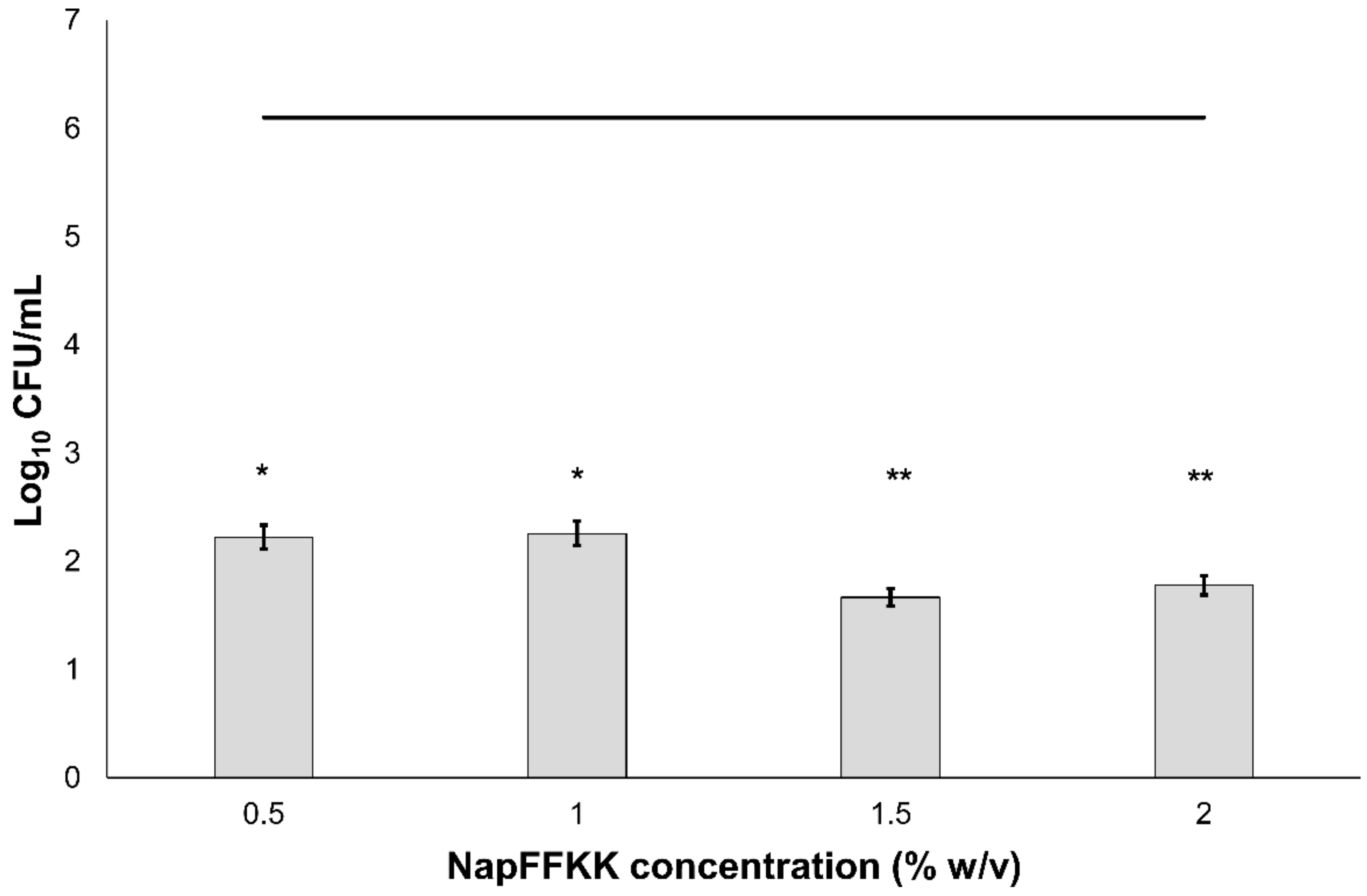
2.2. Fungal Susceptibility
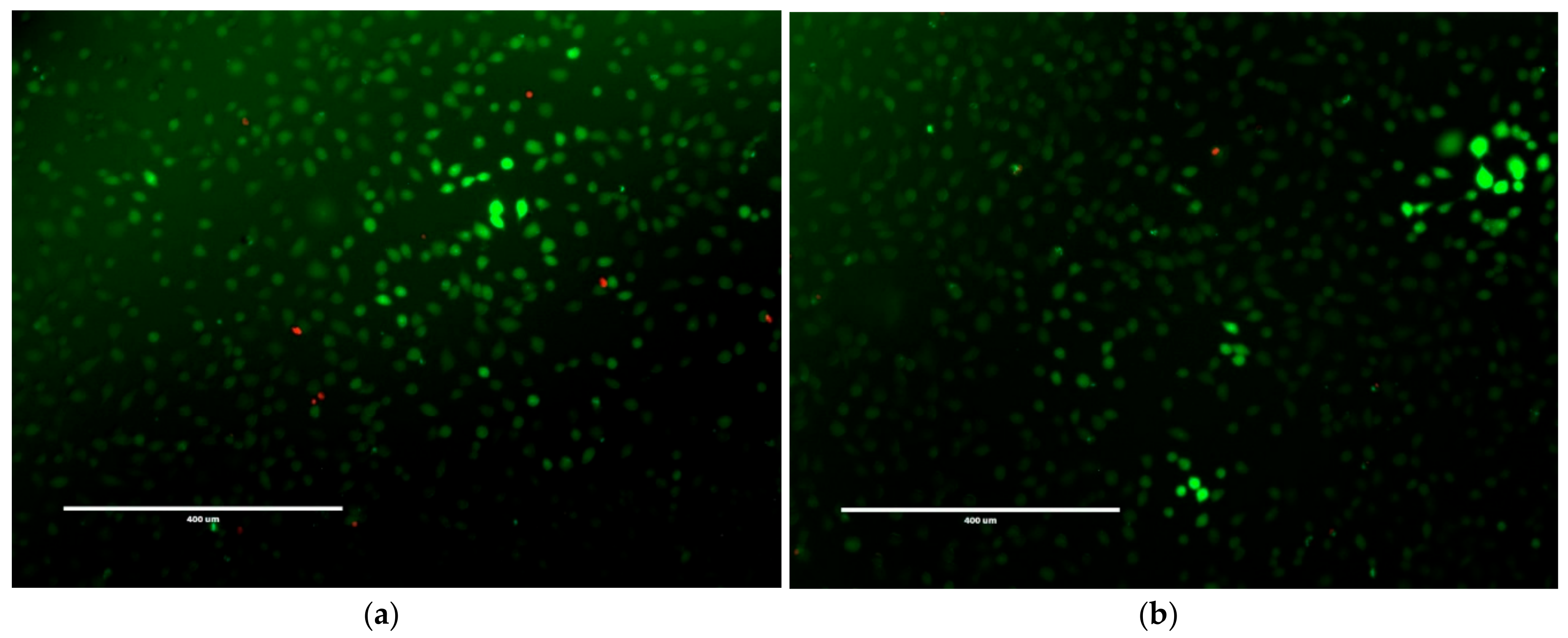
2.3. Mammalian Cell Cytotoxicity and Viability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis
4.2.2. Hydrogel Formulation
4.2.3. Scanning Electron Microscopy
4.2.4. Fungal Susceptibility Assay
4.2.5. Cell Cytotoxicity and Viability Assays
4.2.6. Haemolysis Assay
4.2.7. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kriengkauykiat, J.; Ito, J.I.; Dadwal, S.S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 2011, 3, 175–191. [Google Scholar] [PubMed]
- Venkatesan, P.; Perfect, J.R.; Myers, S.A. Evaluation and management of fungal infections in immunocompromised patients. Dermatol. Ther. 2005, 18, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Orlicka, K.; Barnes, E.; Culver, E.L. Prevention of infection caused by immunosuppressive drugs in gastroenterology. Ther. Adv. Chronic Dis. 2013, 4, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.; Lamont-Friedrich, S.J.; Michl, T.D.; Griesser, H.J.; Coad, B.R. The importance of fungal pathogens and antifungal coatings in medical device infections. Biotechnol. Adv. 2018, 36, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Lin, X. Recent progress on antifungal drug development. Curr. Pharm. Biotechnol. 2011, 12, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in us hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Zaas, A.K.; Alexander, B.D. Echinocandins: Role in antifungal therapy, 2005. Expert Opin. Pharmacother. 2005, 6, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Guillot, J.; Arne, P.; de Hoog, G.S.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: A global health concern with parallels to human disease. Med. Mycol. 2015, 53, 765–797. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Bromley, M.J. Infectious disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging threats in antifungal-resistant fungal pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.M.; Dotson, E.; Sarosi, G.A.; Hage, C.A. Traditional and emerging antifungal therapies. Proc. Am. Thorac. Soc. 2010, 7, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal Resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, I.P.; Kakkar, S. Topical delivery of antifungal agents. Expert Opin. Drug Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. The potential of antimicrobial peptides as biocides. Int. J. Mol. Sci. 2011, 12, 6566–6596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondaryk, M.; Staniszewska, M.; Zielinska, P.; Urbanczyk-Lipkowska, Z. Natural antimicrobial peptides as inspiration for design of a new generation antifungal compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des. 2010, 75, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Current perspectives on echinocandin class drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Breeuwer, P.; van’t Hof, W.; Walgreen-Weterings, E.; Oomen, L.C.; Veerman, E.C.; Amerongen, A.V.; Abee, T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 1999, 274, 7286–7291. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.P.; Gilmore, B.F.; Laverty, G. Evolution of antimicrobial peptides to self-assembled peptides for biomaterial applications. Pathogens 2014, 3, 791–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverty, G.; McCloskey, A.P.; Gilmore, B.F.; Jones, D.S.; Zhou, J.; Xu, B. Ultrashort cationic naphthalene-derived self-assembled peptides as antimicrobial nanomaterials. Biomacromolecules 2014, 15, 3429–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloskey, A.P.; Gilmore, S.M.; Zhou, J.; Draper, E.R.; Porter, S.; Gilmore, B.F.; Xu, B.; Laverty, G. Self-assembling ultrashort NSAID-peptide nanosponges: Multifunctional antimicrobial and anti-inflammatory materials. RSC Adv. 2016, 6, 114738–114749. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Draper, E.R.; Gilmore, B.F.; Laverty, G. Ultrashort self-assembling Fmoc-peptide gelators for anti-infective biomaterial applications. J. Pept. Sci. 2017, 23, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, C.J.; Ryan, D.M.; Nissan, D.A.; Nilsson, B.L. The effect of increasing hydrophobicity on the self-assembly of amphipathic beta-sheet peptides. Mol. Biosyst. 2009, 5, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, S. Antifungal effect of antimicrobial peptides (AMPs LR14) derived from Lactobacillus plantarum strain LR/14 and their applications in prevention of grain spoilage. Food Microbiol. 2014, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Leng, Q.; Begum, M.D.; Woodle, M.C.; Scaria, P.; Chou, S.T.; Mixson, A.J. Peptide-based antifungal therapies against emerging infections. Drugs Future 2010, 35, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A Polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. d-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.; Chan, J.M.; Sardon, H.; Ono, R.J.; Garcia, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.S.; Sinthuvanich, C.; Gaspar, D.; Franquelim, H.G.; Castanho, M.A.; Schneider, J.P. Arginine-rich self-assembling peptides as potent antibacterial gels. Biomaterials 2012, 33, 8907–8916. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yan, J.; Dang, W.; Xie, J.; Yan, B.; Yan, W.; Sun, M.; Zhang, B.; Ma, M.; Zhao, Y.; et al. Dual antifungal properties of cationic antimicrobial peptides polybia-MPI: Membrane integrity disruption and inhibition of biofilm formation. Peptides 2014, 56, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Venuleo, C.; Beri, A.; Oppenheim, F.G. Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast 2005, 22, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Wang, L.; Xu, B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J. Am. Chem. Soc. 2006, 128, 3038–3043. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, J.; Nagaraj, H.; McCloskey, A.P.; Huwaitat, R.; Porter, S.; Albadr, A.; Laverty, G. Peptide therapeutics and the pharmaceutical industry: Barriers encountered translating from the laboratory to patients. Curr. Med. Chem. 2016, 23, 4231–4259. [Google Scholar] [CrossRef] [PubMed]
- Arnusch, C.J.; Ulm, H.; Josten, M.; Shadkchan, Y.; Osherov, N.; Sahl, H.G.; Shai, Y. Ultrashort peptide bioconjugates are exclusively antifungal agents and synergize with cyclodextrin and amphotericin b. Antimicrob. Agents Chemother. 2012, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]








| Formulation Step | Constituent | Quantity |
|---|---|---|
| 1 | NapFFKK-OH | 10 mg pre-weighed |
| 2 | Deionised H2O | 200 µL (in 50 µL aliquots) |
| 3 | 1 M NaOH | 50 µL (in 10 µL aliquots) |
| 4 | Deionised H2O | 200 µL (in 50 µL aliquots) |
| 5 | 0.5 M HCl | 20 µL (in 10 µL aliquots) |
| 6 | Deionised H2O | To 500 µL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albadr, A.A.; Coulter, S.M.; Porter, S.L.; Thakur, R.R.S.; Laverty, G. Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections. Gels 2018, 4, 48. https://doi.org/10.3390/gels4020048
Albadr AA, Coulter SM, Porter SL, Thakur RRS, Laverty G. Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections. Gels. 2018; 4(2):48. https://doi.org/10.3390/gels4020048
Chicago/Turabian StyleAlbadr, Alyaa A., Sophie M. Coulter, Simon L. Porter, Raghu Raj Singh Thakur, and Garry Laverty. 2018. "Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections" Gels 4, no. 2: 48. https://doi.org/10.3390/gels4020048
APA StyleAlbadr, A. A., Coulter, S. M., Porter, S. L., Thakur, R. R. S., & Laverty, G. (2018). Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections. Gels, 4(2), 48. https://doi.org/10.3390/gels4020048