Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO
Abstract
1. Introduction
2. Results and Discussion
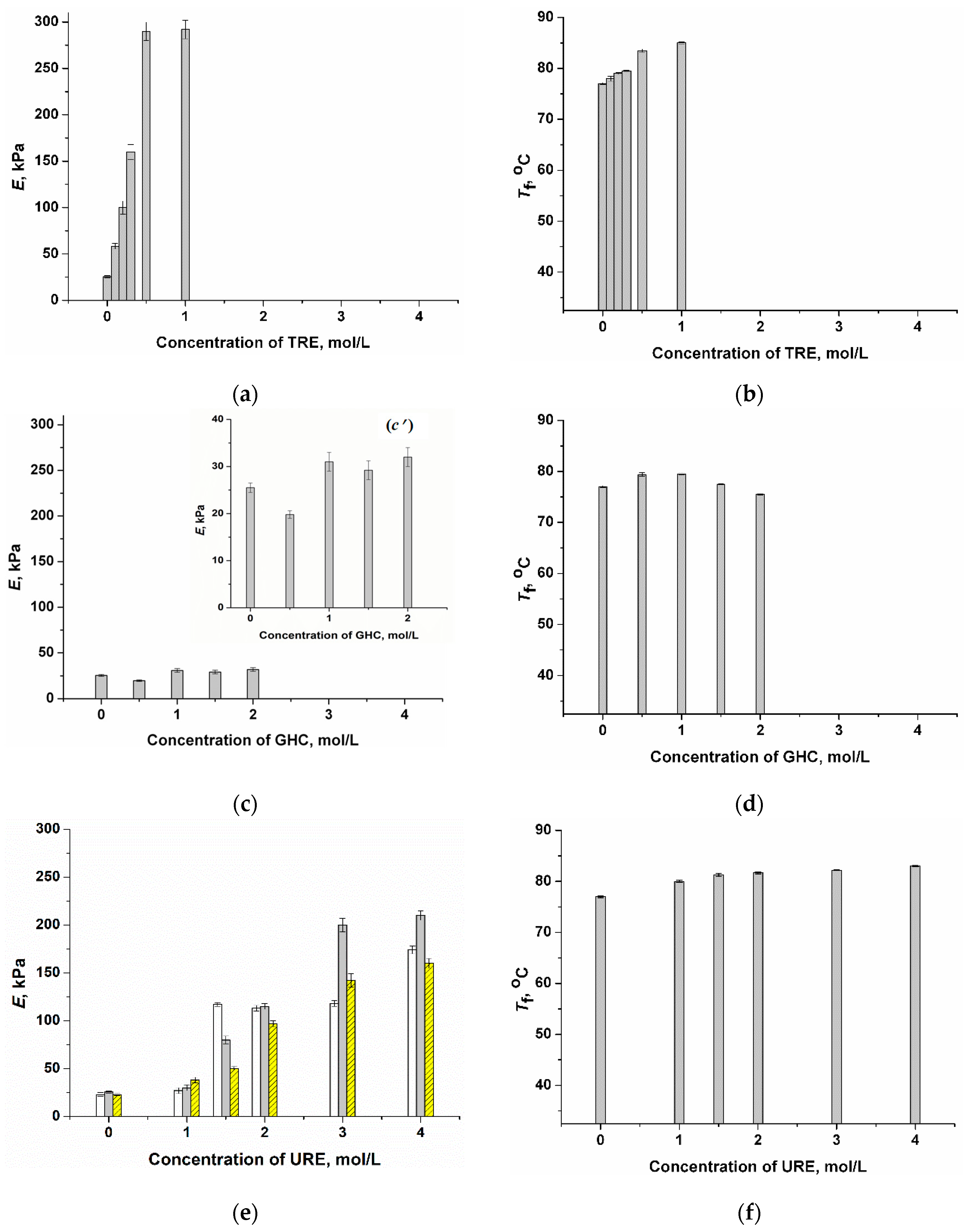
2.1. Influence of Chaotropic and Kosmotropic Additives on the Gel Strength and Heat Endurance of PVACGs Prepared in DMSO Medium
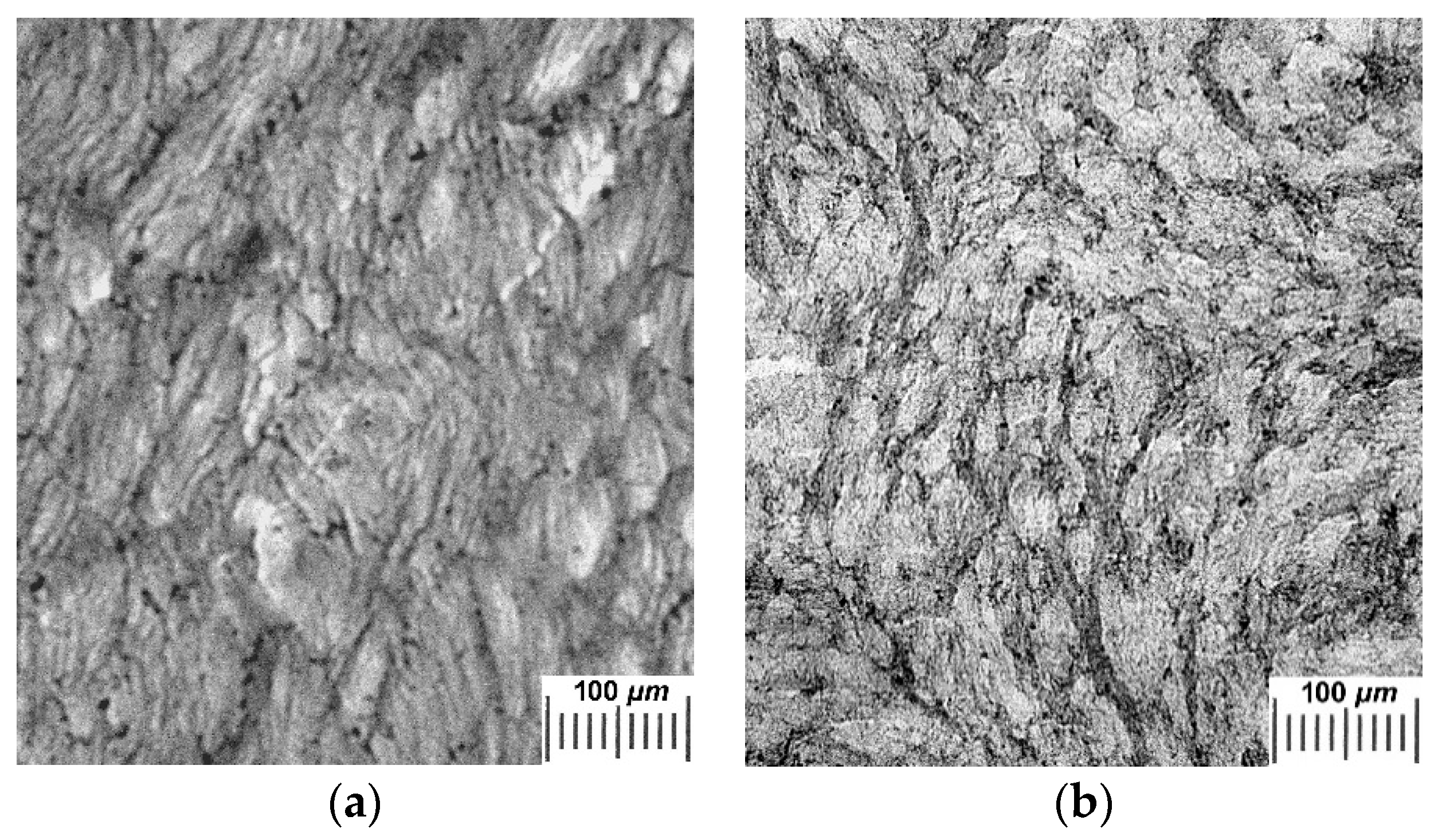
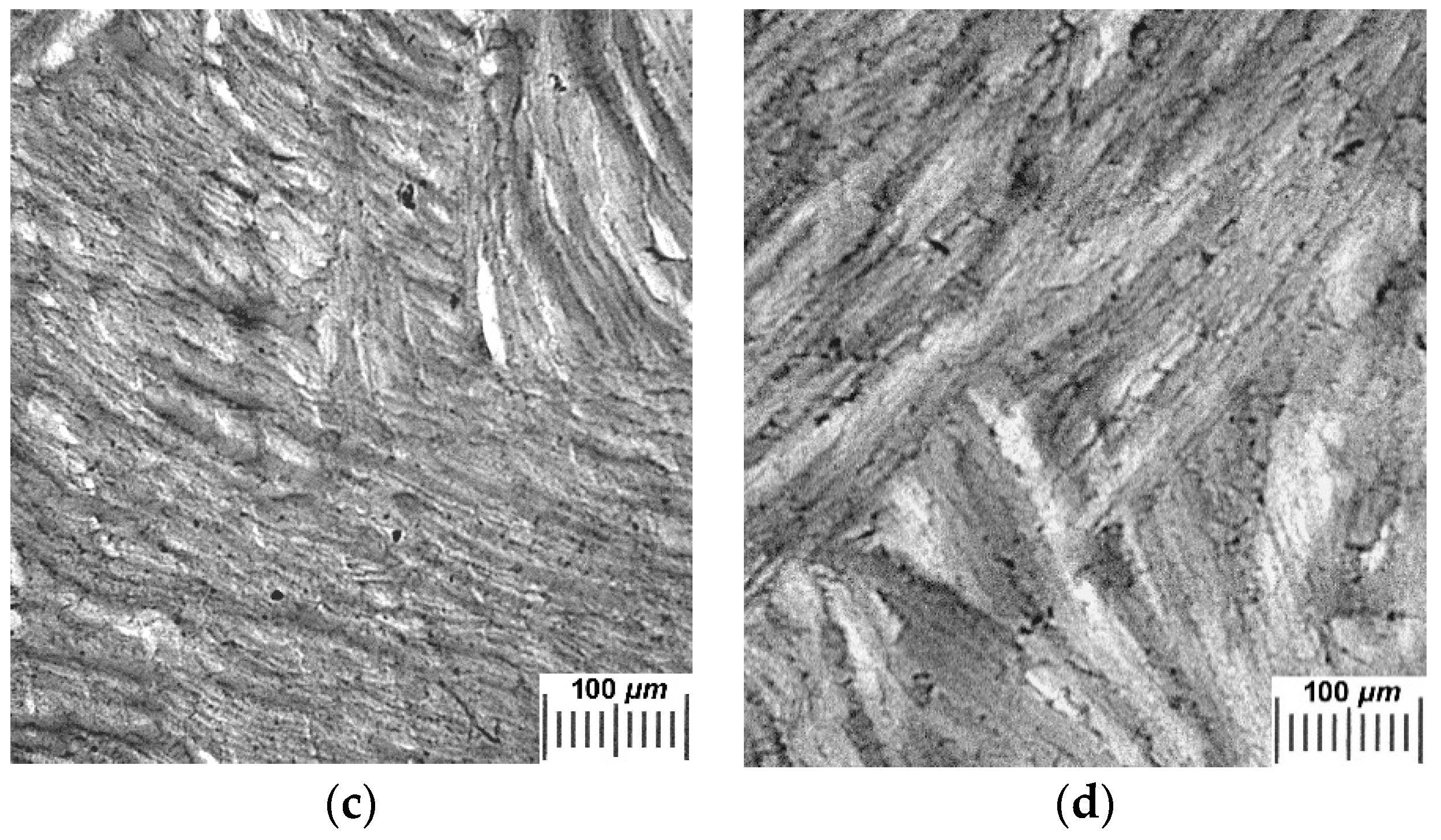
2.2. Influence of THL, GHC, and URE Additives as Components of the Feed PVA/DMSO Solutions on the Macroporous Morphology of the Resultant PVACGs
- additive-free PVACG;
2.3. On Possible Mechanisms of the “Kosmotropic-Like” Influence of Organic Chaotropes on the Freeze–Thaw Gelation of PVA in DMSO
3. Experimental Section
3.1. Materials
3.2. Preparation of Feed PVA Solutions
3.3. Viscometry of Dilute PVA Solutions
3.4. Viscometry of Moderately Concentrated PVA Solutions
3.5. Preparation of PVA Cryogels
3.6. Characterization of PVACG Samples
3.7. Macroporous Morphology of PVACG Samples
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Rus. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Coll. Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef]
- Okay, O. Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Springer: Cham, Switzerland, 2014; p. 330. ISBN 978-3-319-05845-0. [Google Scholar]
- Liu, C.; Tong, G.; Chen, C.; Tan, Z.; Quan, C.; Zhang, C. Polymeric cryogel: Preparation, properties and biomedical applications. Prog. Chem. 2014, 26, 1190–1201. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- Sergeev, G.B.; Batyuk, V.A. Reactions in frozen multicomponent systems. Rus. Chem. Rev. 1976, 45, 391–408. [Google Scholar] [CrossRef]
- Nambu, M. Rubber-like poly(vinyl alcohol) gel. Kobunshi Ronbunshu 1990, 47, 695–703. [Google Scholar] [CrossRef]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncross-linked poly(vinyl alcohol) gels produced by cyclic freezing-thawing processes: A short review. J. Controll. Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Rus. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Aranaz, I.; Ferrer, M.L.; del Monto, F. Production and properties of poly(vinyl alcohol) cryogels: Recent developments. In Macroporous Polymers: Production, Properties and Biological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–115. ISBN 978-1-4200-8461-0. [Google Scholar]
- Lazzeri, L. Progress in bioartificial polymeric materials. Trends Polym. Sci. 1996, 4, 249–252. [Google Scholar]
- Chu, K.C.; Rutt, B.K. Poly(vinyl alcohol) cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.R. Simulation and validation of arterial ultrasound imagining and blood flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwatz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Lamouche, G.; Kennedy, B.F.; Kennedy, K.M.; Bisaillon, C.E.; Curatolo, A.; Campbell, G.; Pazos, V.; Sampson, D.D. Review of tissue simulating phantoms with controllable optical, mechanical and structural properties for use in optical coherence tomography. Biomed. Optics Expr. 2012, 3, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical application. Adv. Polym. Sci. 2014, 263, 283–321. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as a replacement material for damaged articular hyaline cartilage. Materials 2016, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. New generation of macroporous and supermacroporous materials of biotechnological interest—polymeric cryogels. Rus. Chem. Bull. 2008, 57, 1015–1032. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Stolarzewicz, I.; Bialecka-Floriańczyk, E.; Majewska, E.; Krzyczkowska, J. Immobilization of yeast on polymeric supports. Chem. Biochem. Eng. Q. 2011, 25, 135–144. [Google Scholar]
- Mattiasson, B. Cryogels for biotechnological applications. Adv. Polym. Sci. 2014, 263, 245–282. [Google Scholar] [CrossRef]
- Ismailov, A.D.; Alekserova, L.E. Photobiosensors containing luminescent bacteria. Biochemistry 2015, 80, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Butnaru, E.; Cheaburu, C.N.; Yilmaz, O.; Pricope, G.M.; Vasile, C. Poly(vinyl alcohol)/chitosan/montmorillonite nanocomposites for food packing applications: Influence of montmorillonite content. High Perform. Polym. 2016, 28, 1124–1138. [Google Scholar] [CrossRef]
- Altunina, L.K.; Fufaeva, M.S.; Filatov, D.A.; Scarovskaya, L.I.; Rozhdestvenskii, E.A.; Gan-Erdene, T. Effect of cryogel on soil properties. Euroasian Soil Sci. 2014, 47, 425–431. [Google Scholar] [CrossRef]
- Altunina, L.K.; Kuvshinov, V.A.; Dolgikh, S.N. Cryogels—A promising material for underground works in permafrost. In Advances in Geological Storage of Carbon Dioxide; Lombardi, S., Altunina, L.K., Beaubien, S.E., Eds.; Springer: Heidelberg, Germany, 2006; Volume 65, pp. 103–110. ISBN 13 978-1-4020-4469-4. [Google Scholar]
- Vasiliev, N.K.; Pronk, A.D.C.; Shatalina, I.N.; Janssen, F.H.M.E.; Houben, R.W.G. A review on the development of reinforced ice for use as a building material in cold regions. Cold Reg. Sci. Technol. 2015, 115, 56–63. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M.; Tanaka, F. Structure of junction zones in poly(vinyl alcohol) gels by rheological and thermal studies. J. Chim. Phys. Phys. Chim. Biol. 1996, 93, 880–886. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Triolo, R. Slow crystallization kinetics of poly(vinyl alcohol) in confined environment during cryotropic gelation of aqueous solutions. Macromolecules 2006, 39, 9429–9434. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Girolamo, R.D. Kinetic analysis of cryotropic gelation of poly(vinyl alcohol)/water solutions by small-angle neutron scattering. Adv. Polym. Sci. 2014, 263, 159–197. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ando, I.; Ishii, T.; Amiya, S. Structural and dynamical studies of poly(vinyl alcohol) gels by high-resolution solid-state 13C NMR spectroscopy. J. Mol. Struct. 1998, 440, 155–164. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Kobayashi, M.; Ando, I.; Kurosu, H.; Ishii, T.; Amiya, S. A structural and dynamic study of poly(vinyl alcohol) in the gel state by solid-state 13C NMR and 1H pulse NMR. J. Mol. Struct. 1998, 447, 49–59. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Vainerman, E.S.; Mamtsis, A.M.; Rogozhin, S.V. On the possibility of mechanodestruction of poly(vinyl alcohol) molecules under moderate freezing of its concentrated water solutions. Polym. Bull. 1986, 15, 333–340. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Zubov, A.L.; Simenel, I.A. Study of cryostructuration of polymer systems. XII. Poly(vinyl alcohol) cryogels: Influence of low-molecular electrolytes. J. Appl. Polym. Sci. 1996, 61, 1991–1998. [Google Scholar] [CrossRef]
- Patachia, S.; Florea, C.; Friedrich, C.; Thomann, Y. Tailoring of poly(vinyl alcohol) cryogels by salts addition. eXPRESS Polym. Lett. 2009, 3, 320–331. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Sakhno, N.G.; Damshkaln, L.G.; Bakeeva, I.V.; Zubov, V.P.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 31. Influence of the additives of alkaline metals chlorides on physicochemical properties and morphology of poly(vinyl alcohol) cryogels. Colloid J. 2011, 73, 234–243. [Google Scholar] [CrossRef]
- Kolosova, O.Y.; Kurochkin, I.N.; Kurochkin, I.I.; Lozinsky, V.I. Cryostructuring of polymeric systems. 48. Influence of organic chaotropes and kosmotropes on the cryotropic gel-formation of aqueous poly(vinyl alcohol) solutions. Eur. Polym. J. 2018, 102, 169–177. [Google Scholar] [CrossRef]
- Kosmotropes and Chaotropes. Available online: http://www1.lsbu.ac.uk/water/kosmotropes_chaotropes.html#r1567 (accessed on 5 October 2018).
- Rogozhin, S.V.; Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Ivanova, S.A.; Shtil’man, M.I.; Korshak, V.V. Non-covalent cryostructuration in polymer systems. Dokl. Akad. Nauk SSSR 1984, 278, 129–133. [Google Scholar]
- Hyon, S.H.; Cha, W.I.; Ikada, Y. Preparation of transparent poly(vinyl alcohol) hydrogel. Polym. Bull. 1989, 22, 119–122. [Google Scholar] [CrossRef]
- Trieu, H.H.; Qutubuddin, S. Poly(vinyl alcohol) hydrogels. 2. Effects of processing parameters on structure and properties. Polymer 1995, 36, 2531–2539. [Google Scholar] [CrossRef]
- Masri, C.; Chagnon, G.; Favier, D. Influence of processing parameters on the macroscopic mechanical behavior of PVA hydrogels. Mater. Sci. Eng. C 2017, 75, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Leonova, I.M.; Ivanov, R.V.; Bakeeva, I.V. A study of cryostructuring of polymer systems. 46. Physicochemical properties and microstructure of poly(vinyl alcohol) cryogels formed from polymer solutions in mixtures of dimethyl sulfoxide with low-molecular-mass alcohols. Colloid J. 2017, 79, 788–796. [Google Scholar] [CrossRef]
- Horii, F.; Masuda, K.; Kaji, H. CP/MAS 13C NMR spectra of frozen solutions of poly(vinyl alcohol) with different tacticities. Macromolecules 1997, 30, 2519–2520. [Google Scholar] [CrossRef]
- Masuda, K.; Horii, F. CP/MAS 13C NMR analyses of the chain conformation and hydrogen bonding for frozen poly(vinyl alcohol) solutions. Macromolecules 1998, 31, 5810–5817. [Google Scholar] [CrossRef]
- Pritchard, J.G. Poly(vinyl alcohol): Basic Properties and Uses; Gordon & Breach Science Publish: London, UK, 1970; p. 139. ISBN 9780356033358. [Google Scholar]
- Lozinsky, V.I.; Michurov, D.A.; Kolosova, O.Y. Polymeric Composition for the Preparation of Poly(vinyl alcohol) Cryogels and Method for Increasing Their Strength and Heat Endurance. Russian Patent Application No. 2018-104214 (006226). Available online: http://www1.fips.ru/wps/portal/IPS_Ru#1538739639068 (accessed on 5 October 2018).
- Gordon, A.; Ford, R. The Chemist’s Companion: A Handbook of Practical Data, Techniques and References; John Wiley and Sons: New York, NY, USA, 1973; p. 560. ISBN 978-0-471-31590-2. [Google Scholar]
- Fujii, K. Stereochemistry of poly(vinyl alcohol). J. Polym. Sci. Macromol. Rev. 1971, 5, 431–540. [Google Scholar] [CrossRef]
- Eldridge, J.E.; Ferry, J.D. Studies of the cross-linking process in gelatin gels. III. Dependence of melting point on concentration and molecular weight. J. Phys. Chem. 1954, 58, 992–995. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B.; McEvoy, H. Fundamentals of hydrogels and gelation. Br. Polym. J. 1986, 18, 2–7. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly(vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Willcox, P.J.; Howie, D.W.; Schmidt-Rohr, K.; Hoagland, D.A.; Gido, S.P.; Pudjijanto, S.; Kleiner, W.; Venkatraman, S. Microstructure of poly(vinyl alcohol) hydrogels produced by freeze/thaw cycling. J. Polym. Sci. B Polym. Phys. 1999, 37, 3438–3454. [Google Scholar] [CrossRef]
- Trieu, H.; Qutubuddin, S. Poly(vinyl alcohol) hydrogels. I. Microscopic structure by freeze-etching and critical point drying techniques. Colloid Polym. Sci. 1994, 272, 301–309. [Google Scholar] [CrossRef]
- Jiang, Y.; Hussain, H.; Kressler, J. Poly(vinyl alcohol) cryogel formation using biocompatible ice nucleating agents. Macromol. Mater. Eng. 2015, 300, 181–190. [Google Scholar] [CrossRef]
- Hirai, N. Viscosities of concentrated polymer solutions. I. Moderately concentrated solutions. J. Polym. Sci. 1959, 39, 435–443. [Google Scholar] [CrossRef]
- Bohdanecký, M. Huggins viscometric constant of some linear polymers in a series of solvents. Collect. Czech. Chem. Commun. 1970, 35, 1972–1990. [Google Scholar] [CrossRef]
- Haas, D.J.; Harris, D.R.; Mills, H.H. The crystal structure of guanidinium chloride. Acta Crystallogr. 1965, 19, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Marechal, Y. The Hydrogen Bond and the Water Molecule; Elsevier Science: Amsterdam, The Netherlands, 2007; p. 332. ISBN 978-0-444-51957-3. [Google Scholar]




| Solvent | 30 °C | 15 °C | ΔQEη | ΔQEη/[η] | ||
|---|---|---|---|---|---|---|
| [η] | k’ | [η] | k’ | |||
| Water | 0.60 ± 0.03 | 2.0 ± 0.3 | - | - | - | - |
| Water-3 M URE | 0.79 ± 0.03 | 0.8 ± 0.1 | - | - | - | - |
| DMSO | 1.70 ± 0.10 | 0.4 ± 0.1 | 1.30 ± 0.10 | 1.1 ± 0.4 | 29.4 ± 0.9 | 17 ± 2 |
| DMSO-3 M URE | 1.36 ± 0.03 | 0.44 ± 0.05 | 0.98 ± 0.05 | 0.8 ± 0.2 | 26.0 ± 0.9 | 19 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinsky, V.I.; Kolosova, O.Y.; Michurov, D.A.; Dubovik, A.S.; Vasil’ev, V.G.; Grinberg, V.Y. Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO. Gels 2018, 4, 81. https://doi.org/10.3390/gels4040081
Lozinsky VI, Kolosova OY, Michurov DA, Dubovik AS, Vasil’ev VG, Grinberg VY. Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO. Gels. 2018; 4(4):81. https://doi.org/10.3390/gels4040081
Chicago/Turabian StyleLozinsky, Vladimir I., Olga Yu. Kolosova, Dmitrii A. Michurov, Alexander S. Dubovik, Viktor G. Vasil’ev, and Valerij Ya. Grinberg. 2018. "Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO" Gels 4, no. 4: 81. https://doi.org/10.3390/gels4040081
APA StyleLozinsky, V. I., Kolosova, O. Y., Michurov, D. A., Dubovik, A. S., Vasil’ev, V. G., & Grinberg, V. Y. (2018). Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO. Gels, 4(4), 81. https://doi.org/10.3390/gels4040081





