Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes
Abstract
:1. Introduction
2. Designing Supramolecular Gelators
2.1. Challenges and Possibilities
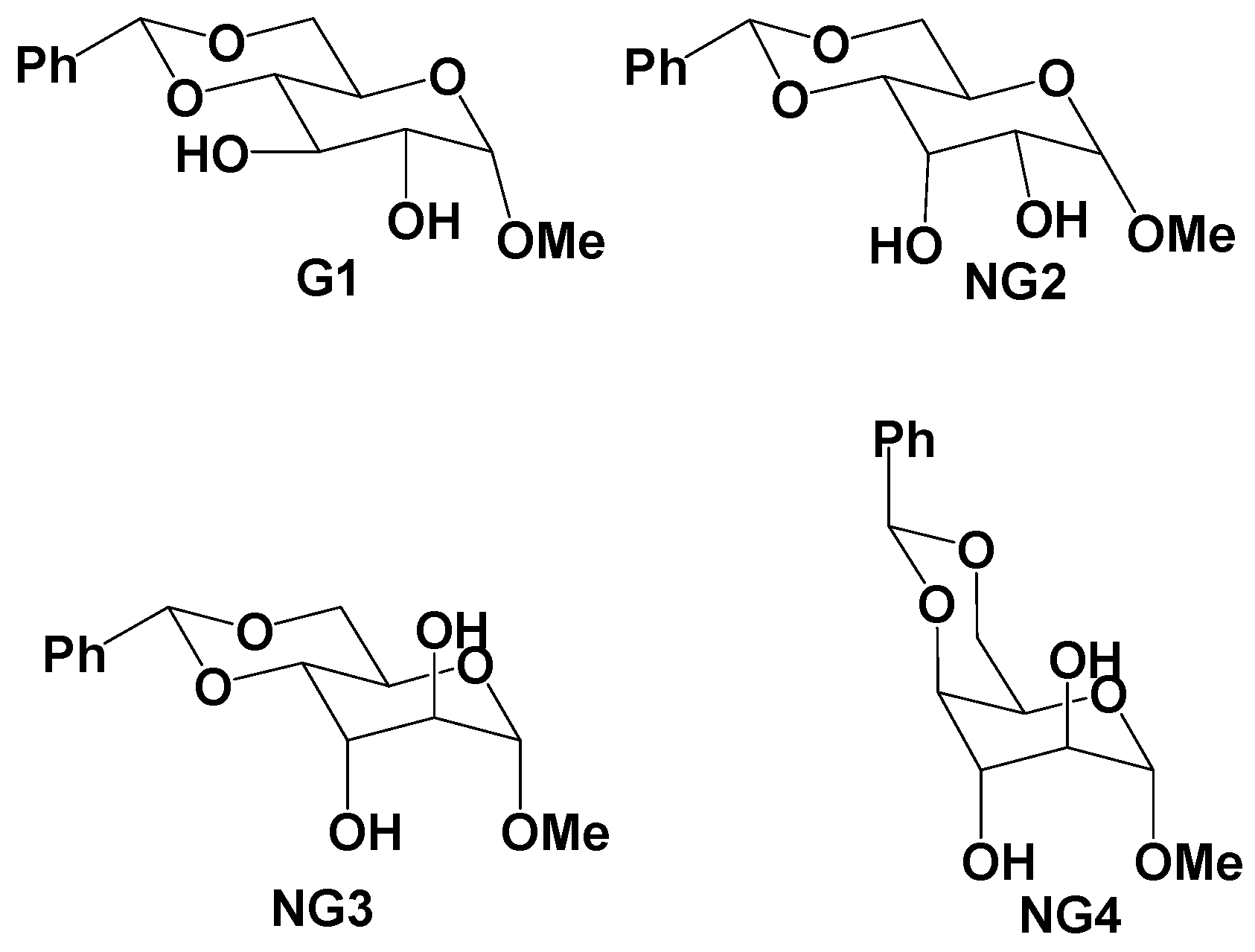
2.2. Developing a Working Hypothesis—Supramolecular Synthon Approach
3. Supramolecular Gels for Various Applications
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Dastidar, P. Supramolecular Gelling Agents: Can They Be Designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The Past, Present, and Future of Molecular Gels. What Is the Status of the Field, and Where Is It Going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with Sense: Supramolecular Materials That Respond to Heat, Light and Sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hanabusa, K. L-Lysine-based low-molecular-weight gelators. Chem. Soc. Rev. 2009, 38, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Smith, D.K.; Feiters, M.C.; Geurts, H.P.M.; Wright, A.C. Two-Component Dendritic Gels: Easily Tunable Materials. J. Am. Chem. Soc. 2003, 125, 9010–9011. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.R.; Chen, L.A.; McDowell, C.; Khan, S.A. Rheological study of crosslinking and gelation in chlorobutyl elastomer systems. Polymer 1996, 37, 5869. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids. Acc. Chem. Res 2006, 39, 489–497. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Lew, A.; Ortony, J. Beyond Covalent Crosslinks: Applications of Supramolecular Gels. Gels 2018, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Weiss, R.G. A Novel Gelator of Organic Liquids and the Properties of Its Gels. Macromolecules 1987, 20, 414–417. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Kamaras, P.; Weiss, R.G. Novel X-ray Method for In Situ Determination of Gelator Strand Structure: Polymorphism of Cholesteryl Anthraquinone-2-carboxylate. Angew. Chem. Int. Ed. Engl. 1996, 35, 1324–1326. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Chemically Reversible Organogels: Aliphatic Amines as “Latent” Gelators with Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 10393–10394. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, A.; Trivedi, D.R.; Dastidar, P. Structural Studies of a New Low Molecular Mass Organic Gelator for Organic Liquids Based on Simple Salt. Chem. Mater. 2003, 15, 2136–2140. [Google Scholar] [CrossRef]
- Luboradzki, R.; Gronwald, O.; Ikeda, I.; Shinkai, S.; Reinhoudt, D.N. An Attempt to Predict the Gelation Ability of Hydrogen-bond-based Gelators Utilizing a Glycoside Library. Tetrahedron 2000, 56, 9595–9599. [Google Scholar] [CrossRef]
- Dastidar, P.; Das, U.K.; Adalder, T.K.; Majumder, J.; Roy, R. Designing Charge-Assisted Hydrogen Bonded Supramolecular Gelators. In Hydrogen Bonded Supramolecular Materials; Li, Z.-T., Wu, L.-Z., Eds.; Springer: Heidelberg, Germany, 2015; pp. 101–131. [Google Scholar]
- Trivedi, D.R.; Ballabh, A.; Dastidar, P.; Ganguly, B. Structure–Property Correlation of a New Family of Organogelators Based on Organic Salts and Their Selective Gelation of Oil from Oil/Water Mixtures. Chem. Eur. J. 2004, 10, 5311–5322. [Google Scholar] [CrossRef]
- Sahoo, P.; Krishna Kumar, D.; Darshak, D.R.; Dastidar, P. An easy access to an organometallic low molecular weight gelator: A crystal engineering approach. Tetrahedron Lett. 2008, 49, 3052–3055. [Google Scholar] [CrossRef]
- Das, U.K.; Trivedi, D.R.; Adarsh, N.N.; Dastidar, P. Supramolecular Synthons in Noncovalent Synthesis of a Class of Gelators Derived from Simple Organic Salts: Instant Gelation of Organic Fluids at Room Temperature via in Situ Synthesis of the Gelators. J. Org. Chem. 2009, 74, 7111–7121. [Google Scholar] [CrossRef]
- Sahoo, P.; Puranik, V.G.; Patra, A.K.; Sastry, P.U.; Dastidar, P. Ferrocene based organometallic gelators: A supramolecular synthon approach. Soft Matter 2011, 7, 3634–3641. [Google Scholar] [CrossRef]
- Amar Ballabh, A.; Darshak, D.R.; Dastidar, P. From Nonfunctional Lamellae to Functional Nanotubes. Org. Lett. 2004, 6, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Adalder, T.K.; Kumar, D.P.; Dastidar, P. High-Throughput Crystal Engineering Based Synthesis of Supramolecular Gels: Blue-Emitting Fluorescent Gold Clusters Synthesized and Stabilized on the Gel-Bed. Cryst. Growth Des. 2014, 14, 11–14. [Google Scholar] [CrossRef]
- Majumder, J.; Dastidar, P. An Easy Access to Organic Salt-Based Stimuli-Responsive and Multifunctional Supramolecular Hydrogels. Chem. Eur. J. 2016, 22, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, M.L.; Xu, B. Molecular hydrogels of therapeutic agents. Chem. Soc. Rev. 2009, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Cherif-Cheikh, R.; Bismuth, F.; Torres, M.L.; Alloza, R.; Bosch, M.T.; Montes, M.; Fuster, E.; Valles, J.; Cordero, J.A.; Peraire, C.; et al. Autogel registered trade mark: a new lanreotide prolonged release formulation. Proc. Int. Symp. Control. Release Bioact. Mater. 1998, 25, 798–799. [Google Scholar]
- Xing, B.G.; Yu, C.W.; Chow, K.H.; Ho, P.L.; Fu, D.G.; Xu, B. Hydrophobic Interaction and Hydrogen Bonding Cooperatively Confer a Vancomycin Hydrogel: A Potential Candidate for Biomaterials. J. Am. Chem. Soc. 2002, 124, 14846–14847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Liang, G.; Ma, M.; Abbah, A.S.; Lu, W.W.; Xu, B. D-Glucosamine-based supramolecular hydrogels to improve wound healing. Chem. Commun. 2007, 8, 843–845. [Google Scholar] [CrossRef]
- Dastidar, P.; Roy, R.; Parveen, R.; Sarkar, K. Supramolecular Synthon Approach in Designing Molecular Gels for Advanced Therapeutics. Adv. Ther. 2019, 2, 1800061. [Google Scholar] [CrossRef]







© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dastidar, P. Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes. Gels 2019, 5, 15. https://doi.org/10.3390/gels5010015
Dastidar P. Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes. Gels. 2019; 5(1):15. https://doi.org/10.3390/gels5010015
Chicago/Turabian StyleDastidar, Parthasarathi. 2019. "Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes" Gels 5, no. 1: 15. https://doi.org/10.3390/gels5010015




