Gelation Kinetics of Hydrogels Based on Acrylamide–AMPS–NVP Terpolymer, Bentonite, and Polyethylenimine for Conformance Control of Oil Reservoirs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Polymer Samples
2.2. Evaluation of the Injectivity and Propagation of the Gelling Systems
2.3. Influence of the Gelant Components and Reservoir Conditions on the Gelation Time and Gel Strength
2.3.1. Polymer Concentration
2.3.2. Crosslinker Concentration
2.3.3. Crosslinker Molecular Weight
2.3.4. Crosslinker Initial pH Value (Gelant pH Value)
2.3.5. Clay Concentration
2.3.6. Clay Type
2.3.7. Temperature
2.3.8. Salinity (Total Dissolved Solids—TDS)
2.3.9. Salt Type
2.4. Overview of the Influence of the Different Parameters Studied on the Gelation Time and Gel Strength
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization of the Samples
4.3. Gelling System Preparation
4.4. Evaluation of the Injectivity, Propagation, Gelation Kinetics, and Gel Strength
4.4.1. Bottle Test
4.4.2. Rheological Tests
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sydansk, R.D.; Romero-Zeron, L. Reservoir Conformance Improvement; Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Liu, Y.; Bai, B.; Wang, Y. Applied technologies and prospects of conformance control treatments in China. Oil Gas Sci. Technol. D’IFP Energ. Nouv. 2010, 65, 859–878. [Google Scholar] [CrossRef]
- Karmakar, G.P.; Chakraborty, C. Improved oil recovery using polymeric gelants: A review. Indian J. Chem. Technol. 2006, 13, 162–167. [Google Scholar]
- Reddy, B.R.; Eoff, L.S.; Crespo, F.; Lewis, C.A. Recent advances in organically crosslinked conformance polymer systems. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, TX, USA, 2013. [Google Scholar]
- Eoff, L.; Dalrymple, E.; Everett, D.; Vasquez, J. Worldwide field applications of a polymeric gel system for conformance applications. SPE Prod. Oper. 2007, 22, 231–235. [Google Scholar] [CrossRef]
- ElKarsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Performance of PAM/PEI gel system for water shut-off in high temperature reservoirs: Laboratory study. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.; Nasr-El-Din, H.; Zitha, P. Gelation kinetics and performance evaluation of an organically crosslinked gel at high temperature and pressure. SPE J. 2008, 13, 337–345. [Google Scholar] [CrossRef]
- Hardy, M.; Botermans, W.; Hamouda, A.; Valdal, J.; Warren, J. The first carbonate field application of a new organically crosslinked water shutoff polymer system. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 1999; pp. 361–376. [Google Scholar]
- Reddy, B.R.; Eoff, L.; Dalrymple, E.; Black, K.; Brown, D.; Rietjens, M. A natural polymer-based cross-linker system for conformance gel systems. SPE J. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- Fernandez, I.J. Evaluation of cationic water-soluble polymers with improved thermal stability. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, TX, USA, 2005. [Google Scholar]
- Sydansk, R.D. A new conformance-improvement-treatment chromium (III) gel technology. In SPE Enhanced Oil Recovery Symposium; Society of Petroleum Engineers: Tulsa, OK, USA, 1988. [Google Scholar]
- Broseta, D.; Marquer, O.; Baylocq, P.; Fery, J.J.; Zaitoun, A. Gel treatment optimization by rheological measurements. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 1999. [Google Scholar]
- Aalaie, J.; Alvand, E.; Hemmati, M.; Sajjadian, V.A. Preparation and probing of the steady shear flow and viscoelastic properties of weakly crosslinked hydrogels based on sulfonated polyacrylamide for oil recovery applications. Polym. Sci. Ser. A 2015, 57, 680–687. [Google Scholar] [CrossRef]
- Dang, T.Q.C.; Chen, Z.; Nguyen, T.B.N.; Bae, W.; Chung, T.; Tu, T.N. The Development and Optimization of a Polymer Conformance Control Technology in Mature Reservoirs: Laboratory Experiments vs. Field Scale Simulation. Energy Sources Part Recovery Util. Environ. Eff. 2014, 36, 1219–1233. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A. Polymer Gels for Water Control: NMR and CT Scan Studies. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2008. [Google Scholar]
- Al-Muntasheri, G.A. Rheology of Gelling Polymers Used in Reservoir Water Shutoff Treatments. Ph.D. Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, 2004. [Google Scholar]
- Willhite, G.P.; Pancake, R.E. Controlling water production using gelled polymer systems. SPE Reserv. Eval. Eng. 2008, 11, 454–465, SPE-89464-PA. [Google Scholar] [CrossRef]
- Johnson, S.; Trejo, J.; Veisi, M.; Willhite, G.P.; Liang, J.-T.; Berkland, C. Effects of divalent cations, seawater, and formation brine on positively charged polyethylenimine/dextran sulfate/chromium (III) polyelectrolyte complexes and partially hydrolyzed polyacrylamide/chromium (III) gelation. J. Appl. Polym. Sci. 2010, 115, 1008–1014. [Google Scholar] [CrossRef]
- Vermolen, E.; Van Haasterecht, M.J.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M.A. Pushing the envelope for polymer flooding towards high-temperature and high-salinity reservoirs with polyacrylamide based ter-polymers. In SPE Middle East Oil and Gas Show and Conference; Society of Petroleum Engineers: Manama, Bahrain, 2011. [Google Scholar]
- Doe, P.H.; Moradi-Araghi, A.; Shaw, J.E.; Stahl, G.A. Development and evaluation of EOR polymers suitable for hostile environments. Part 1: Copolymers of vinylpyrrolidone and acrylamide. SPE Reserv. Eng. 1987, 2, 461–467, SPE-14233-PA. [Google Scholar] [CrossRef]
- Vasquez, J.; Dalrymple, E.D.; Eoff, L.; Reddy, B.R.; Civan, F. Development and evaluation of high-temperature conformance polymer systems. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, TX, USA, 2005. [Google Scholar]
- Wu, Y.; Mahmoudkhani, A.; Watson, P.; Fenderson, T.R.; Nair, M. Development of new polymers with better performance under conditions of high temperature and high salinity. In SPE EOR Conference at Oil and Gas West Asia; Society of Petroleum Engineers: Muscat, Oman, 2012. [Google Scholar]
- Aalaie, J.; Vasheghani-Farahani, E.; Rahmatpour, A.; Semsarzadeh, M.A. Effect of montmorillonite on gelation and swelling behavior of sulfonated polyacrylamide nanocomposite hydrogels in electrolyte solutions. Eur. Polym. J. 2008, 44, 2024–2031. [Google Scholar] [CrossRef]
- Mohammadi, S.; Vafaie Sefti, M.; Baghban Salehi, M.; Mousavi Moghadam, A.; Rajaee, S.; Naderi, H. Hydrogel swelling properties: comparison between conventional and nanocomposite hydrogels for water shutoff treatment. Asia-Pac. J. Chem. Eng. 2015. [Google Scholar] [CrossRef]
- Xu, J.; Chen, D.; Ke, Y.; Yang, L.; Bai, X.; Zhang, G.; Zeng, Z.; Gao, W.; Gong, D. Synthesis and characterization of partially hydrolyzed polyacrylamide nanocomposite weak gels with high molecular weights. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Moghadam, A.M.; Sefti, M.V.; Salehi, M.B.; Naderi, H. Bulk and rheological properties of polyacrylamide hydrogels for water shutoff treatment. Korean J. Chem. Eng. 2014, 31, 532–539. [Google Scholar] [CrossRef]
- Pu, W.-F.; Yang, Y.; Yuan, C.-D. Gelation performance of poly(ethylene imine) crosslinking polymer-layered silicate nanocomposite gel system for potential water-shutoff use in high-temperature reservoirs. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Inoue, Y.; Fukutomi, T.; Chûjô, R. Carbon-13 NMR analysis of the tacticity of polyacrylamide. Polym. J. 1983, 15, 103–105. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A. Acrylamide copolymers: A review of methods for the determination of concentration and degree of hydrolysis. J. Pet. Sci. Eng. 1994, 12, 9–23. [Google Scholar] [CrossRef]
- Jin, S.; Gu, J.; Shi, Y.; Shao, K.; Yu, X.; Yue, G. Preparation and electrical sensitive behavior of poly (N-vinylpyrrolidone-co-acrylic acid) hydrogel with flexible chain nature. Eur. Polym. J. 2013, 49, 1871–1880. [Google Scholar] [CrossRef] [Green Version]
- Lai, N.; Qin, X.; Ye, Z.; Peng, Q.; Zhang, Y.; Ming, Z. Synthesis and evaluation of a water-soluble hyperbranched polymer as enhanced oil recovery chemical. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Levitt, D.B. The Optimal Use of Enhanced Oil RECOVERY Polymers under Hostile Conditions. Dissertation Thesis, The University of Texas at Austin, Austin, TX, USA, 2009. [Google Scholar]
- Parker, W.O.; Lezzi, A. Hydrolysis of sodium-2-acrylamido-2-methylpropanesulfonate copolymers at elevated temperature in aqueous solution via 13 C NMR spectroscopy. Polymer 1993, 34, 4913–4918. [Google Scholar] [CrossRef]
- Ryles, R.G.; Neff, R.E. Thermally stable acrylic polymer for profile modification applications. In Water-Soluble Polymers for Petroleum Recovery; Stahl, G.A., Schulz, D.N., Eds.; Springer: Boston, MA, USA, 1988; pp. 139–146. [Google Scholar]
- Tessarolli, F.G.C.; Gomes, A.S.; Mansur, C.R. Hydrogels applied for conformance-improvement treatment of oil reservoirs. In Hydrogels; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Stahl, G.A.; Schulz, D.N. Water-Soluble Polymers for Petroleum Recovery; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels: a unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002, 14, 1120. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide-clay nanocomposite hydrogels: rheological and light scattering characterization. Macromolecules 2007, 40, 3378–3387. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Interfacial chemistry, particle interactions and improved dewatering behaviour of smectite clay dispersions. Int. J. Miner. Process. 2005, 75, 155–171. [Google Scholar] [CrossRef]
- Diaz, I.; Nava, T.; Deolarte, C.; Castillo, O.; Vasquez, J.; Cancino, V.; Caballero, C. Successfully controlling unwanted gas production in a highly naturally fractured carbonate reservoir. In SPE Western Venezuela Section South American Oil and Gas Congress, SPE-163085-MS; Society of Petroleum Engineers: Maracaibo, Venezuela, 2011. [Google Scholar]
- Stalker, R.; Graham, G.M.; Oliphant, D.; Smillie, M. Potential application of viscosified treatments for improved bullhead scale inhibitor placement in long horizontal wells-a theoretical and laboratory examination. In SPE International Symposium on Oilfield Scale; Society of Petroleum Engineers: Aberdeen, UK, 2004. [Google Scholar]
- Elkarsani, K.S.M. Development of a new cost-effective polymer gel for water control in oil and gas wells. Ph.D. Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, 2013. [Google Scholar]
- Al-Muntasheri, G.A.; Nasr-El-Din, H.A.; Hussein, I.A. A rheological investigation of a high temperature organic gel used for water shut-off treatments. J. Pet. Sci. Eng. 2007, 59, 73–83. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Jia, H.; Pu, W.-F.; Liao, R. Influences of fracture aperture on the water-shutoff performance of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels in hydraulic fractured reservoirs. Energy Fuels 2011, 25, 2616–2624. [Google Scholar] [CrossRef]
- Eggert, R.W.; Willhite, G.P.; Green, D.W. Experimental Measurement of the Persistence of Permeability Reduction in Porous Media Treated With Xanthan/Cr(III) Gel Systems. SPE Reserv. Eng. 1992, 7, 29–35. [Google Scholar] [CrossRef]
- Bryant, S.L.; Rabaioli, M.R.; Lockhart, T.P. Influence of syneresis on permeability reduction by polymer gels. SPE Prod. Facil. 1996, 11, 209–215. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Moore, P.E. Production Responses in Wyoming’s Big Horn Basin Resulting from Application of Acrylamide-Polymer/CrIII-Carboxylate Gels; Society of Petroleum Engineers: Richardson, TX, USA, 1990; SPE-21894-MS. [Google Scholar]
- Man, V.F.-P.; Killeen, Y.M.; Anderson, D.R. High Performance Low Viscoelasticity Foaming Detergent Compositions Employing Extended Chain Anionic Surfactants. U.S. Patent Grant US-9410110-B2, 9 August 2016. [Google Scholar]
- Mészáros, R.; Varga, I.; Gilányi, T. Adsorption of poly (ethyleneimine) on silica surfaces: effect of pH on the reversibility of adsorption. Langmuir 2004, 20, 5026–5029. [Google Scholar] [CrossRef]
- Jayakumar, S. Hydrolyzed Polyacrylamide-Polyethylenimine-Dextran Sulfate Polymer Gel System as a Water Shut-off Agent in Unconventional Gas Reservoirs. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2012. [Google Scholar]
- Rabiee, A. Acrylamide-based anionic polyelectrolytes and their applications: A survey. J. Vinyl Addit. Technol. 2010, 16, 111–119. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Katbab, A.A.; Nabavizadeh, J.; Tabasi, R.Y.; Nejad, M.H. Preparation and characterization of nanocomposite hydrogels based on polyacrylamide for enhanced oil recovery applications. J. Appl. Polym. Sci. 2006, 100, 2096–2103. [Google Scholar] [CrossRef]
- Sirousazar, M.; Kokabi, M.; Hassan, Z.M.; Bahramian, A.R. Mineral kaolinite clay for preparation of nanocomposite hydrogels. J. Appl. Polym. Sci. 2012, 125, E122–E130. [Google Scholar] [CrossRef]
- Rajaee, S.; Salehi, M.B.; Moghadam, A.M.; Sefti, M.V.; Mohammadi, S. Nanocomposite hydrogels adsorption: Experimental investigation and performance on sandstone core. J. Pet. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Güngör, N.; Alemdar, A.; Atici, O.; Ece, I.O. The effect of SDS surfactant on the flow and zeta potential of bentonite suspensions. Mater. Lett. 2001, 51, 250–254. [Google Scholar] [CrossRef]
- Blackmore, A.V.; Warkentin, B.P. Swelling of calcium montmorillonite. Nature 1960, 186, 823. [Google Scholar] [CrossRef]
- Van Olphen, H. Forces between suspended bentonite particles Part II–Calc ium bentonite. In Clays and Clay Minerals, Proc. 6th Natl. Conf; Pergamon Press: Oxford, UK, 1959; pp. 196–206. [Google Scholar]
- Segad, M.; Hanski, S.; Olsson, U.; Ruokolainen, J.; AAkesson, T.; Jönsson, B. Microstructural and swelling properties of Ca and Na montmorillonite:(in situ) observations with cryo-TEM and SAXS. J. Phys. Chem. C 2012, 116, 7596–7601. [Google Scholar] [CrossRef]
- Shainberg, I.; Otoh, H. Size and shape of montmorillonite particles saturated with Na/Ca ions (inferred from viscosity and optical measurements). Isr. J. Chem. 1968, 6, 251–259. [Google Scholar] [CrossRef]
- Tan, X.; Liu, F.; Hu, L.; Reed, A.H.; Furukawa, Y.; Zhang, G. Evaluation of the particle sizes of four clay minerals. Appl. Clay Sci. 2017, 135, 313–324. [Google Scholar] [CrossRef]
- Mouzon, J.; Bhuiyan, I.U.; Hedlund, J. The structure of montmorillonite gels revealed by sequential cryo-XHR-SEM imaging. J. Colloid Interface Sci. 2016, 465, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Gao, D.; Heimann, R.B. Structure and mechanical properties of superabsorbent poly (acrylamide)-montmorillonite composite hydrogels. Polym. Gels Netw. 1993, 1, 225–246. [Google Scholar] [CrossRef]
- Santiago, F.; Mucientes, A.E.; Osorio, M.; Rivera, C. Preparation of composites and nanocomposites based on bentonite and poly (sodium acrylate). Effect of amount of bentonite on the swelling behaviour. Eur. Polym. J. 2007, 43, 1–9. [Google Scholar] [CrossRef]
- Kakadjian, S.; Rauseo, O.; Mejias, F. Dynamic rheology as a method for quantify gel strength of water shutoff systems. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 1999. [Google Scholar]
- Calvet, D.; Wong, J.Y.; Giasson, S. Rheological monitoring of polyacrylamide gelation: Importance of cross-link density and temperature. Macromolecules 2004, 37, 7762–7771. [Google Scholar] [CrossRef]
- Okur, H.I.; Kherb, J.; Cremer, P.S. Cations bind only weakly to amides in aqueous solutions. J. Am. Chem. Soc. 2013, 135, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D.; Portnaya, I.; Faupin, B.; Ramon, O.; Cohen, Y.; Cogan, U.; Mizrahi, S. Interactions between inorganic salts and polyacrylamide in aqueous solutions and gels. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 508–519. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Ward, J.S.; Martin, F.D. Prediction of viscosity for partially hydrolyzed polyacrylamide solutions in the presence of calcium and magnesium ions. Soc. Pet. Eng. J. 1981, 21, 623–631. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Jia, H.; Ren, Q.; Li, Y.M.; Ma, X.P. Evaluation of polyacrylamide gels with accelerator ammonium salts for water shutoff in ultralow temperature reservoirs: Gelation performance and application recommendations. Petroleum 2016, 2, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.; Li, Y.; Li, C.; Fang, H.; Wang, W. Study about how the metal cationic ions affect the properties of partially hydrolyzed hydrophobically modified polyacrylamide (HMHPAM) in aqueous solution. Colloids Surf. Physicochem. Eng. Asp. 2013, 434, 16–24. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Berry, L. Investigating the settling behaviour of saline tailing suspensions using kaolinite, bentonite, and illite clay minerals. Powder Technol. 2018, 326, 228–236. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Ismail, S.; Sultan, A.S.; Ahmad, Z. Performance of fly ash based polymer gels for water reduction in enhanced oil recovery: Gelation kinetics and dynamic rheological studies. Korean J. Chem. Eng. 2017, 1–13. [Google Scholar] [CrossRef]
- Jia, H.; Pu, W.F.; Zhao, J.Z.; Jin, F.Y. Research on the gelation performance of low toxic PEI cross-linking PHPAM gel systems as water shutoff agents in low temperature reservoirs. Ind. Eng. Chem. Res. 2010, 49, 9618–9624. [Google Scholar] [CrossRef]
- Singh, R.; Mahto, V. Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet. Sci. 2017, 1–15. [Google Scholar] [CrossRef]

 F1,
F1,  F2,
F2,  F3, and
F3, and  F4 with polyethylenimine (PEI) pH 7;
F4 with polyethylenimine (PEI) pH 7;  F4,
F4,  F5,
F5,  F6, and
F6, and  F4 with PEI 750,000 kg/kmol and
F4 with PEI 750,000 kg/kmol and  F7.
F7.
 F1,
F1,  F2,
F2,  F3, and
F3, and  F4 with polyethylenimine (PEI) pH 7;
F4 with polyethylenimine (PEI) pH 7;  F4,
F4,  F5,
F5,  F6, and
F6, and  F4 with PEI 750,000 kg/kmol and
F4 with PEI 750,000 kg/kmol and  F7.
F7.

 , 0.2 wt % (F1);
, 0.2 wt % (F1); 
 , 0.6 wt % (F4); and
, 0.6 wt % (F4); and 
 , 1.0 wt % (F7).
, 1.0 wt % (F7).

 , 0.2 wt % (F1);
, 0.2 wt % (F1); 
 , 0.6 wt % (F4); and
, 0.6 wt % (F4); and 
 , 1.0 wt % (F7).
, 1.0 wt % (F7).

 , 0.0 wt % (F2);
, 0.0 wt % (F2); 
 , 0.5 wt % (F4); and
, 0.5 wt % (F4); and 
 , 1.0 wt % (F6).
, 1.0 wt % (F6).

 , 0.0 wt % (F2);
, 0.0 wt % (F2); 
 , 0.5 wt % (F4); and
, 0.5 wt % (F4); and 
 , 1.0 wt % (F6).
, 1.0 wt % (F6).


 , organically modified bentonite composite (brine);
, organically modified bentonite composite (brine); 
 , polycationic bentonite composite (brine);
, polycationic bentonite composite (brine); 
 , sodium bentonite composite (brine);
, sodium bentonite composite (brine); 
 , organically modified bentonite composite (distilled water);
, organically modified bentonite composite (distilled water); 
 , polycationic bentonite composite (distilled water); and
, polycationic bentonite composite (distilled water); and 
 , sodium bentonite nanocomposite (distilled water).
, sodium bentonite nanocomposite (distilled water).

 , organically modified bentonite composite (brine);
, organically modified bentonite composite (brine); 
 , polycationic bentonite composite (brine);
, polycationic bentonite composite (brine); 
 , sodium bentonite composite (brine);
, sodium bentonite composite (brine); 
 , organically modified bentonite composite (distilled water);
, organically modified bentonite composite (distilled water); 
 , polycationic bentonite composite (distilled water); and
, polycationic bentonite composite (distilled water); and 
 , sodium bentonite nanocomposite (distilled water).
, sodium bentonite nanocomposite (distilled water).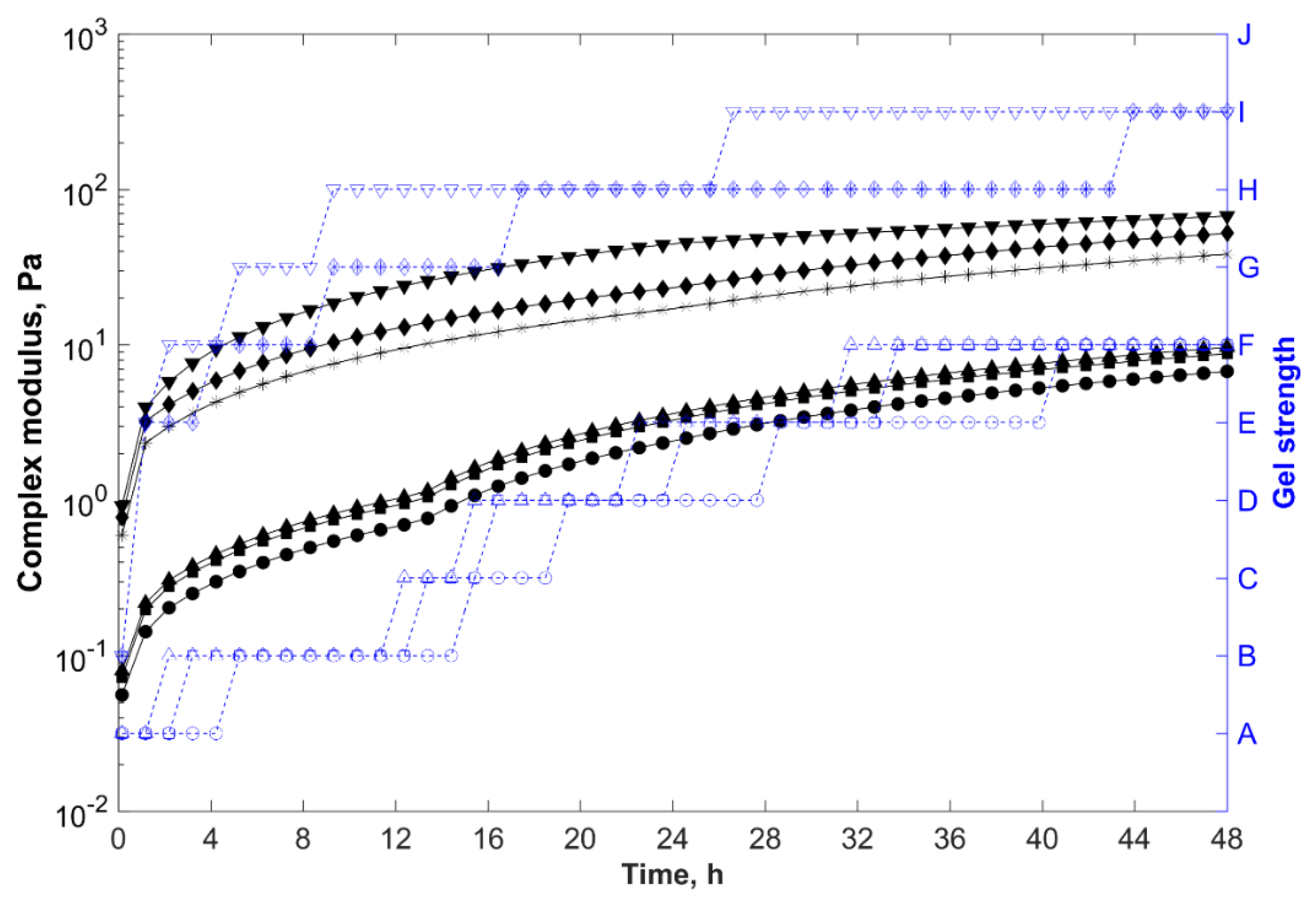

 , 65 °C;
, 65 °C; 
 , 85 °C; and
, 85 °C; and 
 , 105 °C.
, 105 °C.

 , 65 °C;
, 65 °C; 
 , 85 °C; and
, 85 °C; and 
 , 105 °C.
, 105 °C.

 , 33,489 mg/L (injection water);
, 33,489 mg/L (injection water); 
 , 56,012 mg/L (field water); and
, 56,012 mg/L (field water); and 
 , 123,582 mg/L (reservoir water).
, 123,582 mg/L (reservoir water).

 , 33,489 mg/L (injection water);
, 33,489 mg/L (injection water); 
 , 56,012 mg/L (field water); and
, 56,012 mg/L (field water); and 
 , 123,582 mg/L (reservoir water).
, 123,582 mg/L (reservoir water).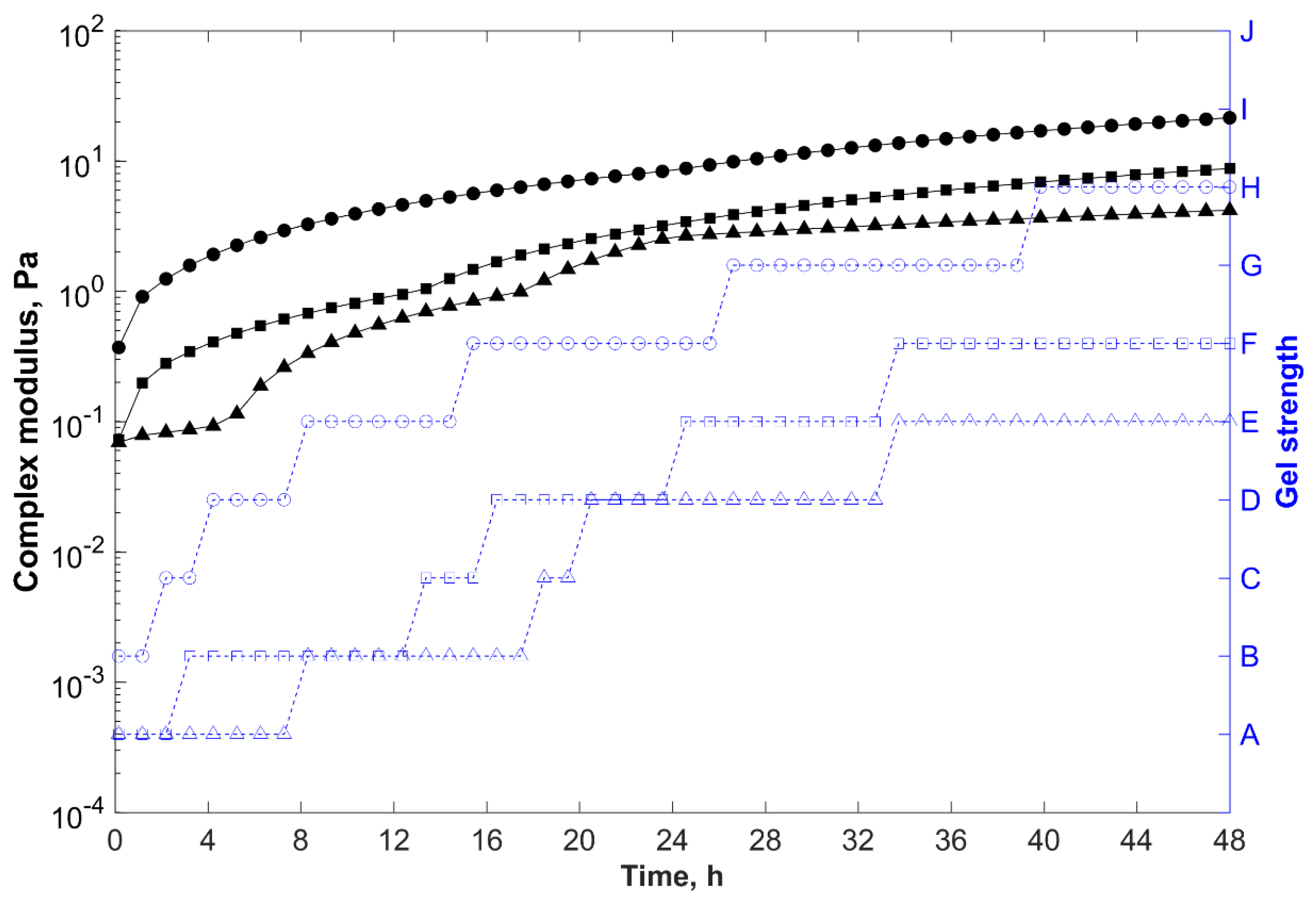

 , distilled water;
, distilled water; 
 , KCl;
, KCl; 
 , NaCl;
, NaCl; 
 , MgCl2; and
, MgCl2; and 
 , CaCl2.
, CaCl2.

 , distilled water;
, distilled water; 
 , KCl;
, KCl; 
 , NaCl;
, NaCl; 
 , MgCl2; and
, MgCl2; and 
 , CaCl2.
, CaCl2.
| Carbon Index | Chemical Shifts (ppm) | Assignment of the Peaks |
|---|---|---|
| C11 | 181 to 179 | Carbonyl-carbon (C=O) of the amide group (−CONH2) |
| C9 | 179 to 177 | Carbonyl-carbon (C=O) of the NVP ring |
| C10 | 177 to 175 | Carbonyl-carbon (C=O) of the AMPS group |
| C8 | 61 to 59 | Methylene-carbon (–CH2) of the AMPS group |
| C7 | 56 to 53 | Carbon linked to CH2–SO3–Na+ of AMPS |
| C6 | 51 to 50 | Methylene-carbons (–CH2) of the NVP ring located further to the carbonyl carbon |
| C5 | 45 to 41 | Methine-carbons (–CH) of the polymer backbone attached to the amide (–CONH2), AMPS-Na or NVP groups |
| C4 | 39 to 34 | Methylene-carbons (–CH2) of the polymer backbone attached to the amide (–CONH2), AMPS-Na or NVP groups |
| C3 | 34 to 33 | Methylene-carbons (–CH2) of the NVP ring located close to the carbonyl carbon |
| C2 | 30 to 27 | Methyl-carbons (–CH3) of the AMPS group |
| C1 | 20 to 19 | Methylene-carbons (–CH2) of the NVP ring |
| Sample | AM Moieties (%) | AMPS Moieties (%) | NVP Moieties (%) | (kg/kmol) | PD |
|---|---|---|---|---|---|
| AMPS–NVP–AM | 48 | 30 | 22 | 2 × 106 | 1.02 |
| Parameters | Gelation Time | Final Gel Strength | |
|---|---|---|---|
| Gelling System Formulation | ↑ polymer concentration | ↓↓ | ↑↑ |
| ↑ crosslinker concentration | ↓↓ | ↑↑ | |
| ↑ clay concentration | ↑ | ↑ | |
| Crosslinker Properties | ↑ crosslinker molecular weight ↑ crosslinker initial pH value (gelant pH value) | ↓↓ ↓↓ | ↑↑ ↑↑ |
| Clay Properties | ↑ divalent exchangeable cations in the intralamellar space | ↑ | ↓ |
| Well or Reservoir Conditions | ↑ temperature | ↓↓ | ↑↑ |
| ↑ salinity | ↑↑ | ↓↓ | |
| ↑ hardness | ↑↑ | ↓↓ | |
| Concentration (mg/L) | |||
|---|---|---|---|
| Ions | Desulfated Seawater (Injection Water) | Field Water (Gel Setting Water Within the Reservoir) | Formation Water (Connate Water) |
| Sodium (Na+) | 11,589 | 18,736 | 40,177 |
| Potassium (K+) | 225 | 943 | 3098 |
| Calcium (Ca2+) | 341 | 1430 | 4696 |
| Magnesium (Mg2+) | 677 | 669 | 646 |
| Chloride (Cl−) | 20,655 | 34,232 | 74,963 |
| Total Dissolved Solids, TDS | 33,489 | 56,012 | 123,582 |
| Formulations | Polymer (wt %) | Clay (wt %) | PEI (wt %) |
|---|---|---|---|
| F1 | 0.2 | 0.8 | 0.5 |
| F2 | 0.6 | 0.8 | 0.0 |
| F3 | 0.6 | 0.0 | 0.5 |
| F4 | 0.6 | 0.8 | 0.5 |
| F5 | 0.6 | 1.6 | 0.5 |
| F6 | 0.6 | 0.8 | 1.0 |
| F7 | 1.0 | 0.8 | 0.5 |
| Test | Objective | Experimental Condition |
|---|---|---|
| Steady shear test with shear rate sweep | Evaluate the rheological behavior and the injectivity of the gelling systems based on the return curve of the samples. | Shear rate: 0.1 to 1000 s−1 Temperature: 25 °C |
| Oscillatory shear test with strain sweep | Determine the linear viscoelasticity region (LVR) of gelling systems and hydrogels. The LVR delimits the critical deformation that can be applied to the sample in order to ensure that its structure is not altered. | Strain: 0.01 to 100; Frequency: 0.1 Hz; Temperature: 65, 85, or 105 °C |
| Oscillatory shear test with time sweep (at constant strain and frequency) | Monitor the evolution of the storage modulus (G’), loss modulus (G”), and complex modulus (G*) as a function of time without any mechanical disturbance or destruction of the elastic structure of the hydrogel. The gelation time of each sample was determined when G* started to increase rapidly (inflection point). | Strain: 0.1 (in the LVR for all tested samples); Frequency: 0.1 Hz; Temperature: 65, 85, or 105 °C; Time: 48 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessarolli, F.G.C.; Souza, S.T.S.; Gomes, A.S.; Mansur, C.R.E. Gelation Kinetics of Hydrogels Based on Acrylamide–AMPS–NVP Terpolymer, Bentonite, and Polyethylenimine for Conformance Control of Oil Reservoirs. Gels 2019, 5, 7. https://doi.org/10.3390/gels5010007
Tessarolli FGC, Souza STS, Gomes AS, Mansur CRE. Gelation Kinetics of Hydrogels Based on Acrylamide–AMPS–NVP Terpolymer, Bentonite, and Polyethylenimine for Conformance Control of Oil Reservoirs. Gels. 2019; 5(1):7. https://doi.org/10.3390/gels5010007
Chicago/Turabian StyleTessarolli, Fernanda G.C., Sara T.S. Souza, Ailton S. Gomes, and Claudia R.E. Mansur. 2019. "Gelation Kinetics of Hydrogels Based on Acrylamide–AMPS–NVP Terpolymer, Bentonite, and Polyethylenimine for Conformance Control of Oil Reservoirs" Gels 5, no. 1: 7. https://doi.org/10.3390/gels5010007
APA StyleTessarolli, F. G. C., Souza, S. T. S., Gomes, A. S., & Mansur, C. R. E. (2019). Gelation Kinetics of Hydrogels Based on Acrylamide–AMPS–NVP Terpolymer, Bentonite, and Polyethylenimine for Conformance Control of Oil Reservoirs. Gels, 5(1), 7. https://doi.org/10.3390/gels5010007






