Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Results
2.1. Scaffold Characterization
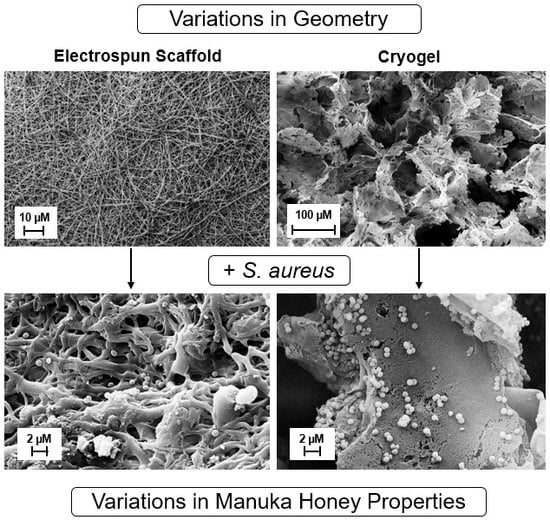
2.1.1. Scanning Electron Microscopy (SEM)
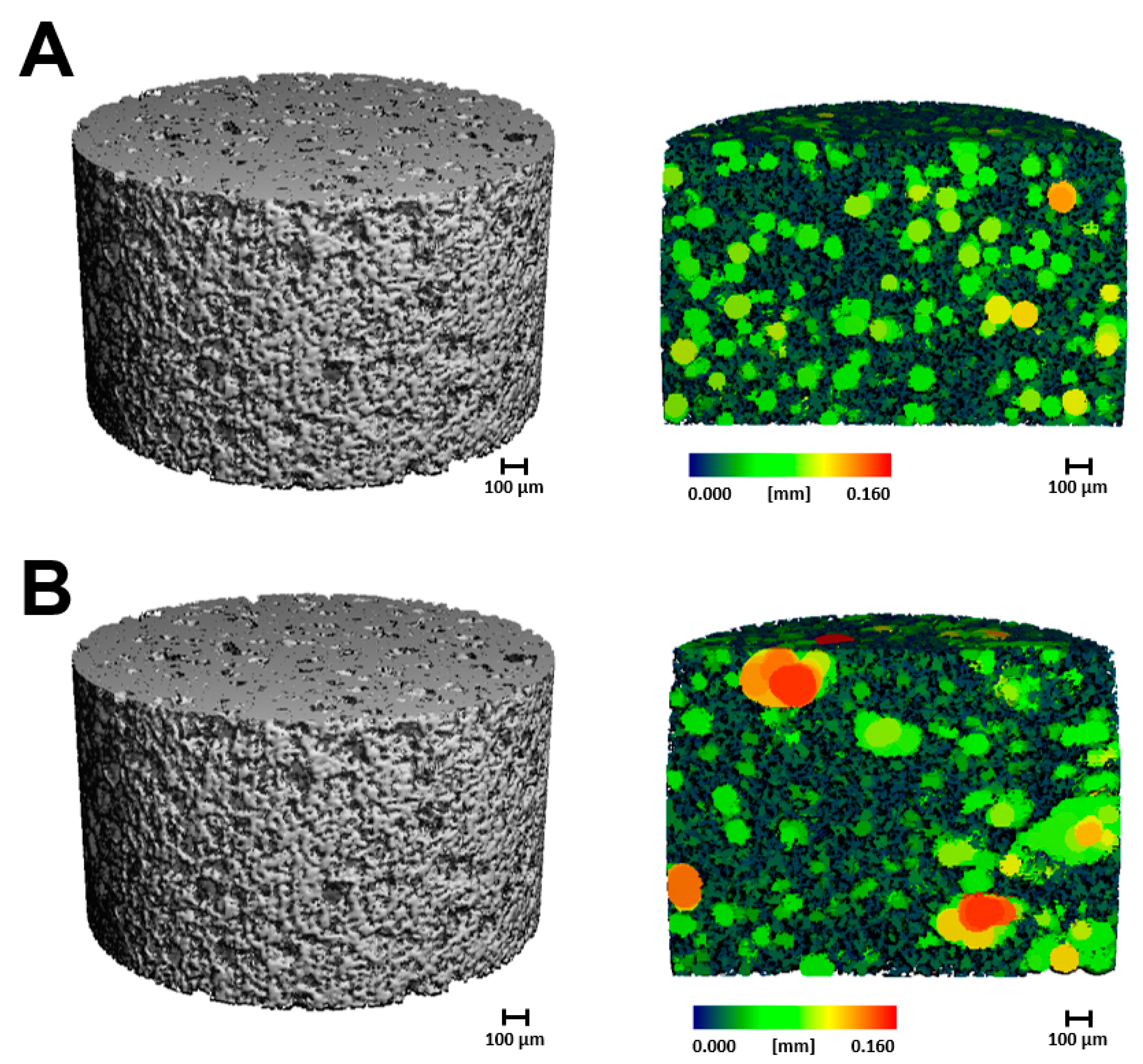
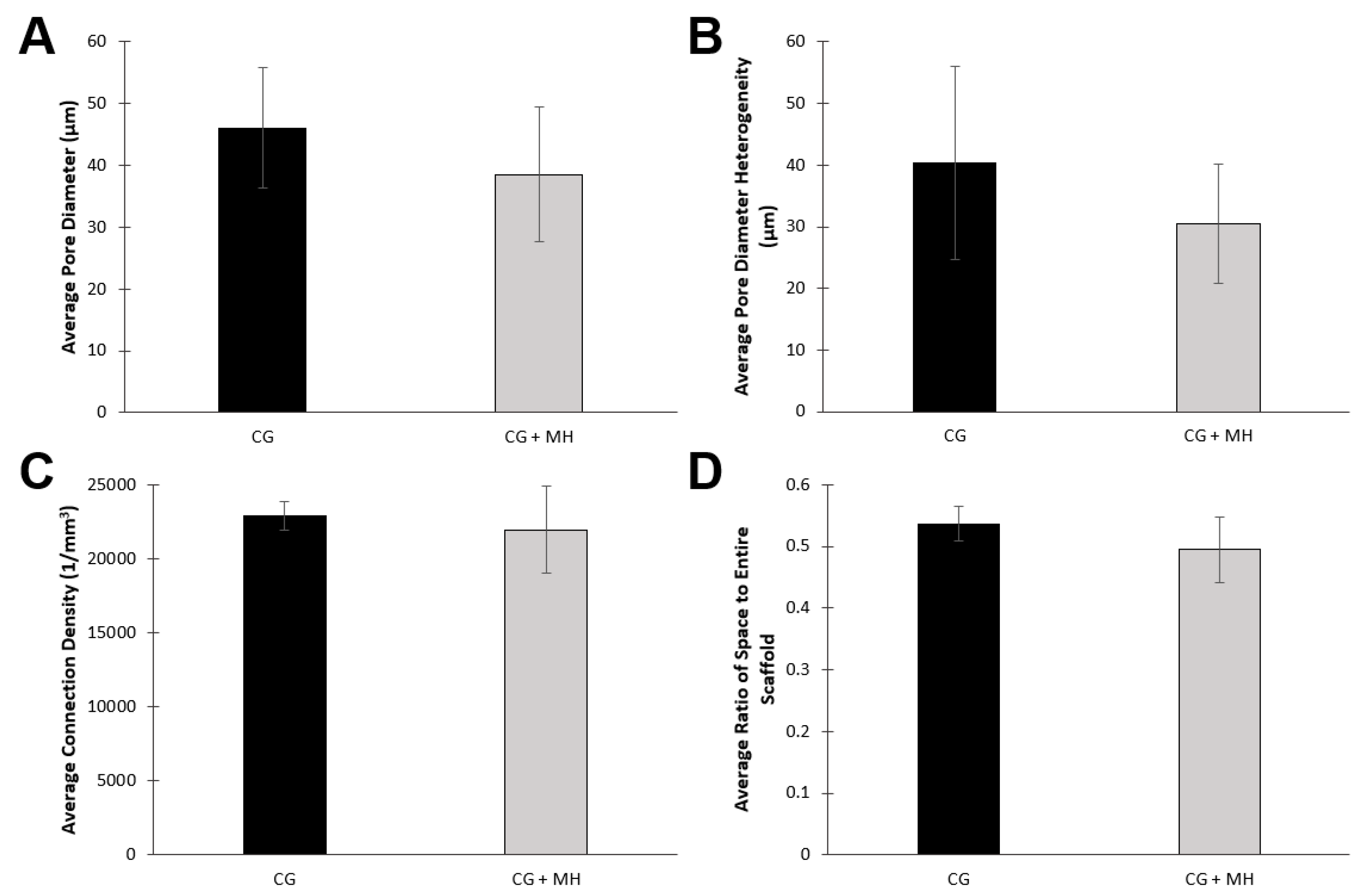
2.1.2. Microcomputed Tomography
2.1.3. Cellular Adhesion
2.2. Manuka Honey Evaluation
2.2.1. Bacterial Clearance
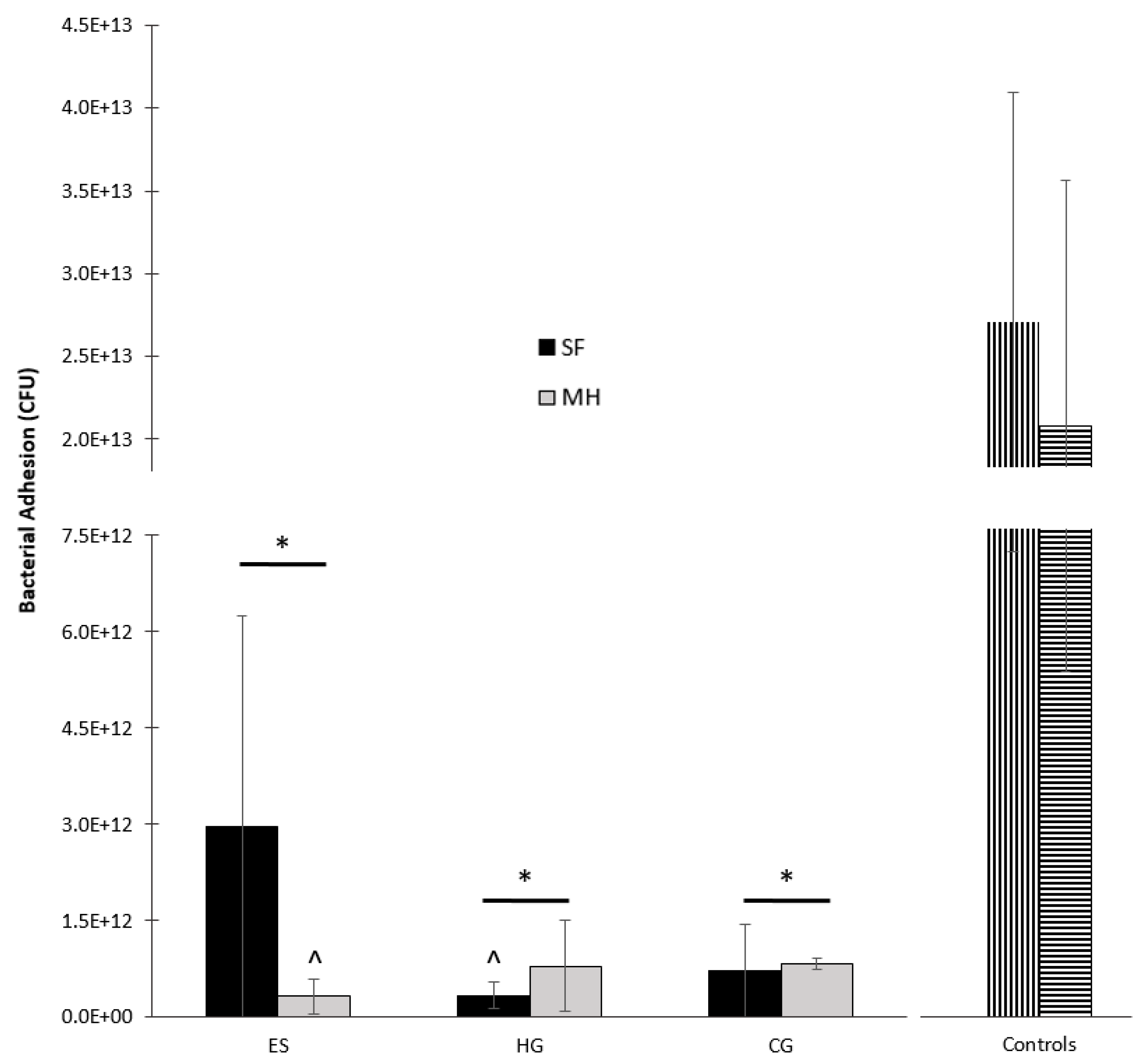
2.2.2. Bacterial Adhesion
3. Discussion
4. Materials and Methods
4.1. Scaffold Fabrication
4.1.1. Electrospun Scaffolds
4.1.2. Hydrogels
4.1.3. Cryogels
4.2. Scaffold Characterization
4.2.1. Scanning Electron Microscopy
4.2.2. Microcomputed Tomography
4.2.3. Cellular Adhesion
4.3. Manuka Honey Evaluation
4.3.1. Bacterial Clearance
4.3.2. Bacterial Adhesion
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Med. Sci. 2013, 16, 731–742. [Google Scholar]
- Speer, S.L.; E Schreyack, G. Manuka Honey: A Tissue Engineering Essential Ingredient. J. Sci. Eng. 2015, 6, 1–3. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.K.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Jenkins, R.; Roberts, A.E.L.; Brown, H.L. On the antibacterial effects of manuka honey: Mechanistic insights. Res. Rep. Biol. 2015, 6, 215–224. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A Preliminary Study on the Potential of Manuka Honey and Platelet-Rich Plasma in Wound Healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Gounari, P.; Skourtis, A.; Panagos, J.; Kazazis, C. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. Gen. Med. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for Wound Healing, Ulcers, and Burns; Data Supporting Its Use in Clinical Practice. Sci. World J. 2011, 11, 766–787. [Google Scholar] [CrossRef]
- Tomblin, V.; Ferguson, L.R.; Han, D.Y.; Murray, P.; Schlothauer, R. Potential pathway of anti-inflammatory effect by New Zealand honeys. Int. J. Gen. Med. 2014, 7, 149–158. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.B.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Dunford, C. The use of honey-derived dressings to promote effective wound management. Prof. Nurse 2005, 20, 35–38. [Google Scholar]
- Cooper, R.; Molan, P.; Krishnamoorthy, L.; Harding, K. Manuka Honey Used to Heal a Recalcitrant Surgical Wound. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 758–759. [Google Scholar] [CrossRef]
- Kamaratos, A.V.; Tzirogiannis, K.N.; Iraklianou, S.A.; Panoutsopoulos, G.I.; Kanellos, I.E.; Melidonis, A.I. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int. Wound J. 2014, 11, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Maqbool, F.; Bahadar, H.; Abdollahi, M. Health Benefits of Manuka Honey as an Essential Constituent for Tissue Regeneration. Curr. Drug Metab. 2017, 18, 881–892. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Bhat, S.; Tripathi, A.; Kumar, A. Supermacroprous chitosan-agarose-gelatin cryogels: In vitro characterization and in vivo assessment for cartilage tissue engineering. J. R. Soc. Interface 2011, 8, 540–554. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.N.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fibers Fabr. 2015, 10, 126–138. [Google Scholar] [CrossRef]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A Comparison of Tissue Engineering Scaffolds Incorporated with Manuka Honey of Varying UMF. BioMed Res. Int. 2017, 2017, 12. [Google Scholar] [CrossRef]
- Bulman, S.E.L.; Goswami, P.; Tronci, G.; Russell, S.J.; Carr, C. Investigation into the potential use of Poly (vinyl alcohol)/Methylglyoxal fibres as antibacterial wound dressing components. J. Biomater. Appl. 2015, 29, 1193–1200. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 193. [Google Scholar] [CrossRef]
- Sasikala, L.; Durai, B.; Rathinamoorthy, R. Manuka honey loaded chitosan hydrogel films for wound dressing applications. Int. J. Pharm. Tech. Res. 2013, 5, 1774–1785. [Google Scholar]
- Muktar, M.Z.; Ismail, W.I.W.; Razak, S.I.A.; Razali, M.H.; Amin, K.A.M. Accelerated Wound Healing of Physically Cross Linked Gellan Gum-Virgin Coconut Oil Hydrogel Containing Manuka Honey. ASM Sci. J. 2018, 11, 166–182. [Google Scholar]
- El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel film loaded with new formula from manuka honey for treatment of chronic wound infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Data on Manuka Honey/Gellan Gum composite hydrogels for cartilage repair. Data Brief. 2018, 20, 831–839. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472. [Google Scholar] [CrossRef]
- Azam, N.A.N.M.; Amin, K.A.M. Influence of Manuka Honey on Mechanical Performance and Swelling Behaviour of Alginate Hydrogel Film. Mater. Sci. Eng. 2018, 440, 012024. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Kumar, A.; Kathuria, N.; Tripathi, A.; Kar, K. Synthesis and characterization of elastic and macroporous chitosan–gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar]
- Kadakia, P.U.; Jain, E.; Hixon, K.R.; Eberlin, C.T.; A Sell, S. Sonication induced silk fibroin cryogels for tissue engineering applications. Mater. Res. Express 2016, 3, 055401. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Carletta, M.N.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 106, 1918–1933. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Balaji, A.; Jaganathan, S.K.; Ismail, A.F.; Rajasekar, R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016, 11, 4339–4355. [Google Scholar]
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A comparison of cryogel scaffolds to identify an appropriate structure for promoting bone regeneration. Biomed. Phys. Eng. Express 2016, 2, 035014. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Carletta, M.N.; Neal, S.M.; Talovic, M.; Dunn, A.J.; Garg, K.; Sell, S.A. Mineralization and antibacterial potential of bioactive cryogel scaffolds in vitro. Int. J. Polym. Mater. Polym. Biomater. 2018, 1–14. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Lu, T.; A Sell, S.; Hixon, K.R.; Eberlin, C.T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H. The calcification potential of cryogel scaffolds incorporated with various forms of hydroxyapatite for bone regeneration. Biomed. Mater. 2017, 12, 25005. [Google Scholar]
- Park, J.-B. The use of hydrogels in bone-tissue engineering. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e115–e118. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M.; Nerlich, M. Composite Hydrogels for Bone Regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef]
- Kemence, N.; Bolgen, N. Gelatin- and hydroxyapatite-based cryogels for bone tissue engineering: Synthesis, characterization, in vitro and in vivo biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef]





| Scaffold | Average Fiber Diameter (µm) | Average Pore Diameter (µm) | Average Pore Area (µm2) |
|---|---|---|---|
| ES | 0.41 ± 0.21 * | 3.28 ± 1.55 | 23.98 ± 17.76 * |
| ES + MH | 0.85 ± 0.40 * | 3.80 ± 1.85 | 16.00 ± 10.70 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels 2019, 5, 21. https://doi.org/10.3390/gels5020021
Hixon KR, Bogner SJ, Ronning-Arnesen G, Janowiak BE, Sell SA. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels. 2019; 5(2):21. https://doi.org/10.3390/gels5020021
Chicago/Turabian StyleHixon, Katherine R., Savannah J. Bogner, Gabriela Ronning-Arnesen, Blythe E. Janowiak, and Scott A. Sell. 2019. "Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds" Gels 5, no. 2: 21. https://doi.org/10.3390/gels5020021
APA StyleHixon, K. R., Bogner, S. J., Ronning-Arnesen, G., Janowiak, B. E., & Sell, S. A. (2019). Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels, 5(2), 21. https://doi.org/10.3390/gels5020021