Segregation Behavior of Polysaccharide–Polysaccharide Mixtures—A Feasibility Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Biopolymer Properties
2.2. Precipiation and Sedimentation Behavior
2.3. Rheological Measurements
2.4. Mixed Biopolymer Systems—Caseinate and Alginate
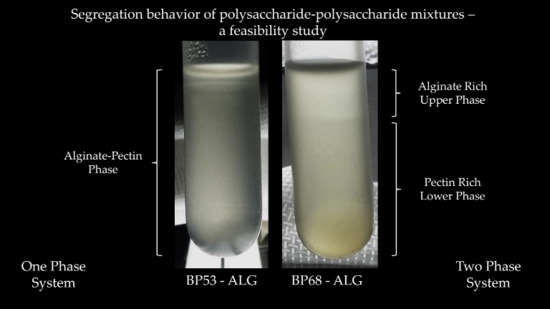
2.5. Mixed Alginate-Beet Pectin Systems
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Characterization of Biopolymer Solutions
4.2.2. Preparation and Characterization of Mixed Biopolymer Systems
4.2.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corredig, M.; Sharafbafi, N.; Kristo, E. Polysaccharide-protein interactions in dairy matrices, control and design of structures. Food Hydrocoll. 2011, 25, 1833–1841. [Google Scholar] [CrossRef]
- Dickinson, E. Particle-based stabilization of water-in-water emulsions containing mixed biopolymers. Trends Food Sci. Technol. 2019, 83, 31–40. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Beaulieu, M.; Schmitt, C.; Sanchez, C. Protein-polysaccharide interactions: Phase-ordering kinetics, thermodynamic and structural aspects. Curr. Opin. Colloid Interface Sci. 2003, 8, 401–414. [Google Scholar] [CrossRef]
- Stenger, C.; Zeeb, B.; Hinrichs, J.; Weiss, J. Formation of concentrated biopolymer particles composed of oppositely charged wpi and pectin for food applications. J. Dispers. Sci. Technol. 2017, 38, 1258–1265. [Google Scholar] [CrossRef]
- Zeeb, B.; Schock, V.; Schmid, N.; Majer, L.; Herrmann, K.; Hinrichs, J.; Weiss, J. Impact of food structure on the compatibility of heated wpi-pectin-complexes in meat dispersions. Food Funct. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; de Hoog, E.H.A.; Oosterveld, A.; Tromp, R.H. Protein-Polysaccharide Interactions to Alter Texture. In Annual Review of Food Science and Technology; Doyle, M.P., Klaenhammer, T.R., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 6, pp. 371–388. [Google Scholar]
- Zeeb, B.; Mi-Yeon, L.; Gibis, M.; Weiss, J. Growth phenomena in biopolymer complexes composed of heated wpi and pectin. Food Hydrocoll. 2018, 74, 53–61. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some thermodynamic considerations in food formulation. Food Hydrocoll. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Ingredient Interactions in Complex Foods: Aggregation and Phase Separation. In Understanding and Controlling the Microstructure of Complex Foods; McClements, D.J., Ed.; Elsevier Woodhead Publishing: Swaston Cambridge, UK, 2007; pp. 185–206. [Google Scholar]
- Eghbal, N.; Choudhary, R. Complex coacervation: Coacervation: Encapsulation and controlled release of active agents in food systems. Lwt Food Sci. Technol. 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Formation of biopolymer particles by thermal treatment of beta-lactoglobulin-pectin complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Protte, K.; Bollow, C.; Sonne, A.; Menéndez-Aguirre, O.; Weiss, J.; Hinrichs, J. Impacts on micro- and macro-structure of thermally stabilised whey protein-pectin complexes: A fluorescence approach. Food Biophys. 2016, 11, 226–234. [Google Scholar] [CrossRef]
- Zeeb, B.; Stenger, C.; Hinrichs, J.; Weiss, J. Formation of concentrated particles composed of oppositely charged biopolymers for food applications—Impact of processing conditions. Food Struct. 2016, 10, 10–20. [Google Scholar] [CrossRef]
- Esquena, J. Water-in-water (w/w) emulsions. Curr. Opin. Colloid Interface Sci. 2016, 25, 109–119. [Google Scholar] [CrossRef]
- Nicolai, T.; Murray, B. Particle stabilized water in water emulsions. Food Hydrocoll. 2017, 68, 157–163. [Google Scholar] [CrossRef]
- Thongkaew, C.; Zeeb, B.; Gibis, M.; Hinrichs, J.; Weiss, J. Initial droplet size impacts ph-induced structural changes in phase-separated polymer dispersions. J. Food Sci. 2016, 81, E1124–E1129. [Google Scholar] [CrossRef] [PubMed]
- Matalanis, A.; Lesmes, U.; Decker, E.A.; McClements, D.J. Fabrication and characterization of filled hydrogel particles based on sequential segregative and aggregative biopolymer phase separation. Food Hydrocoll. 2010, 24, 689–701. [Google Scholar] [CrossRef]
- Comert, F.; Dubin, P.L. Liquid-liquid and liquid-solid phase separation in protein-polyelectrolyte systems. Adv. Colloid Interface Sci. 2017, 239, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Grossmann, L.; Wefers, D.; Bunzel, M.; Weiss, J.; Zeeb, B. Accessibility of transglutaminase to induce protein crosslinking in gelled food matrices-influence of network structure. LWT 2017, 75, 271–278. [Google Scholar] [CrossRef]
- Zeeb, B.; Gibis, M.; Fischer, L.; Weiss, J. Crosslinking of interfacial layers in multilayered oil-in-water emulsions using laccase: Characterization and ph-stability. Food Hydrocoll. 2012, 27, 126–136. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Schliemann, W.; Corke, H. Chemical stability and colorant properties of betaxanthin pigments from celosia argentea. J. Agric. Food Chem. 2001, 49, 4429–4435. [Google Scholar] [CrossRef] [PubMed]
- Syrbe, A.; Bauer, W.; Klostermeyer, H. Polymer science concepts in dairy systems—An overview of milk protein and food hydrocolloid interaction. Int. Dairy J. 1998, 8, 179–193. [Google Scholar] [CrossRef]
- Bryant, C.; McClements, D. Influence of xanthan gum on physical characteristics of heat-denatured whey protein solutions and gels. Food Hydrocoll. 2000, 14, 383–390. [Google Scholar] [CrossRef]
- Brouzet, C.; Mittal, N.; Lundell, F.; Söderberg, L.D. Characterizing the oriental and network dynamics of polydisperse nanofibers on the nanoscale. Macromolecules 2019, 52, 2286–2295. [Google Scholar] [CrossRef]
- Nordenström, M.; Fall, A.; Nyström, G.; Wågberg, L. Formation of colloidal nanocellulose glasses and gels. Langmuir 2017, 33, 9772–9780. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, F.G.; Xu, X.F.; Huan, S.Q.; Gu, J.Y.; McClements, D.J. Impact of polysaccharide molecular characteristics on viscosity enhancement and depletion flocculation. J. Food Eng. 2017, 207, 35–45. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Biopolymers Online: Biology Chemistry Biotechnology Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Zouambia, Y.; Moulai-Mostefa, N.; Krea, M. Structural characterization and surface activity of hydrophobically functionalized extracted pectins. Carbohydr. Polym. 2009, 78, 841–846. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose 2010, 17, 1045–1065. [Google Scholar] [CrossRef]
- Takayama, Y.; Kato, N. Shear-induced structuring for multiple parallel gel filaments obtained from casein-alginate hybrids. Langmuir 2018, 34, 13352–13360. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Van Puyvelde, P.; Moldenaers, P. Interfacial tension of aqueous biopolymer mixtures close to the critical point. Int. J. Biol. Macromol. 2004, 34, 29–35. [Google Scholar] [CrossRef][Green Version]
- Reeve, R.M. A specific hydroxylamine-ferric chloride reaction for histochemical localization of pectin. Stain Technol. 1959, 34, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Hornatowska, J. Visualisation of Pectins and Proteins by Microscopy. STFI-Packforsk Report. 2005. Available online: https://www.researchgate.net/profile/Telse_Zimmermann/post/Does_anyone_have_evidence_for_fluorescence_of_pectin_stain_with_ruthenium_red_in_the_CLSM/attachment/59d63866c49f478072ea560a/AS:273699800846336@1442266525159/download/Hornatowska%2C+2005+Visualisation+of+woody+pectine.pdf (access on 13 May 2018).
- Zeeb, B.; Grossmann, L.; Weiss, J. Accessibility of transglutaminase to induce protein crosslinking in gelled food matrices-impact of membrane structure. Food Biophys. 2016, 11, 176–183. [Google Scholar] [CrossRef]
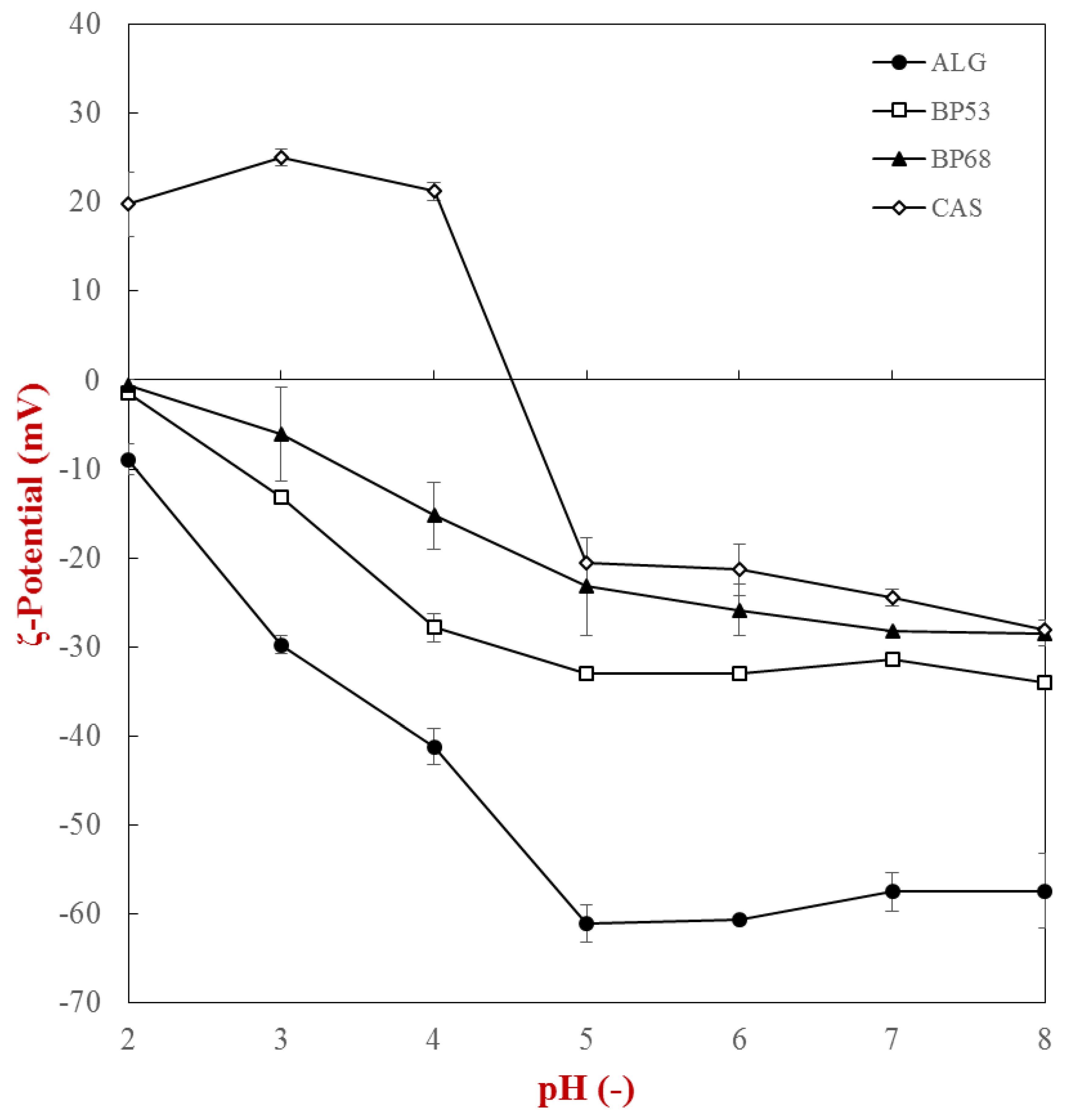
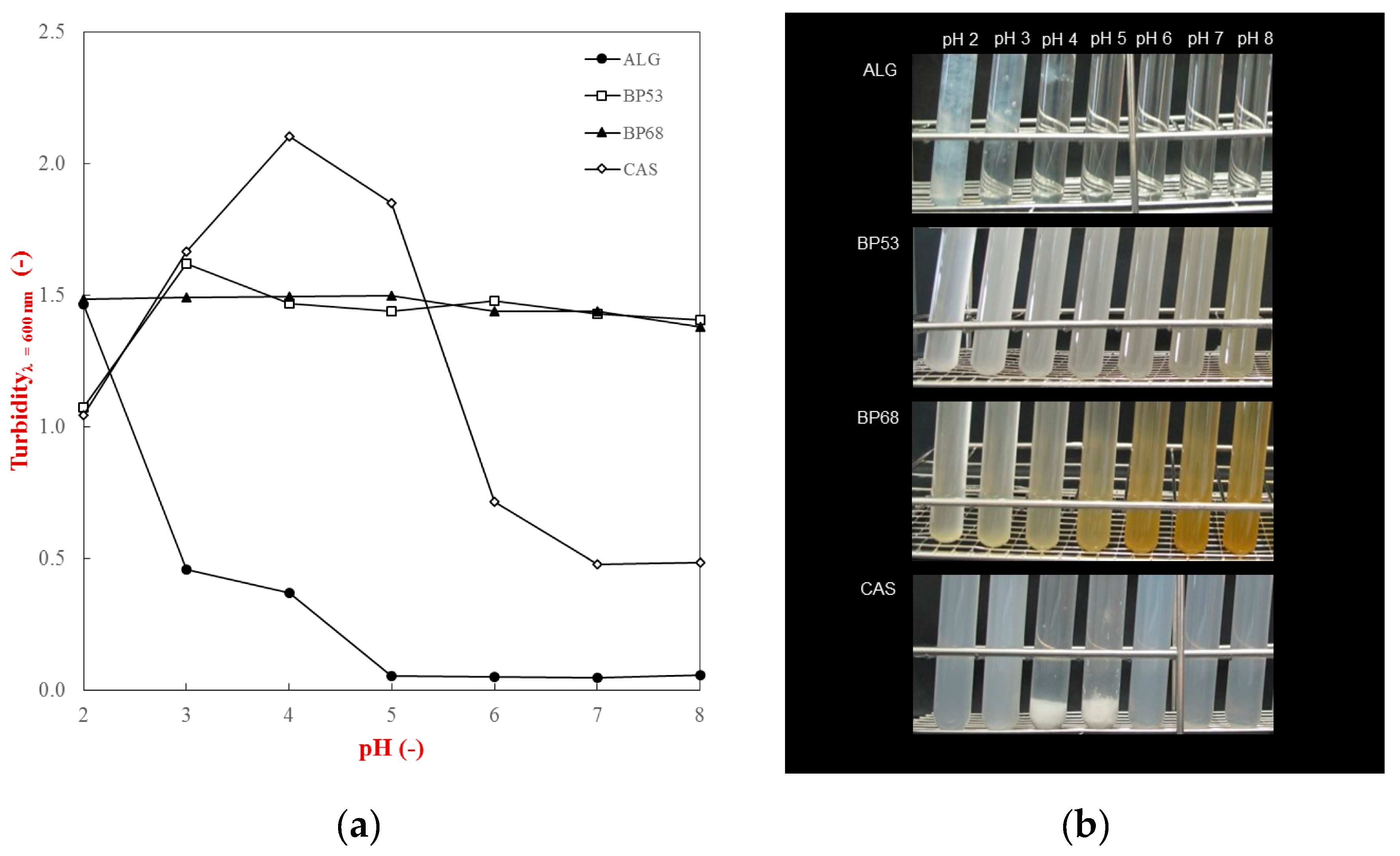

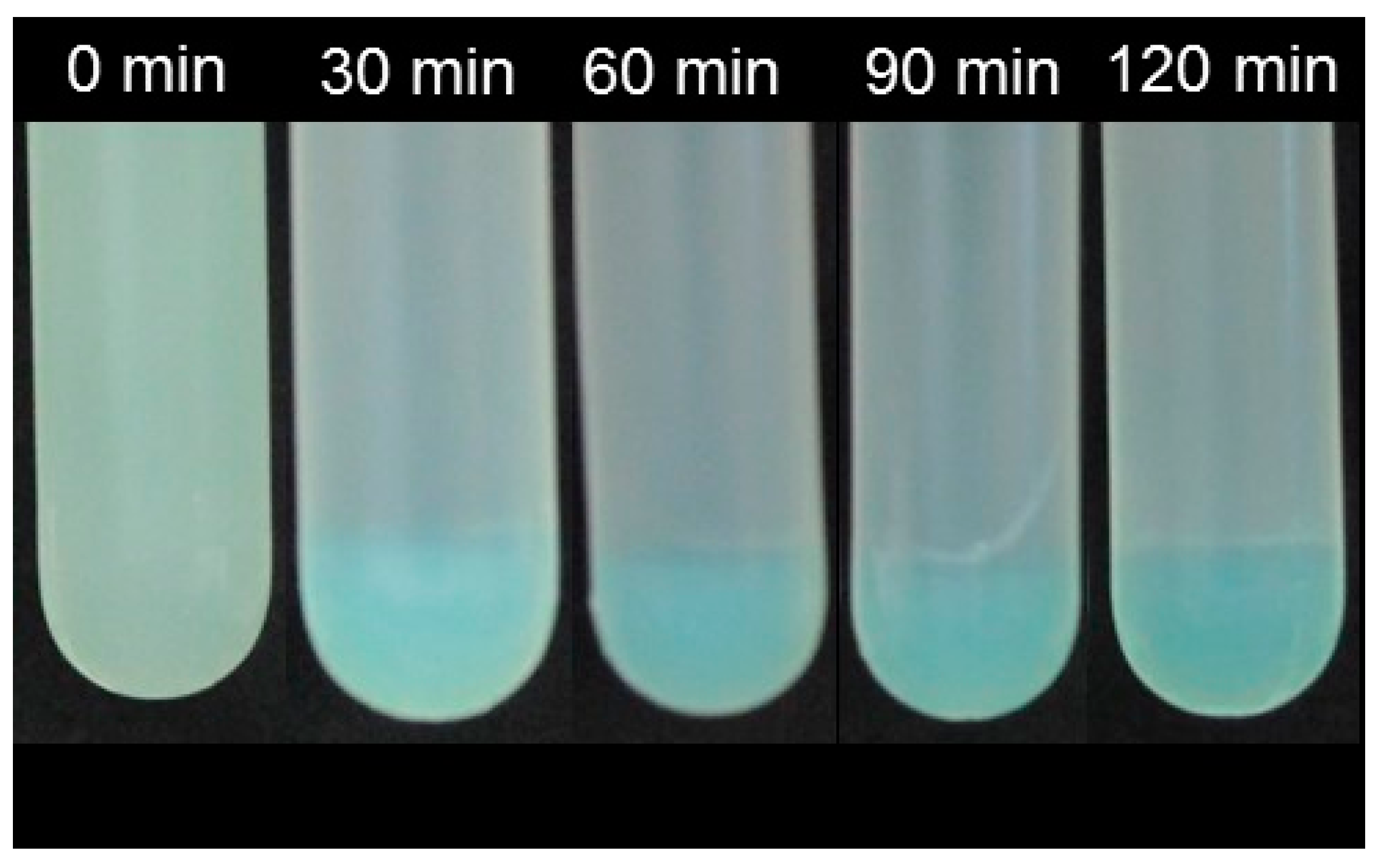
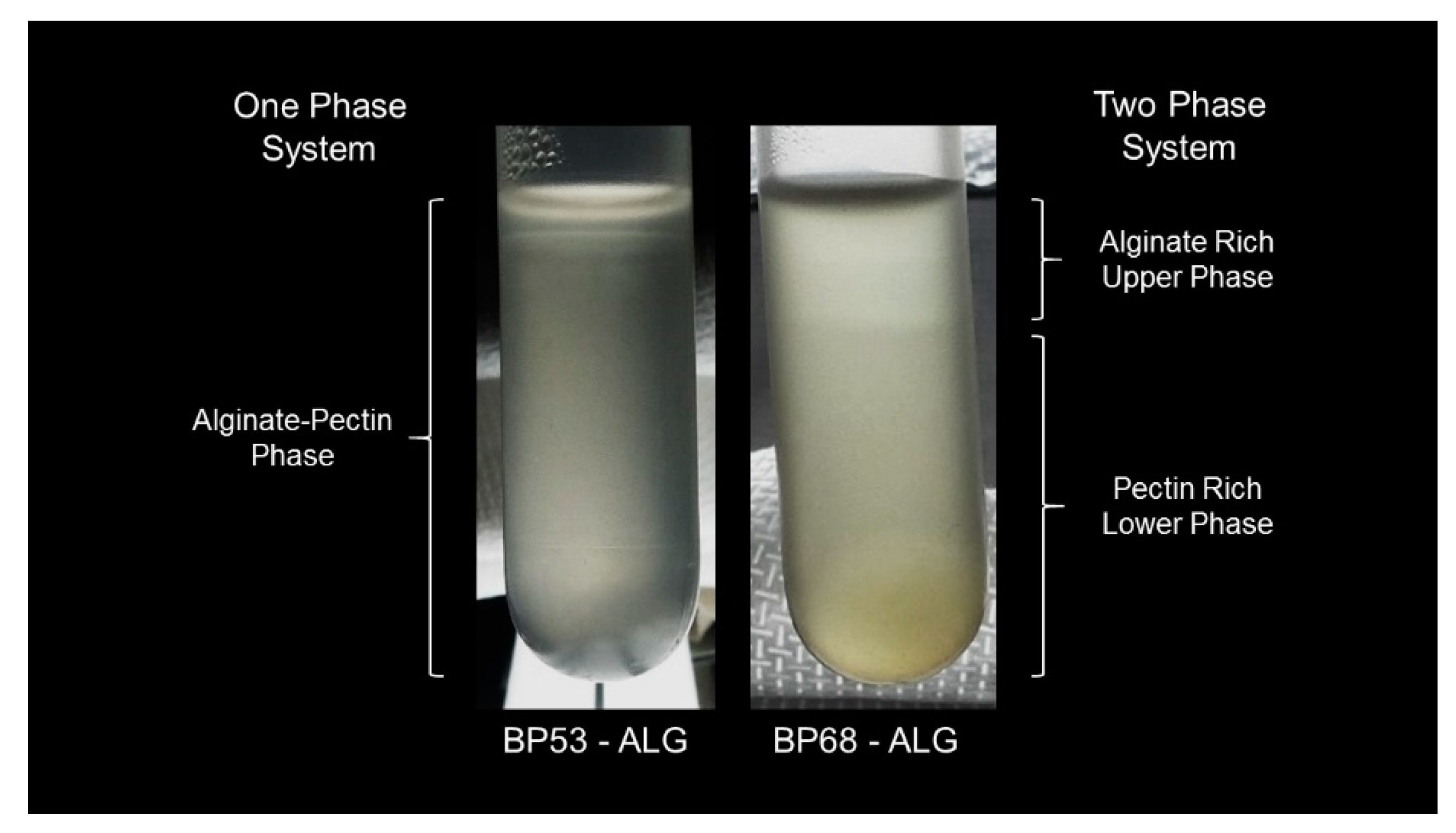
| Phase | Absorbance at λ = 325 nm | a*−value | Calcium Sensitivity 1 |
|---|---|---|---|
| Upper | 0.75 ± 0.00 | −0.15 ± 0.04 | ++ |
| Lower | 1.04 ± 0.01 | 1.56 ± 0.13 | + |
| Alginate | 0.04 ± 0.00 | n.d. | +++ |
| Beet pectin (DE 53%) | 1.11 ± 0.01 | n.d. | – |
| Biopolymer | Abbreviation | Characteristics 1 |
|---|---|---|
| Sodium caseinate | CAS | ≥ 88% protein; ≤ 6% moisture; ≤ 4.5% minerals; ≤ 0.2% fat; ≤ 0.2% lactose |
| Alginate | ALG | Guluronic acid:mannuronic acid = 0.75 ± 0.02 |
| Sugar beet pectin | BP53 | DE° = 53.4%; DAc° = 25.8%; AGA = 65.4%; ferulic acid = 0.75 ± 0.02%; pH = 2.82 |
| Modified sugar beet pectin | BP68 | DE° = 68.3%; DAc° = 20.4%; AGA = 70.0%; pH = 4.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeeb, B.; Jost, T.; McClements, D.J.; Weiss, J. Segregation Behavior of Polysaccharide–Polysaccharide Mixtures—A Feasibility Study. Gels 2019, 5, 26. https://doi.org/10.3390/gels5020026
Zeeb B, Jost T, McClements DJ, Weiss J. Segregation Behavior of Polysaccharide–Polysaccharide Mixtures—A Feasibility Study. Gels. 2019; 5(2):26. https://doi.org/10.3390/gels5020026
Chicago/Turabian StyleZeeb, Benjamin, Theresa Jost, David Julian McClements, and Jochen Weiss. 2019. "Segregation Behavior of Polysaccharide–Polysaccharide Mixtures—A Feasibility Study" Gels 5, no. 2: 26. https://doi.org/10.3390/gels5020026
APA StyleZeeb, B., Jost, T., McClements, D. J., & Weiss, J. (2019). Segregation Behavior of Polysaccharide–Polysaccharide Mixtures—A Feasibility Study. Gels, 5(2), 26. https://doi.org/10.3390/gels5020026