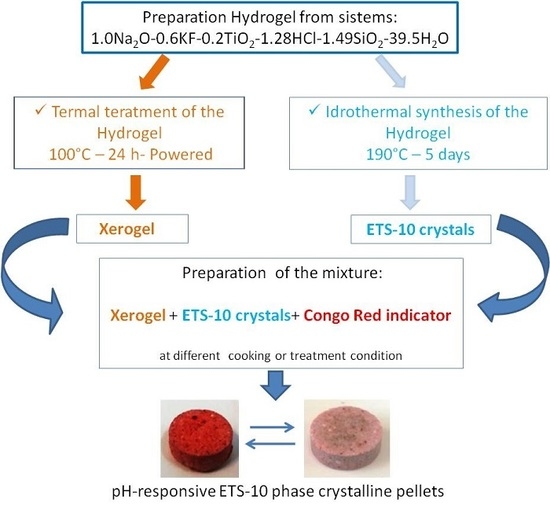
Preparation of ETS-10 Microporous Phase Pellets with Color Change Properties
Abstract
1. Introduction
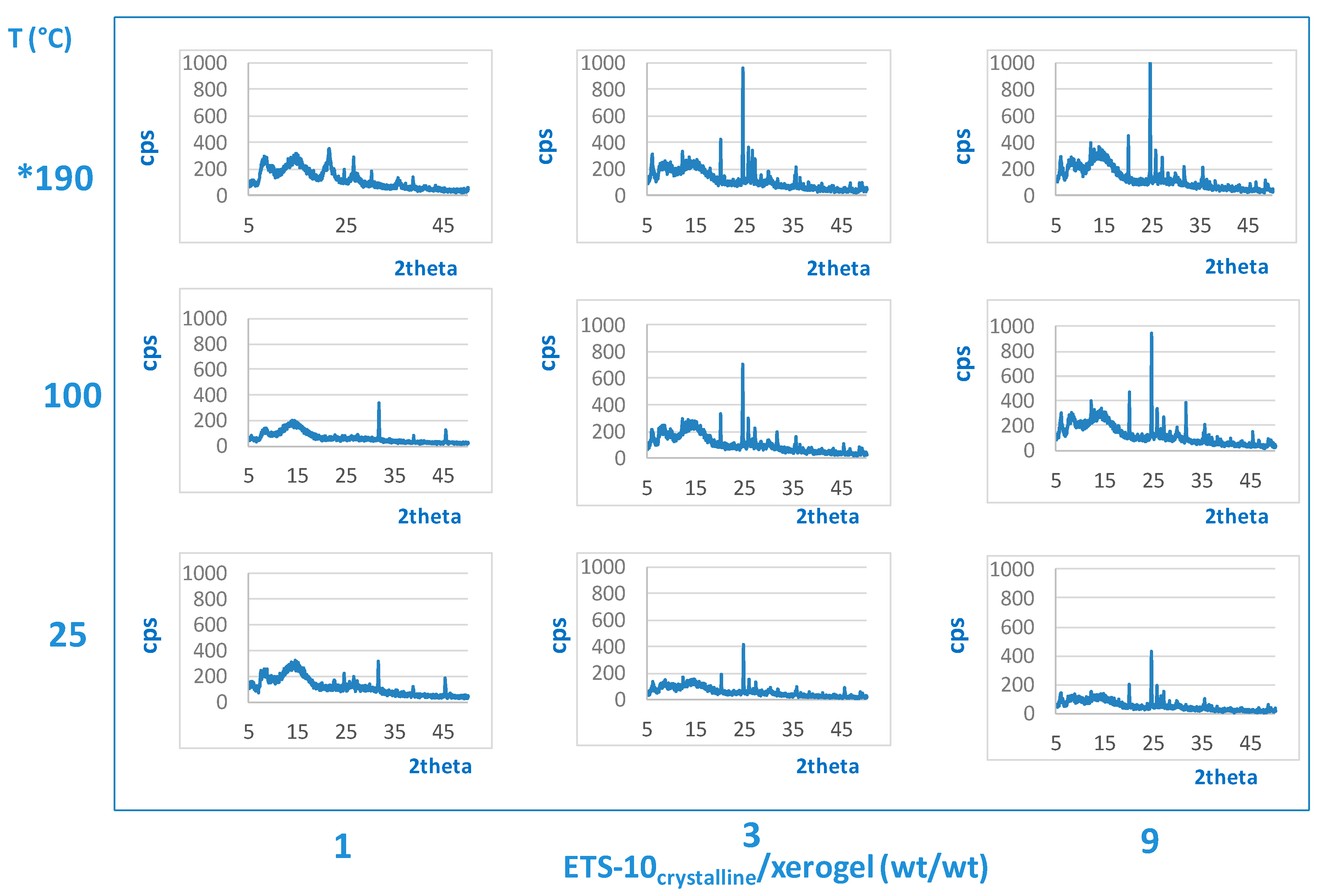
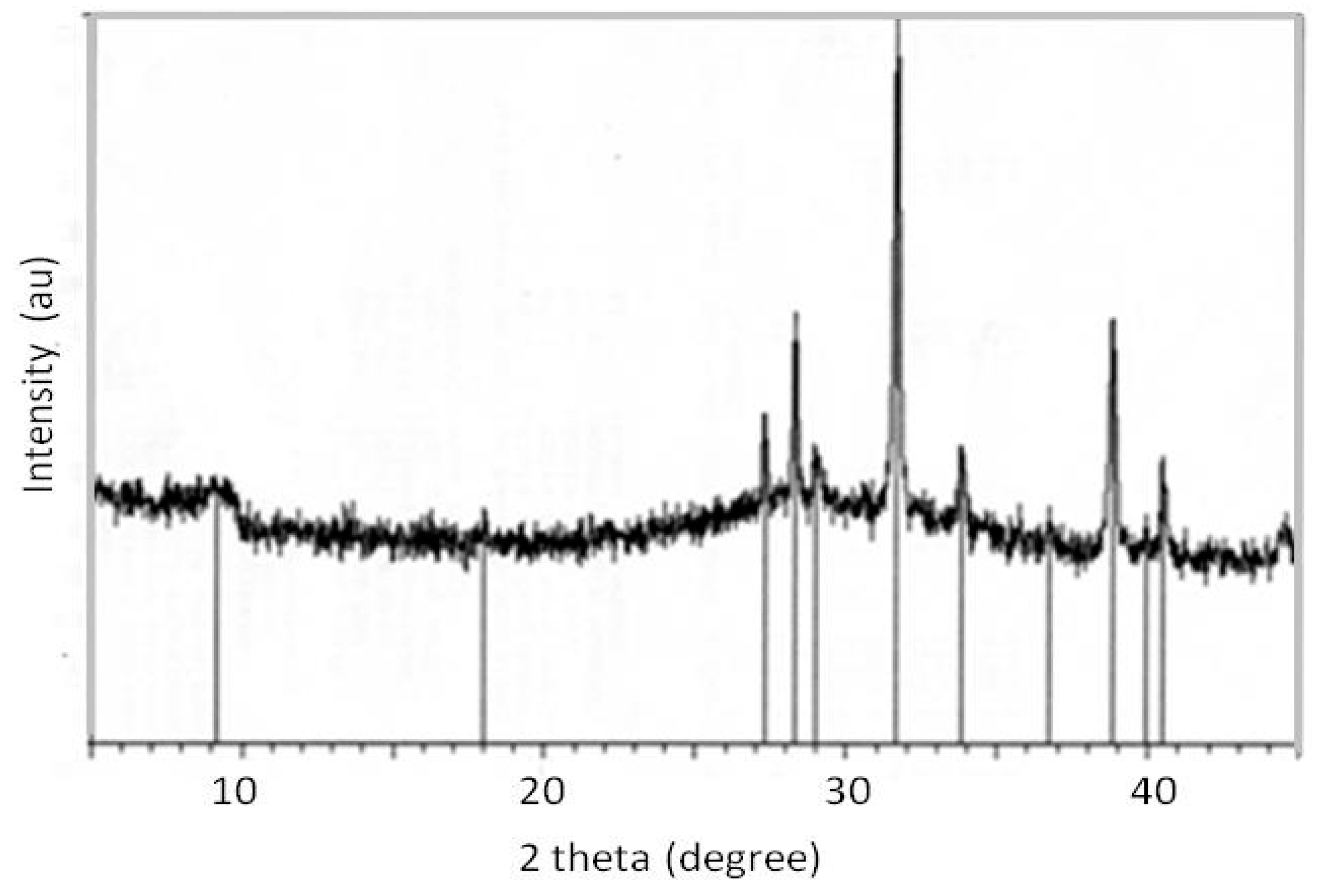
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- De Luca, P.; Carbone, I.; Nagy, J.B. Green building materials: A review of state of the art studies of innovative materials. J. Green Build. 2017, 12, 141–161. [Google Scholar] [CrossRef]
- Reddy, B.V. Sustainable materials for low carbon buildings. Int. J. Low Carbon Technol. 2009, 4, 175–181. [Google Scholar] [CrossRef]
- De Luca, P.; Pane, L.; Vuono, D.; Siciliano, C.; Candamano, S.; Nagy, J.B. Preparation and characterization of natural glues with carbon nanotubes. Environ. Eng. Manag. J. 2017, 16, 1659–1671. [Google Scholar] [CrossRef]
- Ding, G.K. Sustainable construction—The role of environmental assessment tools. J. Environ. Manag. 2008, 86, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Nastro, V.; Vuono, D.; Guzzo, M.; Niceforo, G.; Bruno, I.; De Luca, P. Characterisation of raw materials for production of ceramics. J. Therm. Anal. Calorim. 2006, 84, 181–184. [Google Scholar] [CrossRef]
- De Luca, P.; Roberto, B.; Vuono, D.; Siciliano, C.; Nagy, J.B. Preparation and Optimization of Natural Glues Based on Laricio Pine Resin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012071. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, S.; Li, F.; Lin, Y. Utilization of municipal sewage sludge as additives for the production of eco-cement. J. Hazard. Mater. 2012, 30, 457–465. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; Nagy, J.B. Preparation and Characterization of Plasters with Photodegradative Action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marci, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211, 3–29. [Google Scholar] [CrossRef]
- Ma, Y.; Tong, W.; Zhou, H.; Suib, S.L. A review of zeolite-like porous materials. Microporous Mesoporous Mater. 2000, 37, 243–252. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.M. Zeolites and zeolite-like materials. Pure Appl. Chem. 1986, 58, 1323–1328. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Kuznicki, S.M. Large-Pored Crystalline Titanium Molecular Sieve Zeolites. US Patent 4,853,202, 1 August 1989. [Google Scholar]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Philippou, A.; Mackay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S.; Anderson, M. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Malley, P.J.O.; Philippou, A.; MacKay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Microporous Titanosilicate ETS-10: A Structural Survey. Philos. Mag. B 1995, 71, 813–841. [Google Scholar] [CrossRef]
- Rocha, J.; Anderson, M.W. Microporous Titanosilicates and Other Novel Mixed Octahedral-Tetrahedral Framework Oxides. Eur. J. Inorg. Chem. 2000, 2000, 801–818. [Google Scholar] [CrossRef]
- Yang, X.; Paillaud, J.L.; Van Breukelen, H.; Kessler, H.; Duprey, E. Synthesis of microporous titanosilicate ETS-10 with TiF4 or TiO2. Microporous Mesoporous Mater. 2001, 46, 1–11. [Google Scholar] [CrossRef]
- De Luca, P.; Nastro, A. Synthesis of ETS-10 molecular sieve from systems containing TAABr salts. Stud. Surf. Sci. Catal. 1997, 105, 221–228. [Google Scholar]
- Sudheesh, N.; Shukla, R.S. Rhodium exchanged ETS-10 and ETS-4: Efficient heterogeneous catalyst for hydroaminomethylation. Appl. Catal. A Gen. 2014, 473, 116–124. [Google Scholar] [CrossRef]
- Turta, N.; De Luca, P.; Bilba, N.; Nagy, J.; Nastro, A. Synthesis of titanosilicate ETS-10 in presence of cetyltrimethylammonium bromide. Microporous Mesoporous Mater. 2008, 112, 425–431. [Google Scholar] [CrossRef]
- Veltri, M.; Vuono, D.; De Luca, P.; Nagy, J.B.; Nastro, A. Typical data of a new microporous material obtained from gels with titanium and silicon. J. Therm. Anal. Calorim. 2006, 84, 247–252. [Google Scholar] [CrossRef]
- Turta, N.A.; Veltri, M.; Vuono, D.; De Luca, P.; Bilba, N.; Nastro, A. Effect of crystallization temperature on the synthesis of ETS-4 and ETS-10 titanosilicates. J. Porous Mater. 2009, 16, 527–536. [Google Scholar] [CrossRef]
- Vuono, D.; Guzzo, M.; De Luca, P.; Nagy, J.B. Physico-chemical characterization of zirconium-based self-bonded ETS-4 pellets. J. Therm. Anal. Calorim. 2014, 116, 169–182. [Google Scholar] [CrossRef]
- Pavel, C.; Vuono, D.; De Luca, P.; Bilba, N.; Nagy, J.B.; Nastro, A.; Pavel, C. Synthesis and characterization of ET(P)S-4 and ET(P)S-10. Microporous Mesoporous Mater. 2005, 80, 263–268. [Google Scholar] [CrossRef]
- Al-Attar, L.; Dyer, A.; Blackburn, R. Uptake of Uranium on ETS-10 Microporous Titanosilicate. J. Radioanal. Nucl. Chem. 2000, 246, 451–455. [Google Scholar] [CrossRef]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; Nagy, J.B.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.B.; Coimbra, J.; Otero, M.; Pereira, E.; Duarte, A.C.; Lin, Z.; Rocha, J. Uptake of Hg2+ from aqueous solutions by microporous titano- and zircono-silicates. Quim. Nova 2008, 31, 321–325. [Google Scholar] [CrossRef]
- Zhao, G.X.S.; Lee, J.L.; Chia, P.A. Unusual Adsorption Properties of Microporous Titanosilicate ETS-10 toward Heavy Metal Lead. Langmuir 2003, 19, 1977–1979. [Google Scholar] [CrossRef]
- Lv, L.; Su, F.; Zhao, X.S. Microporous titanosilicate ETS-10 for the removal of divalentheavy metals. Stud. Surf. Sci. Catal. 2005, 156, 933–940. [Google Scholar]
- Pavel, C.; Popa, K.; Bilba, N.; Cecal, A.; Cozma, D.; Pui, A. The sorption of some radiocations on microporous titanosilicate ETS-10. J. Radioanal. Nucl. Chem. 2003, 258, 243–248. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; Nagy, J.B. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- De Luca, P.; Poulsen, T.G.; Salituro, A.; Tedeschi, A.; Vuono, D.; Konya, Z.; Madarász, D.; Nagy, J.B. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 1257–1267. [Google Scholar] [CrossRef]
- Lico, D.; Vuono, D.; Siciliano, C.; Nagy, J.B.; De Luca, P. Removal on unleaded gasoline from water by multi-walled carbon nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Shough, A.M.; Doren, D.J.; Nash, M.; Lobo, R.F. Effects of Vanadium Substitution on the Structure and Photocatalytic Behavior of ETS-10. J. Phys. Chem. C 2007, 111, 1776–1782. [Google Scholar] [CrossRef]
- Guan, G.; Kida, T.; Kusakabe, K.; Kimura, K.; Abe, E.; Yoshida, A. Photocatalytic activity of CdS nanoparticles incorporated in titanium silicate molecular sieves of ETS-4 and ETS-10. Appl. Catal. A Gen. 2005, 295, 71–78. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Nagy, J.B. Activated ceramic materials with deposition of photocatalytic titano-silicate micro-crystals. Sustain. Chem. 2011, 1, 155–165. [Google Scholar]
- Ren, Y.; Gu, M.; Hu, Y.; Yue, B.; Jiang, L.; Kong, Z.; He, H. Preparation and Photocatalytic Activity of Lanthanide Loaded Microporous Titanosilicate ETS-10 Catalysts. Chin. J. Catal. 2012, 33, 123–128. [Google Scholar] [CrossRef]
- Labrés i Xamena, F.X.; Calza, P.; Lamberti, C.; Prestipino, C.; Damin, A.; Bordiga, S.; Pelizzetti, E.; Zecchina, A. Enhancement of the ETS-10 Titanosilicate activity in the shape-selective photocatalytic degradation of large aromatic molecules by controlled defect production. J. Am. Chem. Soc. 2003, 125, 2264–2271. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Southon, P.D.; Adesina, A.A.; Howe, R.F. ETS-10 as a photocatalyst. Int. J. Photoenergy 2003, 5, 131–140. [Google Scholar] [CrossRef]
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Gallo, V. Oxygenated polycyclic aromatic hydrocarbons in size-segregated urban aerosol. J. Aerosol Sci. 2015, 87, 126–134. [Google Scholar] [CrossRef]
- Zhuo, S.; Du, W.; Shen, G.; Li, B.; Liu, J.; Cheng, H.; Xing, B.; Tao, S. Estimating relative contributions of primary and secondary sources of ambient nitrated and oxygenated polycyclic aromatic hydrocarbons. Atmos. Environ. 2017, 159, 126–134. [Google Scholar] [CrossRef]
- Hussein, I.A.S.; Mona, S.M.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar]
- Filice, M.; De Luca, P.; Guido, G.P. Particular matter pollution in university area: Traffic flow analysis. Environ. Eng. Manag. J. 2009, 8, 1407–1412. [Google Scholar] [CrossRef]
- Marcazzan, G.; Valli, G.; Vecchi, R. Factors influencing mass concentration and chemical composition of fine aerosols during a PM high pollution episode. Sci. Total Environ. 2002, 298, 65–79. [Google Scholar] [CrossRef]
- De Luca, P.; Vuono, D.; Filice, M. Self-bonded ets-10 pellets containing iron. Environ. Eng. Manag. J. 2009, 8, 1009–1015. [Google Scholar] [CrossRef]
- De Luca, P.; Nappo, G.; Siciliano, C.; Nagy, J.B. The role of carbon nanotubes and cobalt in the synthesis of pellets of titanium silicates. J. Porous Mater. 2018, 25, 283–296. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; Nagy, J.B. Synthesis of Self-Bonded Pellets of ETS-4 Phase by New Methodology of Preparation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012003. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.; Ferdov, S.; Kalvachev, Y.; Mihailova, B.; Petrov, O.; Kalvachev, Y. Hydrothermal synthesis of microporous titanosilicates. Microporous Mesoporous Mater. 2007, 105, 232–238. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Mastroianni, C.; Siciliano, C.; Nagy, J.B.; Macario, A. Preparation of ETS-10 Microporous Phase Pellets with Color Change Properties. Gels 2019, 5, 42. https://doi.org/10.3390/gels5030042
De Luca P, Mastroianni C, Siciliano C, Nagy JB, Macario A. Preparation of ETS-10 Microporous Phase Pellets with Color Change Properties. Gels. 2019; 5(3):42. https://doi.org/10.3390/gels5030042
Chicago/Turabian StyleDe Luca, Pierantonio, Carmelo Mastroianni, Carlo Siciliano, Janos B. Nagy, and Anastasia Macario. 2019. "Preparation of ETS-10 Microporous Phase Pellets with Color Change Properties" Gels 5, no. 3: 42. https://doi.org/10.3390/gels5030042
APA StyleDe Luca, P., Mastroianni, C., Siciliano, C., Nagy, J. B., & Macario, A. (2019). Preparation of ETS-10 Microporous Phase Pellets with Color Change Properties. Gels, 5(3), 42. https://doi.org/10.3390/gels5030042