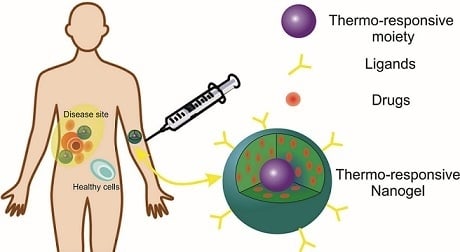
Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications
Abstract
1. Introduction
1.1. Hydrogels
1.2. Nanostructured Materials
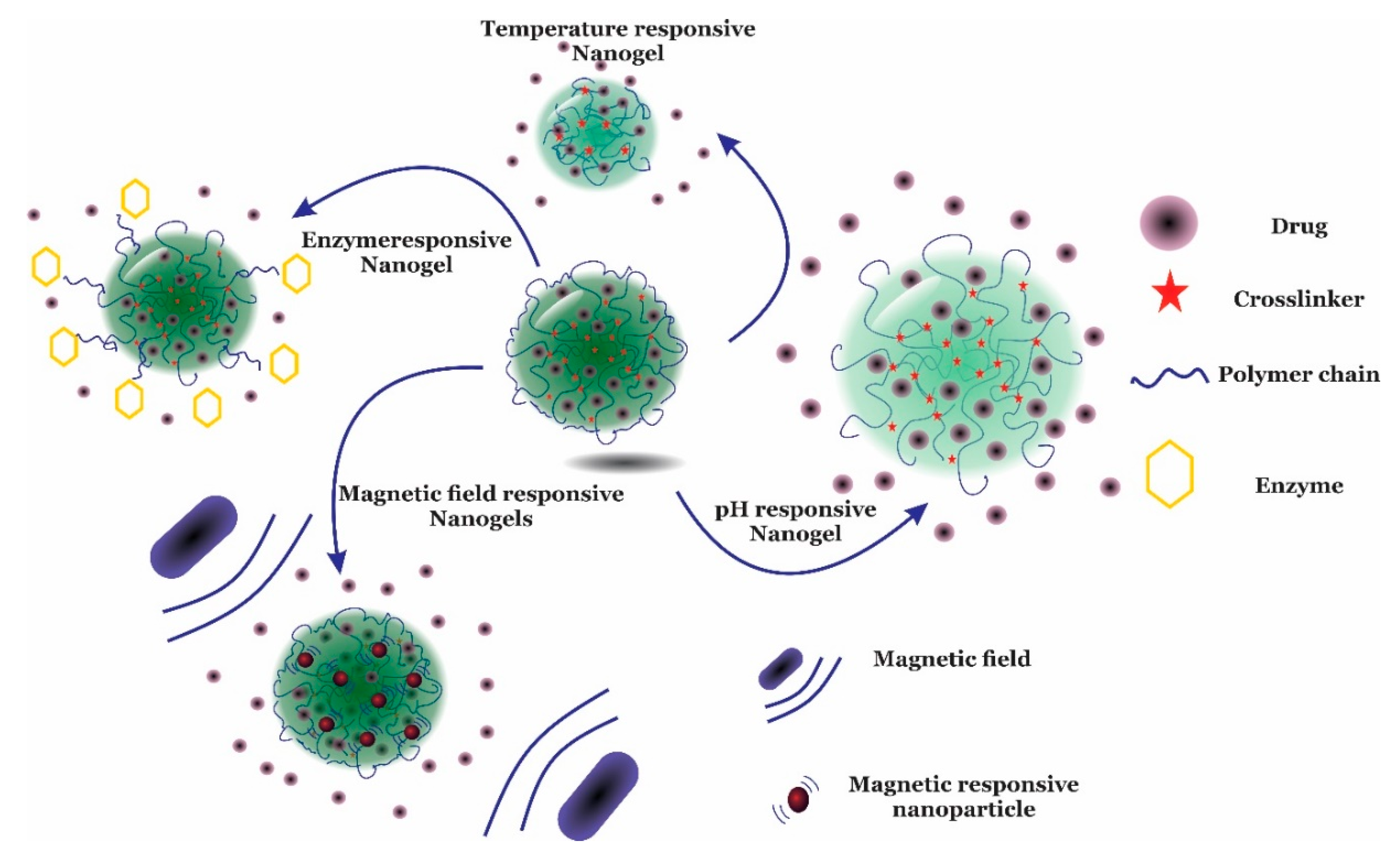
2. Nanogels
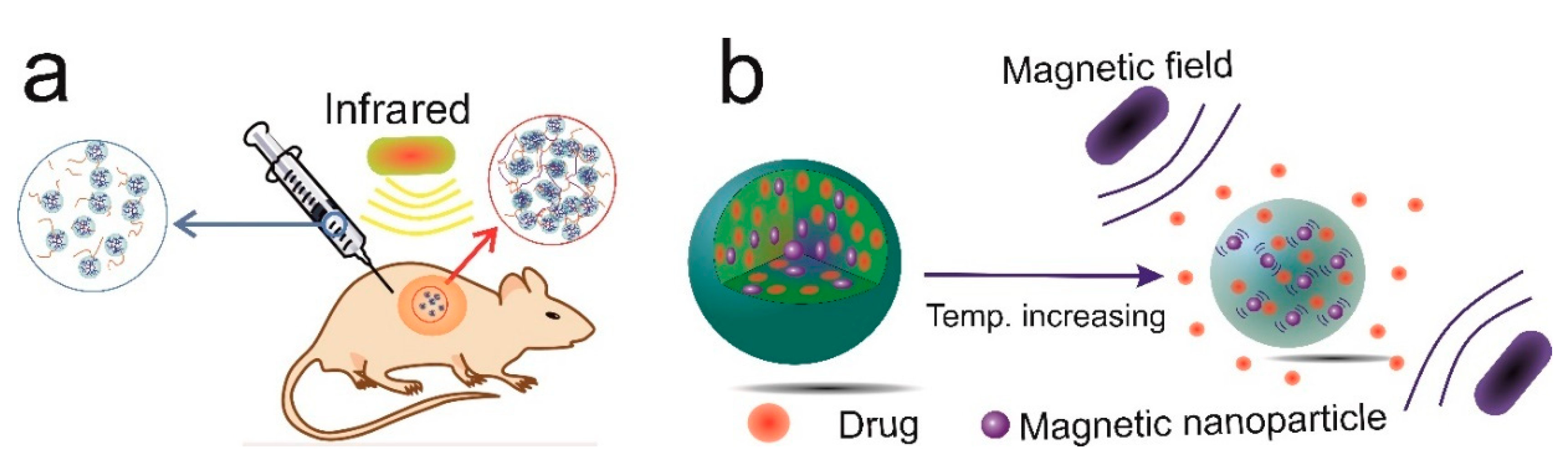
Thermosensitive Nanogels
3. Thermosensitive Polymers
3.1. Polymers Bearing Amide Groups
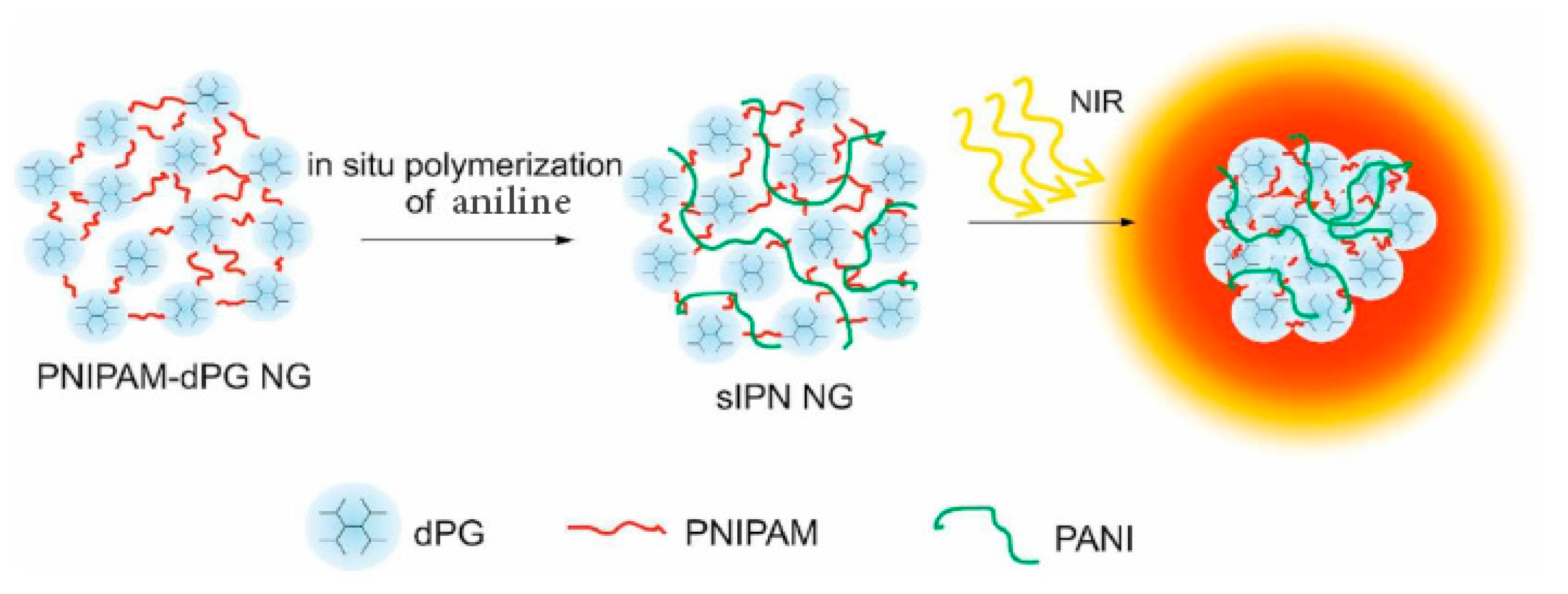
3.1.1. PNIPAM
3.1.2. PNIPMAM
3.1.3. PDEAAM
3.1.4. PVCL
3.2. Polymers Bearing Polyether Groups
PEG
3.3. Polymers Bearing Vinyl Ether Groups
3.3.1. PMEO2MA
3.3.2. OEGMA
3.4. Hydrophilic Polymers Bearing Hydrophobic Groups
3.4.1. Cholesterol-Bearing Polymers
3.4.2. PLLA-Bearing Polymers
3.4.3. PLLA Bearing Polymers
4. Conclusions
Funding
Conflicts of Interest
Appendix A
| Component | Abbreviation | Component | Abbreviation |
|---|---|---|---|
| (2-acetoacetoxyethyl) methacrylate | AAEMA | 1,3-bis(carboxyphenoxy) propane | CPP |
| 1-vinylimidazole | Vim | 2-(2-methoxyethoxy) ethyl meth acrylate) | MeO2MA |
| 2-(5,5-dimethyl-1,3-dioxan-2-yloxy) ethyl acrylate | DMDEA | 2-(acetylthio) ethyl methacrylate | AcSEMA |
| 2,2-bis(2-oxazoline) | BOX | 2-aminoethyl methacrylamide hydrochloride | AEMA |
| 2-dimethyl(aminoethyl) methacrylate | DMAEM | 2-dimethylmaleinimido ethylacrylamide | DMIAAm |
| 2-hydroxyethyl methacrylate | HEMA | 2-lactobionamidoethyl methacrylamide | LAEMAm |
| 2-methacryloyloxyethyl acrylate | MEA | 2-methoxyethyl acrylate | MEA |
| 3-(trimethoxysilyl)propyl methacrylate) | MPMA | 3-{[(2R)-2-(octadecylamino)-3-phenyl propanoyl]amino}butyrate | TEAB |
| 3-acrylamidophenylboronic acid | AAPBA | 3-gluconamidopropyl methacrylamide | GAPMA |
| 4-(2-acryloylaminoethylamino)-7-nitro-2,1,3-benzoxadiazole | NBDAA | 4-acrylamidofluorescein | AFA |
| 5-fluorouracil | 5-Fu | 6-O-vinyladipoyl-D-galactose | ODGal |
| Acetamidophenol | AP | Acrylamide | AAM |
| Acrylamidoglycolic acid | AGA | Acrylic acid | AA |
| Alginate | Alg | Atom transfer radical polymerization | ATRP |
| Basic fibroblast growth factor | bFGF | Beta glactosidase | BG |
| Beta glycerophosphate | β-GP | Bevacizumab | Bz |
| Bis (2-acryloyloxyethyl) disulfide | BADS | Bisacrylamide | BAM |
| Bismuth (III) oxide | Bi2O3 | Bone morphogenetic protein 2 | BMP-2 |
| Bovine serum albumin | BSA | Bupivacaine | BV |
| Butyl methacrylate | BMA | Butyl methylacrylate | PIB |
| Butyl methylacrylate | BMA | Carboxymethyl cellulose sodium salts | CMC |
| Carboxymethyl hexanoyl chitosan | CHChS | Cellulose | Clu |
| Chitosan | CHS | Cholesterol | Chol |
| Chondroitin sulfate | CS | Cisplatin | CIS |
| Cloud point temperature | CPT | Coumarin 102 | C 102 |
| Covinyl pyrrolidone | CVP | Curcumin | Cur |
| Cytochrome C | Cyt C | Dendritic polyglycerol | dPG |
| Deoxyribonucleic acid-acrylamide | DNA-AAM | Dextran | Dex |
| Dextran methacrylates | Dex-MA | Di(ethylene glycol) methyl ethyl methacrylate | DEGMA |
| Diacetone acrylamide | DAAM | Dibucaine | Dc |
| Diclofenac | Df | Dimethyl maleinimido acrylamide | DMIAAM |
| Doxorubicin | Dox | Ethosuximide | ESM |
| Ethylene glycol dimethacrylate | EGDMA | Ethylene glycol dimethacrylate | EGDMA |
| Ethylene glycole | EG | Fibrinogen | Fib |
| Fibroblast growth factor 18 | FGF18 | Food and Drug Administration | FDA |
| Gelatin type A | Gel A | Glutaraldehyde | GA |
| Graphene oxide | GO | Heparin | Hep |
| Hollow gold nanoparticle | HGNP | Human growth hormone | hGH |
| Human serum albumin | HAS | Hyaluronic acid | HA |
| Hydroxymethyl acrylamide | HMAA | Hydroxypropyl cellulose | HP-Clu |
| Indomethacin | Imc | Iodoazomycin Arabinofuranoside | IAZA |
| Itaconic acid | IA | Lewis lung carcinoma | LLC |
| Lidocaine | Lid | Lower critical aggregation concentration | LCAC |
| Lower critical solution temperature | LCST | L-Proline | L-Pro |
| Maleic acid | MA | Maleimide dithiol | MDT |
| Malloapelta B | Mall B | Megestrol acetate | Meg |
| Melatonin | Mt | Mesoporous silica | mSiO2 |
| Methacrylate | MA | Methacrylic Acid | MAA |
| Methotrexate | MTX | Methoxy-poly(ethylene glycol) | Met-PEG |
| Monomethoxy poly(ethylene glycol) | mPEG | Muscone | Mc |
| N, N-di ethylacrylamide | DEAAM | N,N′-methylenebis(acrylamide) | MBAM |
| N,N-diethylacrylamide | DEA | N,N-dimethylacrylamide | DMA |
| N,N-dimethylaminoethyl methacrylate | DMAEMA | N-acryloyl-3-aminophenylboronic acid | APBA |
| N-acryloylglycinamide | NAGA | Naphthalimide-based dye | NPTUA |
| Nile red | NR | Nitrobenzoxadiazole | NDB |
| Nitrobenzoxadiazole | NBD | N-methylolacrylamide | NMA |
| N-tert-butyl acrylamide | NTBA | N-vinylcaprolactam | NVCL |
| N-vinylformamide | VFA | N-vinylpyrrolidone | VP |
| Oligo (ethylene glycol) | OEG | Oligo (ethylene glycol) methacrylates | OEGMA |
| Oligo (ethylene glycol)methyl ether methacrylate | MEO5MA | Oligo (ethylene oxide) monomethyl ether methacrylate | OEOMA |
| Oligo (L-lactide) | OLA | Paclitaxel | PTX |
| Phenylboronic acid | PBA | Phenylethynesulfonamide | PES |
| Phosphate-buffered saline | PBS | Photochromic spiropyran | SP |
| Pluronic F127 | F127 | Poloxamer 407 | P407 |
| Poly (2-(2-methoxyethoxy) ethyl meth acrylate)) | PMEO2MA | Poly (2-(2-methoxyethoxy)ethyl methacrylate) | PMEO2MA |
| Poly (2-(diethylamino)ethyl) methacrylate | PDEAEMA | Poly (2-aminoethyl methacrylamide hydrochloride) | PAEMA |
| Poly (2-Ethoxy-2-oxo-1,3,2-dioxaphospholane) | PEEP | Poly (2-isopropyl-2-oxazoline) | piPOz |
| Poly (2-methacryloyloxyethyl phosphorylcholine) | PMPC | poly (2-methoxyethyl acrylate) | PMEA |
| Poly (2-methylthioethyl glycidyl ether) | PMTEGE | Poly (3,4-ethylenedioxythiophene) | PEDOT |
| Poly (acrylamide) | PAM | Poly (acrylonitrile) | PAN |
| Poly (amino carbonate urethane) | PACU | Poly (caprolactone) | PCL |
| Poly (ether) | PE | Poly (ethyl glycidyl ether) | PEGE |
| Poly (ethylene glycol dimethacrylate) | PEGDMA | Poly (ethylene glycol) diacrylate | PEGDA |
| Poly (ethylene glycol) methacrylates | PEGMA | Poly (ethylene glycol) methyl ether acrylate | PEGMEA |
| Poly (ethylene glycole) | PEG | Poly (ethylene oxide) | PEO |
| Poly (glycidol) | PGL | Poly (glycidyl methyl ether) | PGME |
| Poly (lactide co-glycoside) | PLLA-co-GS | Poly (lactide-glycolic acid) | PLGA |
| Poly (L-alanine) | PLA | Poly (L-aspartic acid) | P(L-Asp) |
| Poly (L-lactide) | PLLA | Poly (L-lysine) | PLL |
| Poly (methacrylic acid) | PMA | Poly (methoxydiethylene glycol methacrylate) | PMEODEGM |
| Poly (methyl glycidyl ether) | PGME | Poly (N, N-di ethylacrylamide) | PDEAAM |
| Poly (N,N-dimethylacrylamide) | PDMA | Poly (N-acryloyl-2,2-dimethyl-1,3-oxazolidine | PADMO |
| Poly (N-isopropyl meth acryl amide) | PNIPMAM | Poly (N-isopropylacrylamide) | PNIPAM |
| Poly (N-n-propylacrylamide) | PNNPAM | Poly (N-vinyl caprolactam) | PVCL |
| Poly (oligo (ethylene glycol) methacrylates) | POEGMA | Poly (organo phosphazene) | POP |
| Poly (phenylboronate ester) acrylate | PPBDEMA | Poly (propylene oxide) | PPO |
| Poly (sodium 2-acrylamido-2-methylpropanesulfonate) | PAMPS | Poly (sodium styrenesulfonate) | PSSNa |
| Poly (urethane) | PU | Poly (vinyl ether) | PVE |
| Poly [(3-acrylamidopropyl)-trimethylammonium chloride] | PAMPTMA | Poly acrylamide | PAA |
| Poly ethylenimine | PEI | Poly Oligo (ethylene oxide) methyl ether methacrylate | POEOMA |
| Poly propylene glycol | PPG | Poly tetra (ethylene glycol) diacrylate | PTEGDA |
| Polyacrylic acid | PAA | Polyamidoamine | PAMAM |
| Polyaniline | PANI | Polyglycerol | PG |
| Polyvinyl alcohol | PVA | Porphyrin | Por |
| Prednisone | Pn | Prilocaine | Pl |
| Propranolol | Ppl | Propyl acrylic acid | PAA |
| Protamine | Pt | Protamine sulfate | PS |
| Pullulan | Plu | Quantum dots | QDs |
| Rhodamine B | RhB | Ricin A | RA |
| Salicylic acid | SCA | Sebacic acid | SA |
| Sodium 2-acrylamido-2-methylpropane sulfonate | AMPS | Sodium alginate | SA |
| spiropyran | SP | Styrene | ST |
| Succinic anhydride | SA | Succinylated pullulan | S-Plu |
| Temozolomide | TZ | Tetraethylene glycol dimethacrylate | TEGDMA |
| Tween 80 | T80 | Vascular endothelial growth factor | VEGF |
References
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Sarwan, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Hybrid thermo-responsive polymer systems and their biomedical applications. Front. Mater. 2020, 7, 73. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) Thermo-Responsive Microgels as Self-Regulated Drug Delivery System. Macromol. Chem. Phys. 2016, 217, 2525–2533. [Google Scholar] [CrossRef]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Release 2016, 223, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, A.S.; Xu, W.; He, X.; Jabbari, E. Gelation and degradation characteristics of in situ photo-crosslinked poly(L-lactide-co-ethylene oxide-co-fumarate) hydrogels. Polymer 2007, 48, 7113–7120. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Thambi, T.; Duong, H.T.T.; Lee, D.S. Poly(amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. pH/thermo-responsive poly(N-isopropylacrylamide-co-maleic acid) hydrogel with a sensor and an actuator for biomedical applications. Polymer 2017, 110, 177–186. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, K.; Horiguchi, S.; Tahara, Y.; Nakai, K.; Kotani, S.; Oseko, F.; Pezzotti, G.; Yamamoto, T.; Kishida, T.; et al. Nanogel tectonic porous 3D scaffold for direct reprogramming fibroblasts into osteoblasts and bone regeneration. Sci. Rep. 2018, 8, 15824. [Google Scholar] [CrossRef]
- Han, L.H.; Suri, S.; Schmidt, C.E.; Chen, S. Fabrication of three-dimensional scaffolds for heterogeneous tissue engineering. Biomed. Microdevices 2010, 12, 721–725. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27, 1–14. [Google Scholar] [CrossRef]
- Vilaça, H.; Castro, T.; Costa, F.M.G.; Melle-Franco, M.; Hilliou, L.; Hamley, I.W.; Castanheira, E.M.S.; Martins, J.A.; Ferreira, P.M.T. Self-assembled RGD dehydropeptide hydrogels for drug delivery applications. J. Mater. Chem. B 2017, 5, 8607–8617. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, N.; Kazeminava, F.; Akbari, A.; Hamishehkar, H.; Jabbari, E.; Kafil, H.S. Synthesis of polyhedral oligomeric silsesquioxane nano-crosslinked poly(ethylene glycol)-based hybrid hydrogels for drug delivery and antibacterial activity. Polym. Int. 2019, 68, 667–674. [Google Scholar] [CrossRef]
- Liao, W.C.; Lilienthal, S.; Kahn, J.S.; Riutin, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. PH-and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017, 8, 3362–3373. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.A. A biodegradable PEG-based micro-cavitary hydrogel as scaffold for cartilage tissue engineering. Eur. Polym. J. 2015, 72, 651–660. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, K.; Lee, H.J.; Chang, S.; Lee, H.; Kim, J.H.; Koh, W.G. Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold. React. Funct. Polym. 2016, 109, 15–22. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Dadashi, S.; Boddohi, S.; Soleimani, N. Preparation, characterization, and antibacterial effect of doxycycline loaded kefiran nanofibers. J. Drug Deliv. Sci. Technol. 2019, 52, 979–985. [Google Scholar] [CrossRef]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Xia, X.; Liang, Y.; Dai, S.; Alsaedi, A.; Hayat, T.; Kong, F.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.; Naki, T. Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules 2018, 23, 1241. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, D.; Jeong, H.; Heo, J.; Hong, J. Drug Loading and Release Behavior Depending on the Induced Porosity of Chitosan/Cellulose Multilayer Nanofilms. Mol. Pharm. 2017, 14, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Frey, M.W. Synthesis, characterization and electrospinning of poly (vinyl caprolactam-co-hydroxymethyl acrylamide) to create stimuli-responsive nanofibers. Polymer 2017, 108, 154–162. [Google Scholar] [CrossRef]
- Shariati, S.R.P.; Moeinzadeh, S.; Jabbari, E. Nanofiber Based Matrices for Chondrogenic Differentiation of Stem Cells. J. Nanosci. Nanotechnol. 2016, 16, 8966–8977. [Google Scholar] [CrossRef]
- Jabbari, E.; Yang, X.; Moeinzadeh, S.; He, X. Drug release kinetics, cell uptake, and tumor toxicity of hybrid VVVVVVKK peptide-assembled polylactide nanoparticles. Eur. J. Pharm. Biopharm. 2013, 84, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; Del, C.; Alonso, A.; Zhou, S. Biocompatible PEG-Chitosan@Carbon Dots Hybrid Nanogels for Two-Photon Fluorescence Imaging, Near-Infrared Light/pH Dual-Responsive Drug Carrier, and Synergistic Therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-Based Polyelectrolyte Complex Nanoparticles from Chitosan, Heparin, and Hyaluronan. Biomacromolecules 2009, 10, 1402–1409. [Google Scholar] [CrossRef]
- Hussain, M.; Xie, J.; Hou, Z.; Shezad, K.; Xu, J.; Wang, K.; Gao, Y.; Shen, L.; Zhu, J. Regulation of Drug Release by Tuning Surface Textures of Biodegradable Polymer Microparticles. ACS Appl. Mater. Interfaces 2017, 9, 14391–14400. [Google Scholar] [CrossRef]
- Bamberger, D.; Hobernik, D.; Konhäuser, M.; Bros, M.; Wich, P.R. Surface Modification of Polysaccharide-Based Nanoparticles with PEG and Dextran and the Effects on Immune Cell Binding and Stimulatory Characteristics. Mol. Pharm. 2017, 14, 4403–4416. [Google Scholar] [CrossRef]
- Soni, S.; Babbar, A.K.; Sharma, R.K.; Maitra, A. Delivery of hydrophobised 5-fluorouracil derivative to brain tissue through intravenous route using surface modified nanogels. J. Drug Target. 2006, 14, 87–95. [Google Scholar] [CrossRef]
- Murphy, E.A.; Majeti, B.K.; Mukthavaram, R.; Acevedo, L.M.; Barnes, L.A.; Cheresh, D.A. Targeted Nanogels: A Versatile Platform for Drug Delivery to Tumors. Mol. Cancer Ther. 2011, 10, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Bickerton, S.; Zhuang, J.; Thayumanavan, S. Ligand-decorated nanogels: Fast one-pot synthesis and cellular targeting. Biomacromolecules 2012, 13, 1515–1522. [Google Scholar] [CrossRef][Green Version]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef] [PubMed]
- Multi-Functional Nanogels for Tumor Targeting and Redox-Sensitive Drug and siRNA Delivery—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27886088/ (accessed on 23 May 2020).
- Li, Y.; Bui, Q.N.; Duy, L.T.M.; Yang, H.Y.; Lee, D.S. One-Step Preparation of pH-Responsive Polymeric Nanogels as Intelligent Drug Delivery Systems for Tumor Therapy. Biomacromolecules 2018, 19, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Jiao, Z.; Ma, L.; Song, P.; Wang, R.; Xiong, Y. Hydrogen bonding induced UCST phase transition of poly(ionic liquid)-based nanogels. Polymer 2016, 98, 287–293. [Google Scholar] [CrossRef]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Dual responsive magnetic composite nanogels for thermo-chemotherapy. Colloids Surf. B Biointerfaces 2017, 155, 304–313. [Google Scholar] [CrossRef]
- Miao, C.; Li, F.; Zuo, Y.; Wang, R.; Xiong, Y. Novel redox-responsive nanogels based on poly(ionic liquid)s for the triggered loading and release of cargos. RSC Adv. 2016, 6, 3013–3019. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.M. Quadruple thermo-photo-redox-responsive random copolypeptide nanogel and hydrogel. Chin. Chem. Lett. 2018, 29, 927–930. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Bile acid-conjugated chondroitin sulfate A-based nanoparticles for tumor-targeted anticancer drug delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541. [Google Scholar] [CrossRef]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Colazo, J.; Berg, D.; Mugo, S.M.; Serpe, M.J. Multiresponsive Nanogels for Targeted Anticancer Drug Delivery. Mol. Pharm. 2017, 14, 2624–2628. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Marek, S.R.; Conn, C.A.; Peppas, N.A. Cationic nanogels based on diethylaminoethyl methacrylate. Polymer 2010, 51, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Nanogels in the race for drug delivery. Nanomedicine 2010, 5, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ke, F.; Mararenko, A.; Wei, Z.; Banerjee, P.; Zhou, S. Responsive polymer-fluorescent carbon nanoparticle hybrid nanogels for optical temperature sensing, near-infrared light-responsive drug release, and tumor cell imaging. Nanoscale 2014, 6, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Son, J.Y.; Choi, J.H.; Kim, I.G.; Lee, Y.; Lee, J.Y.; Park, K.D. Material for the Treatment of Urinary Incontinence. Biomacromolecules 2014, 15, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Tsai, W.-B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef]
- Song, L.; Liang, X.; Yang, S.; Wang, N.; He, T.; Wang, Y.; Zhang, L.; Wu, Q.; Gong, C. Novel polyethyleneimine-R8-heparin nanogel for high-efficiency gene delivery in vitro and in vivo. Drug Deliv. 2018, 25, 122–131. [Google Scholar] [CrossRef]
- Śliwa, T.; Jarzębski, M.; Andrzejewska, E.; Szafran, M.; Gapiński, J. Uptake and controlled release of a dye from thermo-sensitive polymer P(NIPAM-co-Vim). React. Funct. Polym. 2017, 115, 102–108. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, K.; Luo, J.; Lee, J.; Pan, S.; Lam, K.S. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release 2010, 144, 314–323. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, C.; Fan, J.; Chen, S.; Shen, J.; Wu, W.; Zhou, S. A nanogel of on-site tunable pH-response for efficient anticancer drug delivery. Acta Biomater. 2013, 9, 4546–4557. [Google Scholar] [CrossRef]
- Bhuchar, N.; Sunasee, R.; Ishihara, K.; Thundat, T.; Narain, R. Degradable thermoresponsive nanogels for protein encapsulation and controlled release. Bioconjug. Chem. 2012, 23, 75–83. [Google Scholar] [CrossRef]
- Tan, J.P.K.; Tan, M.B.H.; Tam, M.K.C. Application of nanogel systems in the administration of local anesthetics. Local Reg. Anesth. 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Kohli, E.; Han, H.Y.; Zeman, A.D.; Vinogradov, S.V. Formulations of biodegradable Nanogel carriers with 5′-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J. Control. Release 2007, 121, 19–27. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, D.; Liu, L.; Li, X. Development of poly(hydroxyethyl methacrylate) nanogel for effective oral insulin delivery. Pharm. Dev. Technol. 2018, 23, 351–357. [Google Scholar] [CrossRef]
- De Backer, L.; Braeckmans, K.; Stuart, M.C.A.; Demeester, J.; De Smedt, S.C.; Raemdonck, K. Bio-inspired pulmonary surfactant-modified nanogels: A promising siRNA delivery system. J. Control. Release 2015, 206, 177–186. [Google Scholar] [CrossRef]
- Yukia, Y.; Nochi, T.; Kong, I.G.; Takahashi, H.; Sawada, S.I.; Akiyoshi, K.; Kiyono, H. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 2013, 29, 61–72. [Google Scholar] [CrossRef]
- Novel Inulin-Based Mucoadhesive Micelles Loaded With Corticosteroids as Potential Transcorneal Permeation Enhancers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28512019/ (accessed on 23 May 2020).
- Asghar, K.; Qasim, M.; Dharmapuri, G.; Das, D. Investigation on a smart nanocarrier with a mesoporous magnetic core and thermo-responsive shell for co-delivery of doxorubicin and curcumin: A new approach towards combination therapy of cancer. RSC Adv. 2017, 7, 28802–28818. [Google Scholar] [CrossRef]
- Luan, S.; Zhu, Y.; Wu, X.; Wang, Y.; Liang, F.; Song, S. Hyaluronic-Acid-Based pH-Sensitive Nanogels for Tumor-Targeted Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2410–2419. [Google Scholar] [CrossRef]
- Mohtashamian, S.; Boddohi, S.; Hosseinkhani, S. Preparation and optimization of self-assembled chondroitin sulfate-nisin nanogel based on quality by design concept. Int. J. Biol. Macromol. 2018, 107, 2730–2739. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Guo, H.; Li, C.; Yu, D. An injectable and glucose-sensitive nanogel for controlled insulin release. J. Mater. Chem. 2012, 22, 22788–22796. [Google Scholar] [CrossRef]
- Abandansari, H.S.; Abuali, M.; Nabid, M.R.; Niknejad, H. Enhance chemotherapy efficacy and minimize anticancer drug side effects by using reversibly pH- and redox-responsive cross-linked unimolecular micelles. Polymer 2017, 116, 16–26. [Google Scholar] [CrossRef]
- Pikabea, A.; Ramos, J.; Papachristos, N.; Stamopoulos, D.; Forcada, J. Synthesis and characterization of PDEAEMA-based magneto-nanogels: Preliminary results on the biocompatibility with cells of human peripheral blood. J. Polym. Sci. Part Polym. Chem. 2016, 54, 1479–1494. [Google Scholar] [CrossRef]
- Chen, S.; Bian, Q.; Wang, P.; Zheng, X.; Lv, L.; Dang, Z.; Wang, G. Photo, pH and redox multi-responsive nanogels for drug delivery and fluorescence cell imaging. Polym. Chem. 2017, 8, 6150–6157. [Google Scholar] [CrossRef]
- Deng, L.; Zhai, Y.; Lin, X.; Jin, F.; He, X.; Dong, A. Investigation on properties of re-dispersible cationic hydrogel nanoparticles. Eur. Polym. J. 2008, 44, 978–986. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Q.; Li, Y.; Mo, S.; Cai, S.; Liu, L. The synthesis of biodegradable graft copolymer cellulose-graft-poly(L-lactide) and the study of its controlled drug release. Colloids Surf. B Biointerfaces 2008, 66, 26–33. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shu, X.; Shen, Z.; Sun, R.-C. Self-Assembly and Paclitaxel Loading Capacity of Cellulose-graft-poly(lactide) Nanomicelles. J. Agric. Food Chem. 2012, 60, 3900–3908. [Google Scholar] [CrossRef]
- Cho, J.-K.; Park, W.; Na, K. Self-organized nanogels from pullulan- g -poly(L-lactide) synthesized by one-pot method: Physicochemical characterization and in vitro doxorubicin release. J. Appl. Polym. Sci. 2009, 113, 2209–2216. [Google Scholar] [CrossRef]
- Schauperl, M.; Podewitz, M.; Waldner, B.J.; Liedl, K.R. Enthalpic and Entropic Contributions to Hydrophobicity. J. Chem. Theory Comput. 2016, 12, 4600–4610. [Google Scholar] [CrossRef]
- Dhanya, S.; Bahadur, D.; Kundu, G.C.; Srivastava, R. Maleic acid incorporated poly-(N-isopropylacrylamide) polymer nanogels for dual-responsive delivery of doxorubicin hydrochloride. Eur. Polym. J. 2013, 49, 22–32. [Google Scholar] [CrossRef]
- Qian, K.; Ma, Y.; Wan, J.; Geng, S.; Li, H.; Fu, Q.; Peng, X.; Kan, X.; Zhou, G.; Liu, W.; et al. The studies about doxorubicin-loaded p (N-isopropyl-acrylamide-co-butyl methylacrylate) temperature-sensitive nanogel dispersions on the application in TACE therapies for rabbit VX2 liver tumor. J. Control. Release 2015, 212, 41–49. [Google Scholar] [CrossRef]
- Yu, T.; Geng, S.; Li, H.; Wan, J.; Peng, X.; Liu, W.; Zhao, Y.; Yang, X.; Xu, H. The stimuli-responsive multiphase behavior of core-shell nanogels with opposite charges and their potential application in in situ gelling system. Colloids Surf. B Biointerfaces 2015, 136, 99–104. [Google Scholar] [CrossRef]
- Quan, C.Y.; Sun, Y.X.; Cheng, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermosensitive P (NIPAAm-co-PAAc-co-HEMA) nanogels conjugated with transferrin for tumor cell targeting delivery. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Aguirre, G.; Villar-Alvarez, E.; González, A.; Ramos, J.; Taboada, P.; Forcada, J. Biocompatible stimuli-responsive nanogels for controlled antitumor drug delivery. J. Polym. Sci. Part Polym. Chem. 2016, 54, 1694–1705. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, F. Thermo-sensitive and photoluminescent hydrogels: Synthesis, characterization, and their drug-release property. Mater. Sci. Eng. C 2011, 31, 1429–1435. [Google Scholar] [CrossRef]
- Molina, M.; Wedepohl, S.; Calderón, M. Polymeric near-infrared absorbing dendritic nanogels for efficient in vivo photothermal cancer therapy. Nanoscale 2016, 8, 5852–5856. [Google Scholar] [CrossRef]
- Kubota, K.; Hamano, K.; Kuwahara, N.; Fujishige, S.; Ando, I. Characterization of Poly(N-isopropylmethacrylamide) in Water. Polym. J. 1990, 22, 1051–1057. [Google Scholar] [CrossRef]
- Djokpé, E.; Vogt, W. N-isopropylacrylamide and N-isopropylmethacrylamide: Cloud points of mixtures and copolymers. Macromol. Chem. Phys. 2001, 202, 750–757. [Google Scholar] [CrossRef]
- Cors, M.; Wrede, O.; Genix, A.C.; Anselmetti, D.; Oberdisse, J.; Hellweg, T. Core-Shell Microgel-Based Surface Coatings with Linear Thermoresponse. Langmuir 2017, 33, 6804–6811. [Google Scholar] [CrossRef]
- Peters, J.T.; Hutchinson, S.S.; Lizana, N.; Verma, I.; Peppas, N.A. Synthesis and characterization of poly(N-isopropyl methacrylamide) core/shell nanogels for controlled release of chemotherapeutics. Chem. Eng. J. 2018, 340, 58–65. [Google Scholar] [CrossRef]
- Deshpande, S.; Sharma, S.; Koul, V.; Singh, N. Core-shell nanoparticles as an efficient, sustained, and triggered drug-delivery system. ACS Omega 2017, 2, 6455–6463. [Google Scholar] [CrossRef]
- Idziak, I.; Avoce, D.; Lessard, D.; Gravel, D.; Zhu, X.X. Thermosensitivity of Aqueous Solutions of Poly( N,N -diethylacrylamide). Macromolecules 1999, 32, 1260–1263. [Google Scholar] [CrossRef]
- Colonne, M.; Chen, Y.; Wu, K.; Freiberg, S.; Giasson, S.; Zhu, X.X. Binding of streptavidin with biotinylated thermosensitive nanospheres based on poly(N,N-diethylacrylamide-co-2-hydroxyethyl methacrylate). Bioconjug. Chem. 2007, 18, 999–1003. [Google Scholar] [CrossRef]
- Kishi, R.; Matsuda, A.; Miura, T.; Matsumura, K.; Iio, K. Fast responsive poly(N, N-diethylacrylamide) hydrogels with interconnected microspheres and bi-continuous structures. Colloid Polym. Sci. 2009, 287, 505–512. [Google Scholar] [CrossRef]
- Horák, D.; Matulka, K.; Hlídková, H.; Lapčíková, M.; Beneš, M.J.; Jaroš, J.; Hampl, A.; Dvořák, P. Pentapeptide-modified poly(N,N-diethylacrylamide) hydrogel scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98 B, 54–67. [Google Scholar] [CrossRef]
- Scherzinger, C.; Lindner, P.; Keerl, M.; Richtering, W. Cononsolvency of poly(N, N-diethylacrylamide) (PDEAAM) and poly(N-isopropylacrylamide) (PNIPAM) based microgels in water/methanol mixtures: Copolymer vs core-shell microgel. Macromolecules 2010, 43, 6829–6833. [Google Scholar] [CrossRef]
- Ngadaonye, J.I.; Geever, L.M.; Killion, J.; Higginbotham, C.L. Development of novel chitosan-poly(N,N-diethylacrylamide) IPN films for potential wound dressing and biomedical applications. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Blasco, E.; Schmidt, B.V.K.J.; Barner-Kowollik, C.; Piñol, M.; Oriol, L. Dual thermo- and photo-responsive micelles based on miktoarm star polymers. Polym. Chem. 2013, 4, 4506–4514. [Google Scholar] [CrossRef]
- Delaittre, G.; Save, M.; Gaborieau, M.; Castignolles, P.; Rieger, J.; Charleux, B. Synthesis by nitroxide-mediated aqueous dispersion polymerization, characterization, and physical core-crosslinking of pH- and thermoresponsive dynamic diblock copolymer micelles. Polym. Chem. 2012, 3, 1526–1538. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Shi, X.; Qiu, G.; Lu, X. Thermosensitive DEA/DMA copolymer nanogel: Low initiator induced synthesis and structural colored colloidal array’s optical properties. Eur. Polym. J. 2017, 96, 484–493. [Google Scholar] [CrossRef]
- Grazon, C.; Rieger, J.; Sanson, N.; Charleux, B. Study of poly(N,N-diethylacrylamide) nanogel formation by aqueous dispersion polymerization of N,N-diethylacrylamide in the presence of poly(ethylene oxide)-b-poly(N,N-dimethylacrylamide) amphiphilic macromolecular RAFT agents. Soft Matter 2011, 7, 3482–3490. [Google Scholar] [CrossRef]
- Lu, X.; Sun, M.; Barron, A.E. Non-ionic, thermo-responsive DEA/DMA nanogels: Synthesis, characterization, and use for DNA separations by microchip electrophoresis. J. Colloid Interface Sci. 2011, 357, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Grazon, C.; Charleux, B.; Alaimo, D.; Jérôme, C. Pegylated thermally responsive block copolymer micelles and nanogels via in situ RAFT aqueous dispersion polymerization. J. Polym. Sci. Part Polym. Chem. 2009, 47, 2373–2390. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, H.; Tang, D.; Lv, H.; Ren, Y.; Gu, S. Fabrication of PVCL-co-PMMA nanofibers with tunable volume phase transition temperatures and maintainable shape for anti-cancer drug release. RSC Adv. 2015, 5, 64944–64950. [Google Scholar] [CrossRef]
- Roh, Y.H.; Moon, J.Y.; Hong, E.J.; Kim, H.U.; Shim, M.S.; Bong, K.W. Microfluidic fabrication of biocompatible poly(N-vinylcaprolactam)-based microcarriers for modulated thermo-responsive drug release. Colloids Surf. B Biointerfaces 2018, 172, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.; Crawford, K.; Baruti, O.; Abdulahad, A.; Webster, M.; Puetzer, J.; Ryu, C.; Bonassar, L.J.; Mendenhall, J. The effect of hypoxia on thermosensitive poly(N-vinylcaprolactam) hydrogels with tunable mechanical integrity for cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1863–1873. [Google Scholar] [CrossRef]
- Kutsevol, N.; Glamazda, A.; Chumachenko, V.; Harahuts, Yu.; Stepanian, S.G.; Plokhotnichenko, A.M.; Karachevtsev, V.A. Behavior of hybrid thermosensitive nanosystem dextran-graft-PNIPAM/gold nanoparticles: Characterization within LCTS. J. Nanoparticle Res. 2018, 20, 236. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, W.; Teng, L.; Jin, M.; Lu, B.; Ren, L.; Wang, Y. Graphene Oxide Hybrid Supramolecular Hydrogels with Self-Healable, Bioadhesive and Stimuli-Responsive Properties and Drug Delivery Application. Macromol. Mater. Eng. 2018, 303, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef]
- Callejas-Fernández, J.; Ramos, J.; Forcada, J.; Moncho-Jordá, A. On the scattered light by dilute aqueous dispersions of nanogel particles. J. Colloid Interface Sci. 2015, 450, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kehren, D.; Molano Lopez, A.C.; Pich, A. Nanogel-modified polycaprolactone microfibres with controlled water uptake and degradability. Polymer 2014, 55, 2153–2162. [Google Scholar] [CrossRef]
- Madhusudana Rao, K.; Mallikarjuna, B.; Krishna Rao, K.S.V.; Siraj, S.; Chowdoji Rao, K.; Subha, M.C.S. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surf. B Biointerfaces 2013, 102, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Huang, X.; Yin, G.; Bu, M.; Pu, X.; Chen, X.; Liao, X.; Huang, Z. Thermosensitive star polymer pompons with a core-arm structure as thermo-responsive controlled release drug carriers. RSC Adv. 2018, 8, 15604–15612. [Google Scholar] [CrossRef]
- Cao, P.; Li, W.; Sun, X.; Liang, Y.; Gao, X.; Li, X.; Song, Z.; Liang, G. Gene delivery by a cationic and thermosensitive nanogel promoted established tumor growth inhibition. Nanomed. 2015, 10, 1585–1597. [Google Scholar] [CrossRef]
- Shakoori, Z.; Ghanbari, H.; Omidi, Y.; Pashaiasl, M.; Akbarzadeh, A.; Jomeh Farsangi, Z.; Rezayat, S.M.; Davaran, S. Fluorescent multi-responsive cross-linked P(N-isopropylacrylamide)-based nanocomposites for cisplatin delivery. Drug Dev. Ind. Pharm. 2017, 43, 1283–1291. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Zhu, W.; Ye, Z.; Yu, Y.; Xu, Z.; Ren, J.; Li, P. Dual-Stimuli-Responsive, Polymer-Microsphere-Encapsulated CuS Nanoparticles for Magnetic Resonance Imaging Guided Synergistic Chemo-Photothermal Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1690–1701. [Google Scholar] [CrossRef]
- Poorgholy, N.; Massoumi, B.; Jaymand, M. A novel starch-based stimuli-responsive nanosystem for theranostic applications. Int. J. Biol. Macromol. 2017, 97, 654–661. [Google Scholar] [CrossRef]
- Le, P.N.; Pham, D.C.; Nguyen, D.H.; Tran, N.Q.; Dimitrov, V.; Ivanov, P.; Xuan, C.N.; Nguyen, H.N.; Nguyen, C.K. Poly (N-isopropylacrylamide)-functionalized dendrimer as a thermosensitive nanoplatform for delivering malloapelta B against HepG2 cancer cell proliferation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Rao, Z.; Li, H.; Wu, Y.; Zhao, J.; Rong, J. Controlled protein adsorption and delivery of thermosensitive poly (N-isopropylacrylamide) nanogels. J. Mater. Chem. B 2017, 5, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.; Nguyen, N.H.; Nguyen, C.K.; Tran, N.Q. Smart dendrimer-based nanogel for enhancing 5-fluorouracil loading efficiency against MCF7 cancer cell growth. Bull. Mater. Sci. 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Verma, N.K.; Purohit, M.P.; Equbal, D.; Dhiman, N.; Singh, A.; Kar, A.K.; Shankar, J.; Tehlan, S.; Patnaik, S. Targeted Smart pH and Thermoresponsive N,O-Carboxymethyl Chitosan Conjugated Nanogels for Enhanced Therapeutic Efficacy of Doxorubicin in MCF-7 Breast Cancer Cells. Bioconjug. Chem. 2016, 27, 2605–2619. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.; Huang, W.; Chang, Y.; Shen, M.; Chen, H.; Chern, C.; Chiu, H. Doxorubicin-Loaded Nanogel Assemblies with pH/Thermo-triggered Payload Release for Intracellular Drug Delivery. Macromol. Chem. Phys. 2014, 215, 1332–1341. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Ai, X.; Zhang, J.; Zhou, J. Design and Fabrication of Temperature-Sensitive Nanogels with Controlled Drug Release Properties for Enhanced Photothermal Sterilization. Chem. Eur. J. 2017, 23, 18180–18186. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z. A novel thermo-sensitive nanogel composing of poly(N-isopropylacrylamide) grafted onto alginate-modified graphene oxide for hydrophilic anticancer drug delivery. J. Iran. Chem. Soc. 2018, 15, 121–129. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Luo, W.; Qian, Q.; Li, Q.; Han, B.; Li, Y. Triple cell-responsive nanogels for delivery of drug into cancer cells. Colloids Surf. B Biointerfaces 2018, 163, 362–368. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Zhang, Y.; Xia, Y.; Yun, L.; Zhang, Q.; Zhang, T.; Chen, X.; Chen, H.; Li, W. A nanogel with passive targeting function and adjustable polyplex surface properties for efficient anti-tumor gene therapy. RSC Adv. 2016, 6, 84445–84456. [Google Scholar] [CrossRef]
- Naga Sravan Kumar Varma, V.; Shivakumar, S.; Fathima, S.J.; Radha, V.; Khanum, F. PH and thermosensitive 5-fluorouracil loaded poly (NIPAM-: Co -AAc) nanogels for cancer therapy. RSC Adv. 2016, 6, 105495–105507. [Google Scholar] [CrossRef]
- Lee, S.H.; Bui, H.T.; Vales, T.P.; Cho, S.; Kim, H.J. Multi-color fluorescence of pNIPAM-Based nanogels modulated by dual stimuli-responsive FRET processes. Dyes Pigments 2017, 145, 216–221. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; An, Y.; Li, Y.; Liu, Y.; Wang, L.; Guo, J.; Wang, J.; Zhou, J. Thermoresponsive Nanogel-Encapsulated PEDOT and HSP70 Inhibitor for Improving the Depth of the Photothermal Therapeutic Effect. Adv. Funct. Mater. 2016, 26, 4749–4759. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, P.; Chen, J.; Zhu, C.; Mao, Z.; Gao, C. Application of melatonin-loaded poly(N-isopropylacrylamide) hydrogel particles to reduce the toxicity of airborne pollutes to RAW264.7 cells. J. Colloid Interface Sci. 2017, 490, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z. Drug release study by a novel thermo sensitive nanogel based on salep modified graphene oxide. J. Polym. Res. 2017, 24. [Google Scholar] [CrossRef]
- Pikabea, A.; Ramos, J.; Forcada, J. Production of cationic nanogels with potential use in controlled drug delivery. Part. Part. Syst. Charact. 2014, 31, 101–109. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, K.Y.; Tong, X.; Liu, Y.; Hu, C.; Liu, S.; Yu, Q.; Zhao, Q.; Huang, W. Phosphorescent Polymeric Thermometers for In Vitro and In Vivo Temperature Sensing with Minimized Background Interference. Adv. Funct. Mater. 2016, 26, 4386–4396. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, X.; Wang, G. Selective Release of Hydrophobic and Hydrophilic Cargos from Multi-Stimuli-Responsive Nanogels. ACS Appl. Mater. Interfaces 2016, 8, 28888–28896. [Google Scholar] [CrossRef]
- Carmona-Moran, C.A.; Zavgorodnya, O.; Penman, A.D.; Kharlampieva, E.; Bridges, S.L.; Hergenrother, R.W.; Singh, J.A.; Wick, T.M. Development of gellan gum containing formulations for transdermal drug delivery: Component evaluation and controlled drug release using temperature responsive nanogels. Int. J. Pharm. 2016, 509, 465–476. [Google Scholar] [CrossRef]
- Aguirre, G.; Ramos, J.; Forcada, J. Synthesis of new enzymatically degradable thermo-responsive nanogels. Soft Matter 2013, 9, 261–270. [Google Scholar] [CrossRef]
- Gonzalez-Ayon, M.A.; Cortez-Lemus, N.A.; Zizumbo-Lopez, A.; Licea-Claverie, A. Nanogels of poly(N-vinylcaprolactam) core and polyethyleneglycol shell by surfactant free emulsion polymerization. Soft Mater. 2014, 12, 315–325. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Baby, T.; Chennazhi, K.P.; Jayakumar, R. Multi drug loaded thermo-responsive fibrinogen-graft-Poly (N-vinyl caprolactam) nanogels for breast cancer drug delivery. J. Biomed. Nanotechnol. 2015, 11, 392–402. [Google Scholar] [CrossRef]
- Peng, H.; Xu, W.; Pich, A. Temperature and pH dual-responsive Poly (vinyl lactam) copolymers functionalized with amine side groups: Via RAFT polymerization. Polym. Chem. 2016, 7, 5011–5022. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R.; Pich, A. Dual Responsive Poly (N-vinylcaprolactam) Based Degradable Microgels for Drug Delivery. Part. Part. Syst. Charact. 2017, 34, 1–9. [Google Scholar] [CrossRef]
- Wu, J.Z.; Bremner, D.H.; Li, H.Y.; Sun, X.Z.; Zhu, L.M. Synthesis and evaluation of temperature- and glucose-sensitive nanoparticles based on phenylboronic acid and N-vinylcaprolactam for insulin delivery. Mater. Sci. Eng. C 2016, 69, 1026–1035. [Google Scholar] [CrossRef]
- Etchenausia, L.; Deniau, E.; Brûlet, A.; Forcada, J.; Save, M. Cationic Thermoresponsive Poly (N-vinylcaprolactam) Microgels Synthesized by Emulsion Polymerization Using a Reactive Cationic Macro-RAFT Agent. Macromolecules 2018, 51, 2551–2563. [Google Scholar] [CrossRef]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, J.; Wang, C.; Li, C.; Kong, D.; Zhao, Q. Galactose-functionalized multi-responsive nanogels for hepatoma-targeted drug delivery. Nanoscale 2015, 7, 3137–3146. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Lu, L.; Liu, T.; Lv, F. Fluorescent nanogel based on four-arm PEG-PCL copolymer with porphyrin core for bioimaging. Mater. Sci. Eng. C 2016, 61, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Góis, J.R.; Serra, A.C.; Coelho, J.F.J. Synthesis and characterization of new temperature-responsive nanocarriers based on POEOMA-b-PNVCL prepared using a combination of ATRP, RAFT and CuAAC. Eur. Polym. J. 2016, 81, 224–238. [Google Scholar] [CrossRef]
- Berger, S.; Ornatsky, O.; Baranov, V.; Winnik, M.A.; Pich, A. Hybrid nanogels by encapsulation of lanthanide-doped LaF3nanoparticles as elemental tags for detection by atomic mass spectrometry. J. Mater. Chem. 2010, 20, 5141–5150. [Google Scholar] [CrossRef]
- Ye, Z.; Li, Y.; An, Z.; Wu, P. Exploration of Doubly Thermal Phase Transition Process of PDEGA-b-PDMA-b-PVCL in Water. Langmuir 2016, 32, 6691–6700. [Google Scholar] [CrossRef]
- Cui, Q.; Wu, F.; Wang, E. Thermosensitive behavior of poly(ethylene Glycol)-based block copolymer (peg-b-PADMO) controlled via self-assembled microstructure. J. Phys. Chem. B 2011, 115, 5913–5922. [Google Scholar] [CrossRef]
- Felberg, L.E.; Doshi, A.; Hura, G.L.; Sly, J.; Piunova, V.A.; Swope, W.C.; Rice, J.E.; Miller, R.; Head-Gordon, T. Structural transition of nanogel star polymers with pH by controlling PEGMA interactions with acid or base copolymers. Mol. Phys. 2016, 114, 3221–3231. [Google Scholar] [CrossRef]
- Peters, J.T.; Verghese, S.; Subramanian, D.; Peppas, N.A. Surface hydrolysis-mediated PEGylation of poly(N-isopropyl acrylamide) based nanogels. Regen. Biomater. 2017, 4, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kadlubowski, S.; Matusiak, M.; Jenczyk, J.; Olejniczak, M.N.; Kozanecki, M.; Okrasa, L. Radiation-induced synthesis of thermo-sensitive, gradient hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate. Radiat. Phys. Chem. 2014, 100, 23–31. [Google Scholar] [CrossRef]
- París, R.; Quijada-Garrido, I. Temperature- and pH-responsive behaviour of poly (2-(2-methoxyethoxy) ethyl methacrylate-co-N,N-dimethylaminoethyl methacrylate) hydrogels. Eur. Polym. J. 2010, 46, 2156–2163. [Google Scholar] [CrossRef]
- Shen, W.; Chang, Y.; Liu, G.; Wang, H.; Cao, A.; An, Z. Biocompatible, Antifouling, and Thermosensitive Core−Shell Nanogels Synthesized by RAFT Aqueous Dispersion Polymerization. Macromolecules 2011, 44, 2524–2530. [Google Scholar] [CrossRef]
- Biglione, C.; Sousa-Herves, A.; Menger, M.; Wedepohl, S.; Calderón, M.; Strumia, M.C. Facile ultrasonication approach for the efficient synthesis of ethylene glycol-based thermoresponsive nanogels. RSC Adv. 2015, 5, 15407–15413. [Google Scholar] [CrossRef]
- Zhang, C.; Maric, M. Statistical terpolymers with thermo-responsive fluorescence response in an ionic liquid: Effects of solvatophilicity on LCST phase separation and reversibility. Polym. Chem. 2014, 5, 4926–4938. [Google Scholar] [CrossRef]
- Rajan, R.; Matsumura, K. Tunable Dual-Thermoresponsive Core–Shell Nanogels Exhibiting UCST and LCST Behavior. Macromol. Rapid Commun. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Cao, H.; Guo, F.; Chen, Z.; Kong, X.Z. Preparation of Thermoresponsive Polymer Nanogels of Oligo (Ethylene Glycol) Diacrylate-Methacrylic Acid and Their Property Characterization. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly (NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, T.; Zhong, Q.; Wang, J. Thermoresponsive polymers based on oligo (ethylene glycol) methyl ether methacrylate and modified substrates with thermosensitivity. Macromol. Res. 2017, 25, 206–213. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, X.; Ruan, W.; Shang, Y.; Liu, H. A thermo-sensitive OEGMA-based polymer: Synthesis, characterization and interactions with surfactants in aqueous solutions with and without salt. Colloid Polym. Sci. 2017, 295, 327–340. [Google Scholar] [CrossRef]
- Alejo, T.; Prieto, M.; García-Juan, H.; Andreu, V.; Mendoza, G.; Sebastián, V.; Arruebo, M. A facile method for the controlled polymerization of biocompatible and thermoresponsive oligo (ethylene glycol) methyl ether methacrylate copolymers. Polym. J. 2018, 50, 203–211. [Google Scholar] [CrossRef]
- Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum covalent shell cross-linked micelles designed to deliver doxorubicin for synergistic combination cancer therapy. Int. J. Nanomed. 2017, 12, 3697–3710. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Liu, T.; Hu, X.; Zhang, G.; You, Y.; Liu, S. Biomaterials Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef]
- Wang, L.H.; Xu, X.M.; Hong, C.Y.; Wu, D.C.; Yu, Z.Q.; You, Y.Z. Biodegradable large compound vesicles with controlled size prepared via the self-assembly of branched polymers in nanodroplet templates. Chem. Commun. 2014, 50, 9676–9678. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Zhang, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Multi-responsive nanogels containing motifs of ortho ester, oligo(ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release 2011, 152, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Cheng, Y.; Theato, P.; Zhu, M. Thermo-induced double phase transition behavior of physically cross-linked hydrogels based on oligo(ethylene glycol) methacrylates. Macromol. Chem. Phys. 2015, 216, 2230–2240. [Google Scholar] [CrossRef]
- Alejo, T.; Andreu, V.; Mendoza, G.; Sebastian, V.; Arruebo, M. Controlled release of bupivacaine using hybrid thermoresponsive nanoparticles activated via photothermal heating. J. Colloid Interface Sci. 2018, 523, 234–244. [Google Scholar] [CrossRef]
- Sheng, W.; Liu, T.; Liu, S.; Wang, Q.; Li, X.; Guang, N. Temperature and pH responsive hydrogels based on polyethylene glycol analogues and poly (methacrylic acid) via click chemistry. Polym. Int. 2015, 64, 1415–1424. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(ethylene glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-Frequency, Magnetic-Field-Responsive Drug Release from Magnetic Nanoparticle/Organic Hybrid Based on Hyperthermic Effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Liu, K.; Yan, J.; Hu, G.; Zhang, A. Comblike Thermoresponsive Polymers with Sharp Transitions: Synthesis, Characterization, and Their Use as Sensitive Colorimetric Sensors. Macromolecules 2011, 44, 8614–8621. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. A multifuntional nanoplatform based on responsive fluorescent plasmonic ZnO-Au@PEG hybrid nanogels. Adv. Funct. Mater. 2011, 21, 2830–2839. [Google Scholar] [CrossRef]
- Zhao, A.; Zhou, S.; Zhou, Q.; Chen, T. Thermosensitive micelles from PEG-based ether-anhydride triblock copolymers. Pharm. Res. 2010, 27, 1627–1643. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, J. A thermosensitive and biodegradable physical gel with chemically crosslinked nanogels as the building block. Macromol. Rapid Commun. 2008, 29, 751–756. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.Q.; Wang, Y.C.; Wang, J. Template-free synthesis of biodegradable nanogels with tunable sizes as potential carriers for drug delivery. J. Mater. Chem. 2009, 19, 7856–7863. [Google Scholar] [CrossRef]
- Ko, D.Y.; Moon, H.J.; Jeong, B. Temperature-sensitive polypeptide nanogels for intracellular delivery of a biomacromolecular drug. J. Mater. Chem. B 2015, 3, 3525–3530. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Q.; An, Z. Development of thermosensitive copolymers of poly (2-methoxyethyl acrylate-co-poly (ethylene glycol) methyl ether acrylate) and their nanogels synthesized by RAFT dispersion polymerization in water. Polym. Chem. 2012, 3, 504–513. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Intracellular delivery of DNA and enzyme in active form using degradable carbohydrate-based nanogels. Mol. Pharm. 2012, 9, 3160–3170. [Google Scholar] [CrossRef]
- Ulasan, M.; Yavuz, E.; Bagriacik, E.U.; Cengeloglu, Y.; Yavuz, M.S. Biocompatible thermoresponsive PEGMA nanoparticles crosslinked with cleavable disulfide-based crosslinker for dual drug release. J. Biomed. Mater. Res. Part A 2015, 103, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Aktan, B.; Chambre, L.; Sanyal, R.; Sanyal, A. “Clickable” Nanogels via Thermally Driven Self-Assembly of Polymers: Facile Access to Targeted Imaging Platforms using Thiol-Maleimide Conjugation. Biomacromolecules 2017, 18, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Wang, Y.; Zhou, A.; Kumar, P.; Narain, R. Galactose-based thermosensitive nanogels for targeted drug delivery of iodoazomycin arabinofuranoside (IAZA) for theranostic management of hypoxic hepatocellular carcinoma. Biomacromolecules 2015, 16, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, Y.; Teng, L.; Lu, B.; Ren, L.; Wang, Y. Antimicrobial colloidal hydrogels assembled by graphene oxide and thermo-sensitive nanogels for cell encapsulation. J. Colloid Interface Sci. 2018, 513, 314–323. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Li, J.; Pan, G.; Shi, D.; Ren, J. Preparation, characterization, biotoxicity, and biodistribution of thermo-responsive magnetic complex micelles formed by Mn0.6Zn0.4Fe2O4and a PCL/PEG analogue copolymer for controlled drug delivery. J. Mater. Chem. B 2017, 5, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Oh, J.K. Intracellular delivery cellulose-based bionanogels with dual temperature/pH-response for cancer therapy. Colloids Surf. B Biointerfaces 2015, 133, 246–253. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Core-shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef]
- Liras, M.; Quijada-Garrido, I.; García, O. QDs decorated with thiol-monomer ligands as new multicrosslinkers for the synthesis of smart luminescent nanogels and hydrogels. Polym. Chem. 2017, 8, 5317–5326. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6, 5827–5831. [Google Scholar] [CrossRef]
- Fernandes Stefanello, T.; Szarpak-Jankowska, A.; Appaix, F.; Louage, B.; Hamard, L.; De Geest, B.G.; Van Der Sanden, B.; Nakamura, C.V.; Auzély-Velty, R. Thermoresponsive hyaluronic acid nanogels as hydrophobic drug carrier to macrophages. Acta Biomater. 2014, 10, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable Hydrogel for Sustained Protein Release by Salt-Induced Association of Hyaluronic Acid Nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Sakiyama, M.; Takeda, S.; Nishimura, T.; Mukai, S.A.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Self-Assembled Nanogels of Cholesterol-Bearing Hydroxypropyl Cellulose: A Thermoresponsive Building Block for Nanogel Tectonic Materials. Langmuir 2016, 32, 12283–12289. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Huang, C.; Lee, Y. Biomimetic Porous Scaffolds Made from Poly(L-lactide)-g-chondroitin Sulfate Blend with Poly(L-lactide) for Cartilage Tissue Engineering. Biomacromolecules 2006, 7, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Ouchi, T.; Ohya, Y. Biodegradable nanogels prepared by self-assembly of poly(L-lactide)-grafted dextran: Entrapment and release of proteins. Macromol. Biosci. 2008, 8, 1044–1052. [Google Scholar] [CrossRef]
- Na, K.; Lee, K.H.; Lee, D.H.; Bae, Y.H. Biodegradable thermo-sensitive nanoparticles from poly (L-lactic acid)/poly (ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur. J. Pharm. Sci. 2006, 27, 115–122. [Google Scholar] [CrossRef]
- Scaffaro, R.; Re, G.L.; Rigogliuso, S.; Ghersi, G. 3D polylactide-based scaffolds for studying human hepatocarcinoma processes in vitro. Sci. Technol. Adv. Mater. 2012, 13, 045003. [Google Scholar] [CrossRef]
- Wu, J.; Su, Z.-G.; Ma, G.-H. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int. J. Pharm. 2006, 315. [Google Scholar] [CrossRef]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Won, D.A.; Kim, M.; Tae, G. Systemic modulation of the stability of pluronic hydrogel by a small amount of graphene oxide. Colloids Surf. B Biointerfaces 2015, 128, 515–521. [Google Scholar] [CrossRef]
- Sharma, G.; Kamboj, S.; Thakur, K.; Negi, P.; Raza, K.; Katare, O.P. Delivery of Thermoresponsive-Tailored Mixed Micellar Nanogel of Lidocaine and Prilocaine with Improved Dermatokinetic Profile and Therapeutic Efficacy in Topical Anaesthesia. AAPS Pharm. Sci.Technol. 2017, 18, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, E.; Morimoto, N.; Kujawa, P.; Ozawa, Y.; Winnik, F.M.; Akiyoshi, K. Self-assembled nanogels of cholesteryl-modified polysaccharides: Effect of the polysaccharide structure on their association characteristics in the dilute and semidilute regimes. Biomacromolecules 2007, 8, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-Assembled pH-Sensitive Cholesteryl Pullulan Nanogel As a Protein Delivery Vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.I.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, W.; Na, K. Succinylated polysaccharide-based thermosensitive polyelectrostatic complex for protein drug delivery. J. Bioact. Compat. Polym. 2014, 29, 81–92. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Dou, Q.; Karim, A.A.; Loh, X.J. Modification of thermal and mechanical properties of PEG-PPG-PEG copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Wu, L.; Gou, M.L.; Qian, Z.Y.; Zhang, W.S.; Liu, J. Long-lasting infiltration anaesthesia by lidocaine-loaded biodegradable nanoparticles in hydrogel in rats. Acta Anaesthesiol. Scand. 2009, 53, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Sahu, A.; Vilos, C.; Kamaly, N.; Jo, S.M.; Lee, J.H.; Tae, G. Bioinspired Heparin Nanosponge Prepared by Photo-crosslinking for Controlled Release of Growth Factors. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Seo, B.B.; Song, S.C. Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 2013, 34, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef]
- Lučovnik, M.; Mandić, N.T.; Lozar Krivec, J.; Kolenc, U.; Jeverica, S. Prevalenca kolonizacije z bakterijo Streptococcus agalactiae pri nosečnicah v Sloveniji v obdobju 2013-2014. Zdr. Vestn. 2016, 85, 393–400. [Google Scholar] [CrossRef]
- Guo, W.; Yang, C.; Lin, H.; Qu, F. P(EO-co-LLA) functionalized Fe3O4@mSiO2nanocomposites for thermo/pH responsive drug controlled release and hyperthermia. Dalton Trans. 2014, 43, 18056–18065. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.S.; Jung, Y.S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly(L-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Hsiao, M.H.; Larsson, M.; Larsson, A.; Evenbratt, H.; Chen, Y.Y.; Chen, Y.Y.; Liu, D.M. Design and characterization of a novel amphiphilic chitosan nanocapsule-based thermo-gelling biogel with sustained in vivo release of the hydrophilic anti-epilepsy drug ethosuximide. J. Control. Release 2012, 161, 942–948. [Google Scholar] [CrossRef]
- Edlich, A.; Gerecke, C.; Giulbudagian, M.; Neumann, F.; Hedtrich, S.; Schäfer-Korting, M.; Ma, N.; Calderon, M.; Kleuser, B. Specific uptake mechanisms of well-tolerated thermoresponsive polyglycerol-based nanogels in antigen-presenting cells of the skin. Eur. J. Pharm. Biopharm. 2017, 116, 155–163. [Google Scholar] [CrossRef]
- Tong, N.A.N.; Nguyen, T.P.; Cuu Khoa, N.; Tran, N.Q. Aquated cisplatin and heparin-pluronic nanocomplexes exhibiting sustainable release of active platinum compound and NCI-H460 lung cancer cell antiproliferation. J. Biomater. Sci. Polym. Ed. 2016, 27, 709–720. [Google Scholar] [CrossRef]
- Wang, G.; Nie, Q.; Zang, C.; Zhang, B.; Zhu, Q.; Luo, G.; Wang, S. Self-Assembled Thermoresponsive Nanogels Prepared by Reverse Micelle Positive Micelle Method for Ophthalmic Delivery of Muscone, a Poorly Water-Soluble Drug. J. Pharm. Sci. 2016, 105, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.; Qiu, R.; Shi, L.; Wu, W.; Zhou, S. Responsive fluorescent Bi2O3@PVA hybrid nanogels for temperature-sensing, dual-modal imaging, and drug delivery. Biomaterials 2012, 33, 3058–3069. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Yan, H.; Kim, C. Thermoresponsive Gelatin Nanogels. ACS Macro Lett. 2014, 3, 1210–1214. [Google Scholar] [CrossRef]
- An, D.; Zhao, D.; Li, X.; Lu, X.; Qiu, G.; Shea, K.J. Synthesis of surfactant-free hydroxypropylcellulose nanogel and its dual-responsive properties. Carbohydr. Polym. 2015, 134, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; Bagheri, M. Dual-responsive semi-IPN copolymer nanogels based on poly (itaconic acid) and hydroxypropyl cellulose as a carrier for controlled drug release. J. Polym. Res. 2017, 24, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; An, Z.; Wu, P. UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-acryloylglycinamide-co-diacetone acrylamide). Macromolecules 2017, 50, 2175–2182. [Google Scholar] [CrossRef]
- Nagahama, K.; Hashizume, M.; Yamamoto, H.; Ouchi, T.; Ohya, Y. Hydrophobically modified biodegradable poly (ethylene glycol) copolymers that form temperature-responsive nanogels. Langmuir 2009, 25, 9734–9740. [Google Scholar] [CrossRef]

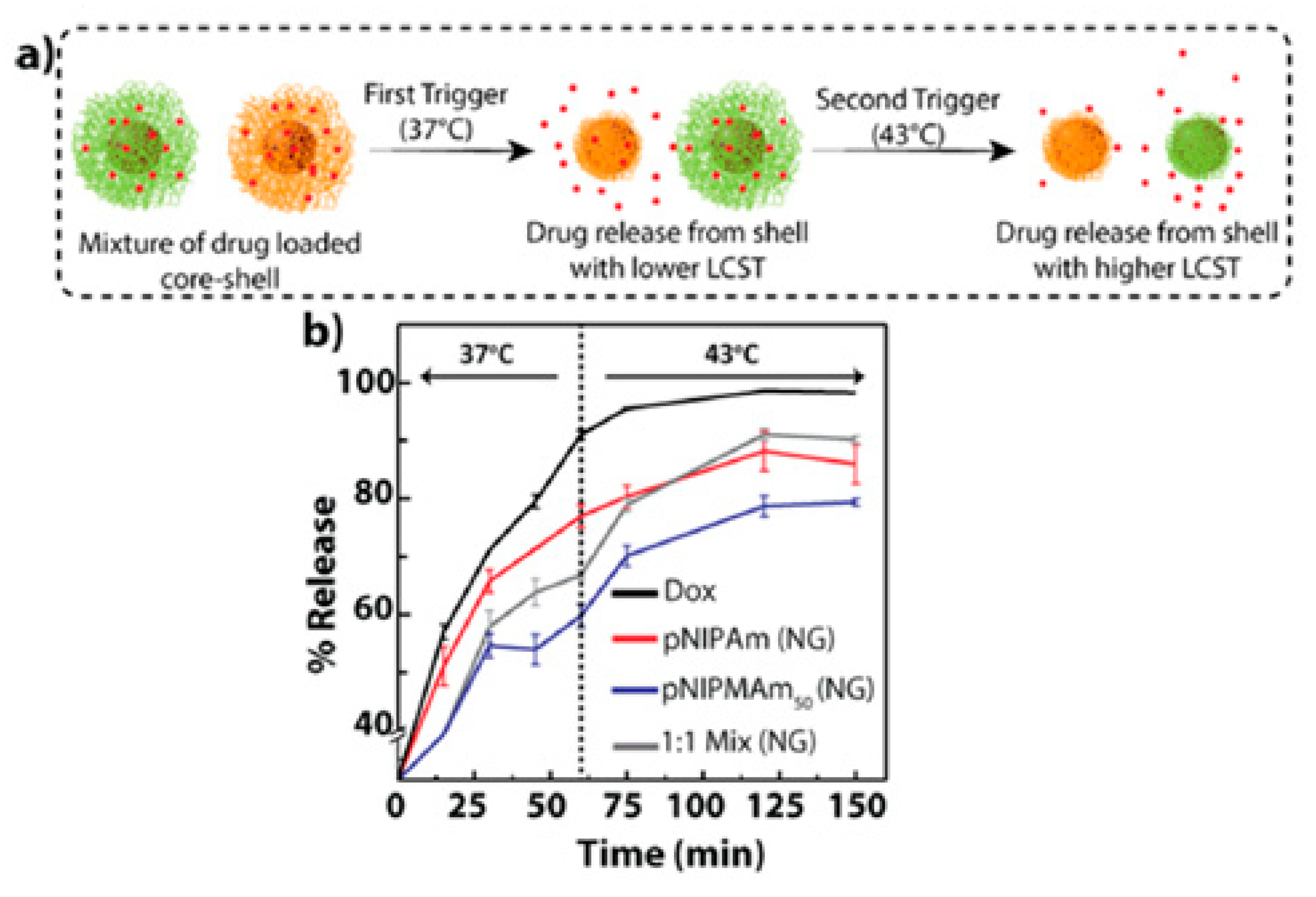

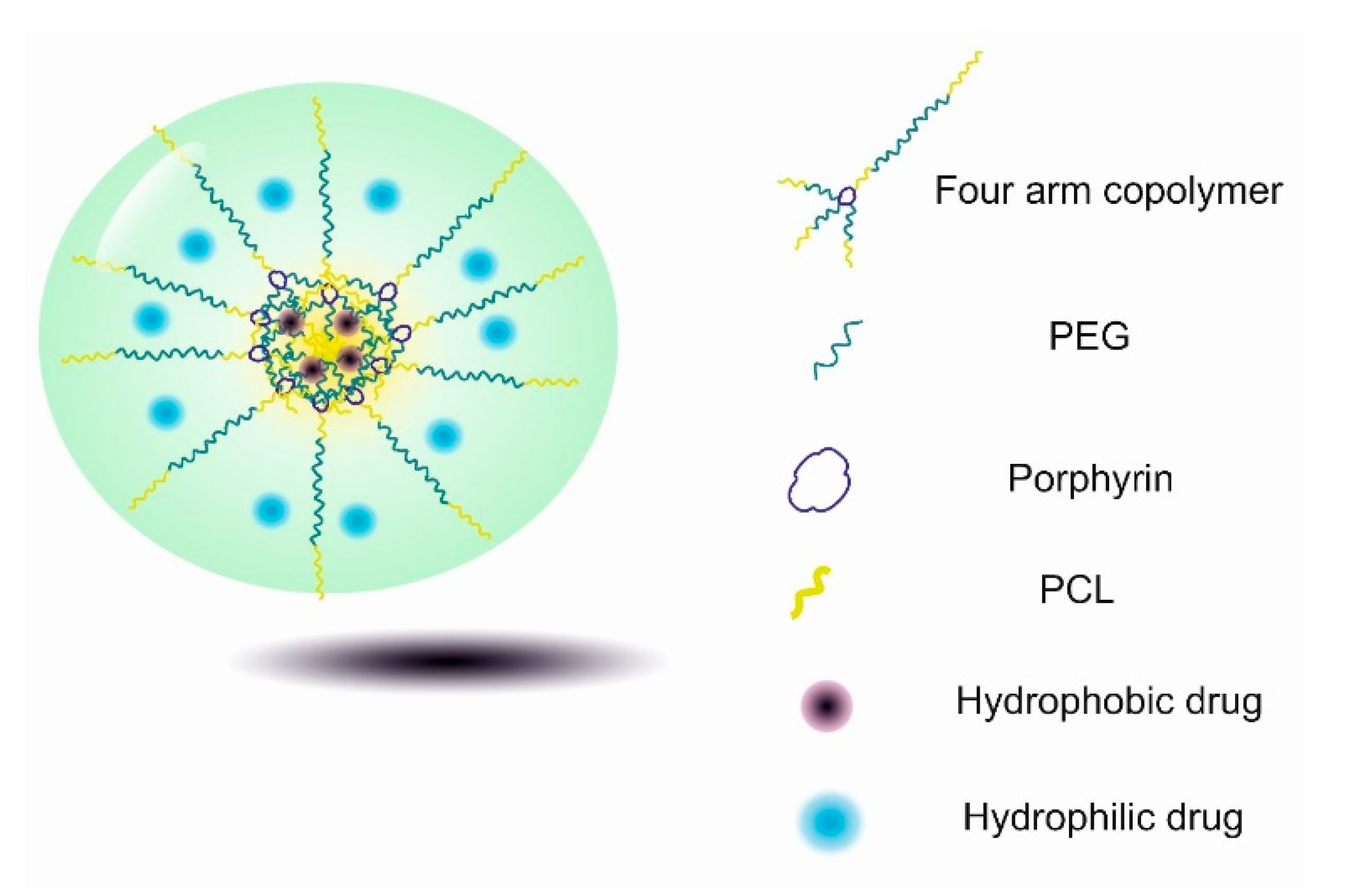


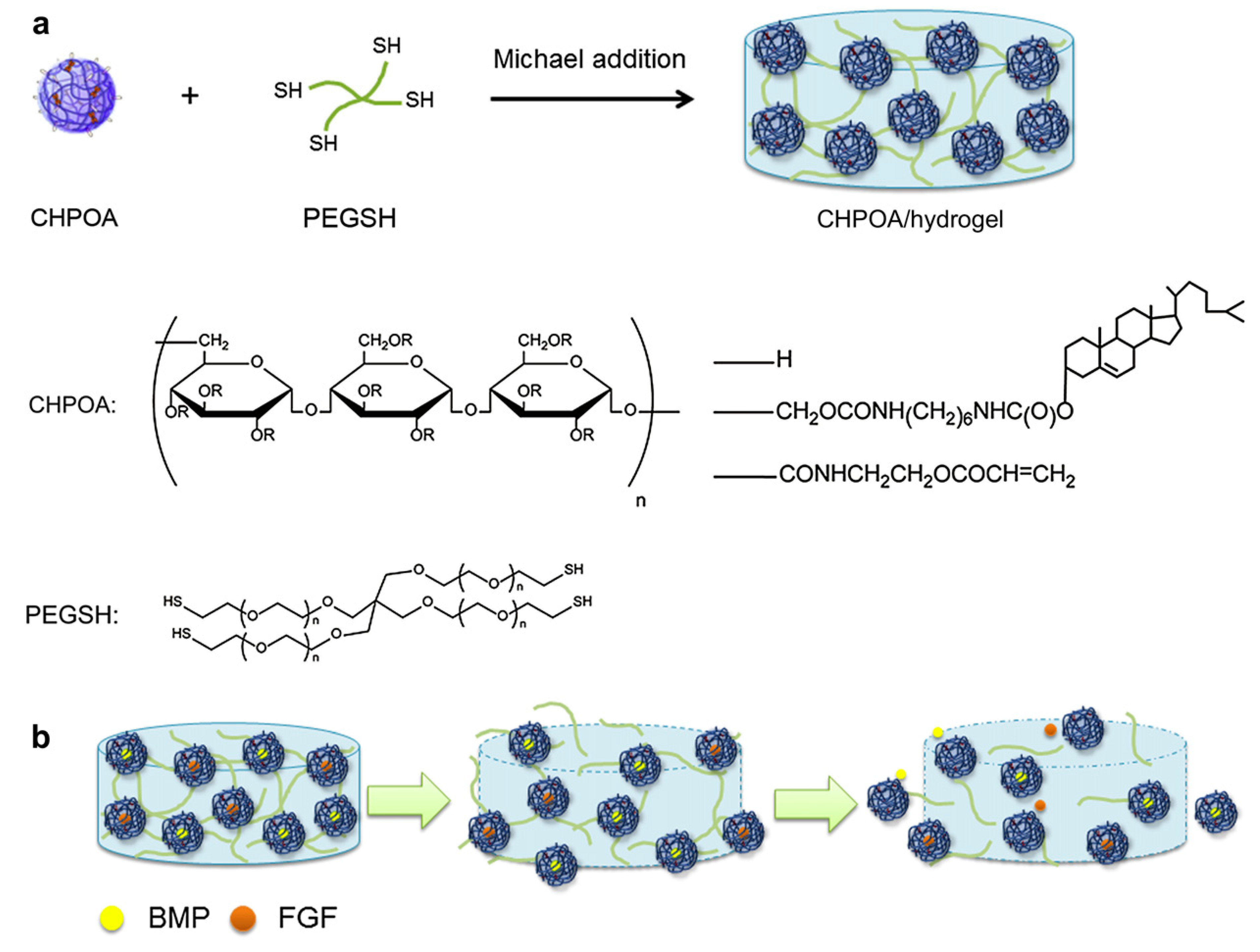
| Component * | Thermosensitive Part | Size (nm) | Therapeutics | Application/Properties | Interactions | Ref. |
|---|---|---|---|---|---|---|
| P(NIPAM-AA) | NIPAM | 125–325 | - | heart repairing | hydrophobic and electrostatic | [106] |
| PTEGDA-b- P(NIPAM-co-NMA) | NIPPAM | 300–480 | DOX | thermo-responsive | hydrophilic | [107] |
| PEI-g-PNIPAM | NIPAM | 200–350 | plasmid gene P53 | pH sensitive shell/temperature sensitive core | ionic | [108] |
| P(NIPAM-co- DMAEMA-co-AFA) | NIPAM | 100 | Cis | thermo-responsive | hydrophobic and hydrophilic | [109] |
| CS-NIPAM -MAA- | NIPAM | 235 | DOX | pH-/thermo-sensitive | electrostatic | [110] |
| starch-g -PNIPAM/ Fe3O4 | NIPAM | 67–79 | MTX | magnetic and temperature responsive | hydrophobic and hydrophilic | [111] |
| NIPAM- (PAMAM) | NIPAM | 200 | Mall B | drug delivery system against cancer cells | hydrophobic and hydrophilic | [112] |
| (PNIPAM) | NIPAM | 356 | BSA | controlled protein delivery | hydrophobic and hydrophilic | [113] |
| PAMAM G3 –PNIPAM | NIPAM | 200 | 5-Fu | enhancing 5-fluorouracil loading; cancer therapy | hydrophobic and hydrophilic | [114] |
| P(NIPPAM-AMPS)- TEGDMA | NIPAM | 199–2211 | DOX | pH-/thermo-sensitive | covalent | [115] |
| NIPAM- (dPG) -PANI | NIPAM | 155–240 | Anti-cancer drug | efficient in-vivo photothermal cancer therapy | hydrophobic and hydrophilic | [79] |
| mPEG-NIPAM- AA-MEA | NIPPAM | 52–144 | DOX | pH-/thermo-sensitive | electrostatic | [116] |
| PEDOT-NIPAM | NIPPAM | 264 | Cur | thermo-responsive | ionic | [117] |
| PNIPAM/(SA-GO) | NIPPAM | 75–375 | DOX | thermo-responsive | electrostatic | [118] |
| Alg-NIPAM | NIPPAM | 180 | DOX | redox-, pH- and thermo-sensitive | electrostatic | [119] |
| PNIPAM-g-PEI | NIPAM | 300 | Toxic protein Ricin A (RA) encoding plasmid DNA (pRA-EGFR) | thermo-responsive | hydrophobic and hydrophilic | [120] |
| (NIPAM-co-AA) | NIPAM | 70–130 | 5-Fu | pH-/thermo-sensitive | hydrophobic and hydrophilic | [121] |
| P(NIPAM-NBD-SP) | NIPAM | 90–130 | - | thermo-responsive | covalent | [122] |
| NIPAM- PEDOT-PES | NIPPAM | 195–295 | DOX | thermo-responsive | hydrophobic and hydrophilic | [123] |
| NIPAM-AA-PEGDA | NIPAM | 178–954 | Mt | thermo-responsive | hydrophobic and hydrophilic | [124] |
| Salep-GO-NIPAM | NIPAM | 93 | Df and DOX | thermo-responsive | hydrophobic and hydrophilic | [125] |
| PDEAEMA-Fe3O4 | PDEAAM | 150–320 | - | magnetic and thermo-sensitive | electrostatic | [66] |
| PDEAEMA - EGDMA | PDEAAM | 160–360 | - | pH-/thermo-sensitive | electrostatic | [126] |
| PAA- b-PDEAAM | PDEAAM | 10–110 | - | thermo-responsive | hydrophobic and hydrophilic | [92] |
| (DEA)/(DMA) | PDEAAM | 165–288 | - | thermo-responsive | hydrophobic and hydrophilic | [95] |
| (DEA)/(DMA) | PDEAAM | 280–440 | - | thermo-responsive | ionic | [93] |
| (PDEAAM) | PDEAAM | 65–185 | - | thermo-responsive | hydrophobic and hydrophilic | [127] |
| PEO-b-PDEAAM | PDEAAM | 30–150 | - | thermo-responsive | hydrophobic and hydrophilic | [94] |
| PDEAEMA | PDEAAM | 200–800 | Coumarin | thermo-responsive | covalent | [128] |
| PVCL-PAA | PVCL | 175–300 | Diclofenac | thermo-responsive | [129] | |
| PVCL-Dex-MA | PVCL | 100–400 | - | thermo-responsive | hydrophobic | [130] |
| PVCL-PEGMA | PVCL | 80–420 | - | thermo-responsive | hydrophilic | [131] |
| Fib-g-PVCL | PVCL | 150–170 | 5-Fu and Meg | thermo-responsive | ionic | [132] |
| PVCL | PVCL | 140–280 | - | nanogel with microfiber | hydrophobic and hydrophilic | [104] |
| PVCL-co-VFA and P(VP-co-VFA) | PVCL | 70–180 | - | pH-/thermo-sensitive | ionic | [133] |
| PDEAEMA/PVCL Dex-MA | PVCL | 700–500 | DOX | - | [77] | |
| PVCL-AGA | PVCL | 50–100 | 5-Fu | pH-/thermo-sensitive | hydrophobic and hydrophilic | [105] |
| PVCL-co-IA | PVCL | 140–360 | DOX | pH-/thermo-sensitive | hydrophobic and hydrophilic | [134] |
| P(VCL-co-AAPBA) | PVCL | 120–250 | Insulin | thermo-responsive | hydrophobic and hydrophilic and electrostatic | [135] |
| PVCL-PEGDA | PVCL | 50–120 | - | thermo-responsive | hydrophobic and hydrophilic | [103] |
| P(AETAC-X) - PNVCL | PVCL | 155–770 | - | thermo-responsive | ionic | [136] |
| P(ODGal- VCL-MAA) | PVCL | 100–190 | DOX | redox-, pH- and thermo-responsive | hydrophobic and hydrophilic | [137] |
| Por–PEG–PCL | PVCL | 100–250 | - | thermo-responsive | electrostatic attraction and hydrophobic interaction | [138] |
| POEOMA- b-PVCL | PVCL | 150–920 | NR as drug model | thermo-responsive | hydrophobic and hydrophilic | [139] |
| P(VCL/AAEMA /OEGMA) | PVCL | 90–135 | - | thermo-responsive | hydrophobic and hydrophilic | [140] |
| PDEGA-b- PDMA-b-PVCL | PVCL | 20–400 | - | thermo-responsive | covalent | [141] |
| Component * | Thermosensitive Part | Size (nm) | Therapeutics | Application/Properties | Interactions | Ref. |
|---|---|---|---|---|---|---|
| ZnO-Au @PEG | PEG | 15–57 | TZ | thermo-responsive | hydrophobic and hydrophilic | [166] |
| (PEG-b-PADMO) | PEG | 10–80 | - | thermo-responsive | covalent | [142] |
| P(PEG-CPP-SA) | PEG | 80–215 | DOX | thermo-responsive | hydrophobic and hydrophilic | [167] |
| PEG-PPG-PEG | PEG | 12*322 | - | thermo-responsive | hydrophobic and hydrophilic | [168] |
| PEEP-PEG-PEEP | PEG | 150–650 | DOX | thermo-responsive | hydrophobic and hydrophilic | [169] |
| PEG-PLL-PLA-HA | PEG | 160–220 | BSA | thermo-responsive | hydrophobic and hydrophilic | [170] |
| P(MEA-co-PEGMEA) | PEGMEA | 28–100 | thermo-responsive | ionic | [171] | |
| LAEMA-b-(PEGMA-co-LAEMA) | PEGMA | 34–315 | Pt, BSA, BG | thermo-responsive | hydrophobic and hydrophilic | [172] |
| PEGMA-CVP | PEGMA | 85–205 | RhB | thermo-responsive | ionic | [173] |
| PEGMA- Maleimide-dithiol | PEGMA | 10–192 | - | thermo-responsive | hydrophilic | [174] |
| Hg NPs@ P(MEO2MA -co-OEGMA) | MEO2MA, OEGMA | 65 | Bupivacaine | thermo-responsive | covalent and electrostatic | [161] |
| MEO2MA-PEGMA | MEO2MA | 40–80 | - | thermo-responsive | hydrophilic | [147] |
| DMDEA- OEGMA-BADS | OEGMA | 17–58 | Paclitaxel, DOX | thermo-responsive | hydrophobic | [159] |
| P[(LAEMA-MA)-b -(DEGMA-MBAM-LAEMA)] | DEGMA | 60–180 | IAZA | thermo-responsive | hydrophobic | [175] |
| MEO2MA – OEGMA-HEMA | MEO2MA, OEGMA | 71–180 | RhB as label | thermo-responsive | covalent | [148] |
| MEO2MA-OEGMA | MEO2MA, OEGMA | 45 | DOX | thermo-responsive | hydrophobic and hydrophilic | [176] |
| PCL-b-P(MEO2MA-co-OEGMA) Mn-Zn-Fe2O4 | MEO2MA, OEGMA | 33–129 | DOX | temperature and magnetic responsive | hydrophobic and hydrophilic | [177] |
| Clay/ P(MEO2MA -co- POEGMA) | MEO2MA, OEGMA | 200–400 | - | nanogel/hydrogel nanocomposite | hydrophobic and hydrophilic | [160] |
| MEO2MA-ChS @Carbon QDs | MEO2MA | 125–350 | DOX | pH-/thermo-sensitive | electrostatic | [26] |
| CMC-MEO2MA-OEOMA-DMA | MEO2MA | 10 | DOX | pH-/thermo-sensitive | electrostatic | [178] |
| Ag-Au @ MEO2MA-HA | MEO2MA | 10*60 | TZ | HA as targeting, bimetallic NP as imaging | hydrophobic and hydrophilic | [179] |
| QDs-SEMA- PMEO2MA | MEO2MA | 6 | - | smart luminescent | hydrophobic and hydrophilic | [180] |
| Ag/Au @PS- MEO2MA-co- MEO5MA | MEO2MA | 20–40 | Cur | thermo-responsive | hydrophobic and hydrophilic | [181] |
| P(MEO2MA-co-OEGMA-co-MAA) | OEGMA | 260–650 | DOX | temperature and magnetic sensitive | hydrophobic and hydrophilic and electrostatic | [163] |
| dPG-OEGMA-DEGMA | OEGMA | 50–200 | - | thermo-responsive | hydrophobic and hydrophilic | [182] |
| HA-P(DEGMA-co-OEGMA) | OEGMA | 150–214 | hydrophobic dye | thermo-responsive | hydrophobic and hydrophilic | [183] |
| P(MEODEGM- AEMA-MPC) | MEODEGM | 45–282 | insulin | thermo-responsive | Ionic and electrostatic | [54] |
| Component * | Thermosensitive Part | Size (nm) | Therapeutics | Application/Properties | Interactions | Ref. |
|---|---|---|---|---|---|---|
| POP-PS | POP | 250–600 | hGH | thermo-responsive | ionic and hydrophobic and hydrophilic | [205] |
| cholesterol bearing HP-Clu | Chl | 29–82 | - | thermo-responsive | hydrophobic and hydrophilic | [185] |
| PLLA-ChS Nisin | PLLA | 180–300 | nisin | target delivery for infection disease | esterification | [206] |
| Succinylated pullulan -g- PLLA | PLLA | 190–520 | lysozyme | thermo-responsive | electrostatic and hydrophobic interactions | [198] |
| S-Plu-g-OLLA | PLLA | 250–450 | amino acids | thermo-responsive | electrostatic | [207] |
| Plu-g-PLLA | PLLA | 202–341 | DOX | thermo-responsive | hydrophobic and hydrophilic | [71] |
| Fe3O4@mSiO2-PEO-PLA | PLLA | 85–150 | DOX | thermo-responsive | esterification | [208] |
| Plu-g- PLLA | PLLA | 120–160 | DOX | thermo-responsive | hydrophobic | [209] |
| F-127 and Hep | F-127 | 50–525 | bFGF, HGF VEGF, BMP-2, | thermo-responsive | ionic | [204] |
| ChS - β-GP | β-GP | 100–500 | ethosuximide | thermo-responsive | hydrophobic | [210] |
| P(GME-co-EGE) | P(GME-co-EGE) | 110–160 | - | thermo-responsive | hydrophobic | [211] |
| F-127 and T80 | F-127 | 32.5 | Lid and Pl | thermo-responsive | hydrophobic and hydrophilic | [193] |
| F-127 and Hep | F-127 | 133 | Cis | thermo-responsive | hydrophobic and hydrophilic | [212] |
| PEO-PPO- PEO | PPO | 60–360 | Mc | thermo-responsive | hydrophobic and hydrophilic | [213] |
| Bi2O3 @PVA | PVA | 80–185 | TZ | thermo-responsive | hydrophobic and hydrophilic and covalent | [214] |
| Gel A-GA | Gel A | 60–250 | - | thermo-responsive | - | [215] |
| HP-Clu and PMMA | HP-Clu | 150–240 | - | pH-/thermo-sensitive | hydrophobic and hydrophilic | [216] |
| HP-Clu- (PIA-co-PMA) | HP-Clu | 100–610 | DOX | pH-/thermo-sensitive | electrostatic | [217] |
| NAGA -DAAM | NAGA | 50–600 | - | thermo-responsive | hydrophobic and hydrophilic | [218] |
| P(L-Asp-co- PEG)- capryl | caprylic acid | 7–180 | - | thermo-responsive | hydrophobic interaction | [219] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaeini-Hesaroeiye, S.; Razmi Bagtash, H.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. https://doi.org/10.3390/gels6030020
Ghaeini-Hesaroeiye S, Razmi Bagtash H, Boddohi S, Vasheghani-Farahani E, Jabbari E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels. 2020; 6(3):20. https://doi.org/10.3390/gels6030020
Chicago/Turabian StyleGhaeini-Hesaroeiye, Sobhan, Hossein Razmi Bagtash, Soheil Boddohi, Ebrahim Vasheghani-Farahani, and Esmaiel Jabbari. 2020. "Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications" Gels 6, no. 3: 20. https://doi.org/10.3390/gels6030020
APA StyleGhaeini-Hesaroeiye, S., Razmi Bagtash, H., Boddohi, S., Vasheghani-Farahani, E., & Jabbari, E. (2020). Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels, 6(3), 20. https://doi.org/10.3390/gels6030020