Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. High Concentration of rCOL Increases Construct Thickness but Does Not Support the Formation of an EC Network
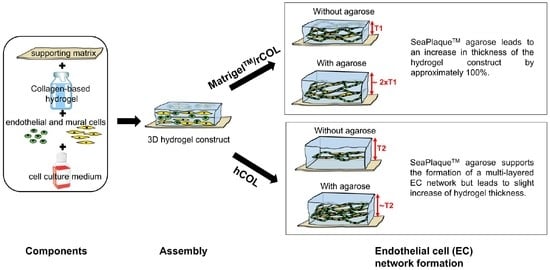
2.2. Agarose-Supplementation Supports 3D EC Network Formation in MatrigelTM/rCOL and hCOL
2.3. The Supplementation of MatrigelTM/rCOL Construct with SeaPlaqueTM Agarose Leads to Thicker Hydrogel Constructs
2.4. Supplementation of hCOL Construct with SeaPlaqueTM Agarose Leads to Thinner Cords but a Multi-Layered EC Network
2.5. hASCs/HUVECs Interaction in Agarose-Containing Constructs
3. Conclusions
4. Materials and Methods
4.1. Ethics Statement
4.2. Preparation of Small Intestinal Submucosa (SIS)
4.3. Human Umbilical Vein Endothelial Cells (HUVECs)
4.4. Isolation of Adult Human Adipose Tissue-Derived Stromal Cells (hASCs)
4.5. Preparation of Lentiviral Supernatants and Cell Transduction
4.6. Preparation of 0.4% SeaPlaqueTM Agarose
4.7. Generation of 3D Hydrogel Constructs in Serum-Free Culture
4.8. Assessment of hASCs/HUVECs Interaction Using Immunofluorescence Staining
4.9. Assessment of Constructs Thickness
4.10. 3D Reconstruction and Quantification of the EC Network
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bae, H.; Puranik, A.S.; Gauvin, R.; Edalat, F.; Carrillo-Conde, B.; Peppas, N.A.; Khademhosseini, A. Building vascular networks. Sci. Transl. Med. 2012, 4, 160ps23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, M.; Eberli, D.; Gobet, R.; Salemi, S. Tissue Engineering in Pediatric Bladder Reconstruction-The Road to Success. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram-Liebig, G.; Barbagli, G.; Heidenreich, A.; Fahlenkamp, D.; Romano, G.; Rebmann, U.; Standhaft, D.; van Ahlen, H.; Schakaki, S.; Balsmeyer, U.; et al. Results of Use of Tissue-Engineered Autologous Oral Mucosa Graft for Urethral Reconstruction: A Multicenter, Prospective, Observational Trial. Ebiomedicine 2017, 23, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget 2016, 7, 58671–58683. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.L.; Wong, J.L.; Vapniarsky, N.; Griffiths, L.G. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 2016, 92, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Song, W.; Chiu, A.; Wang, L.H.; Schwartz, R.E.; Li, B.; Bouklas, N.; Bowers, D.T.; An, D.; Cheong, S.H.; Flanders, J.A.; et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 2019, 10, 4602. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shaul, S.; Landau, S.; Merdler, U.; Levenberg, S. Mature vessel networks in engineered tissue promote graft-host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2955–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.B.; Kim, D.H.; Yoon, J.K.; Park, D.B.; Kim, H.S.; Shin, Y.M.; Baek, W.; Kang, M.L.; Kim, H.J.; Sung, H.J. Microchannel network hydrogel induced ischemic blood perfusion connection. Nat. Commun. 2020, 11, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Lin, R.Z.; Melero-Martin, J.M. Bioengineering human vascular networks: Trends and directions in endothelial and perivascular cell sources. Cell. Mol. Life Sci. 2019, 76, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Pultar, M.; Holnthoner, W. Ex vivo engineering of blood and lymphatic microvascular networks. Vasc. Biol. 2019, 1, H17–H22. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shandalov, Y.; Egozi, D.; Koffler, J.; Dado-Rosenfeld, D.; Ben-Shimol, D.; Freiman, A.; Shor, E.; Kabala, A.; Levenberg, S. An engineered muscle flap for reconstruction of large soft tissue defects. Proc. Natl. Acad. Sci. USA 2014, 111, 6010–6015. [Google Scholar] [CrossRef] [Green Version]
- Allen, P.; Kang, K.T.; Bischoff, J. Rapid onset of perfused blood vessels after implantation of ECFCs and MPCs in collagen, PuraMatrix and fibrin provisional matrices. J. Tissue Eng. Regen. Med. 2015, 9, 632–636. [Google Scholar] [CrossRef]
- Knezevic, L.; Schaupper, M.; Mühleder, S.; Schimek, K.; Hasenberg, T.; Marx, U.; Priglinger, E.; Redl, H.; Holnthoner, W. Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices. Front. Bioeng. Biotechnol. 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Freiman, A.; Shandalov, Y.; Rozenfeld, D.; Shor, E.; Segal, S.; Ben-David, D.; Meretzki, S.; Egozi, D.; Levenberg, S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 2016, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Manikowski, D.; Andree, B.; Samper, E.; Saint-Marc, C.; Olmer, R.; Vogt, P.; Strauss, S.; Haverich, A.; Hilfiker, A. Human adipose tissue-derived stromal cells in combination with exogenous stimuli facilitate three-dimensional network formation of human endothelial cells derived from various sources. Vasc. Pharmacol. 2018, 106, 28–36. [Google Scholar] [CrossRef]
- Andree, B.; Ichanti, H.; Kalies, S.; Heisterkamp, A.; Strauss, S.; Vogt, P.M.; Haverich, A.; Hilfiker, A. Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci. Rep. 2019, 9, 5437. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Principles of Regenerative Medicine, 3rd ed.; Elsevier: San Diego, CA, USA, 2018; p. 1454. [Google Scholar]
- Gros, T.; Sakamoto, J.S.; Blesch, A.; Havton, L.A.; Tuszynski, M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 2010, 31, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Melo, J.S. Preparation of a sponge-like biocomposite agarose–chitosan scaffold with primary hepatocytes for establishing an in vitro 3D liver tissue model. RSC Adv. 2015, 5, 30701–30710. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, T.A.; Jain, A.; Tanner, K.; MacKay, J.L.; Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 2010, 31, 1875–1884. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Gehrke, S.H.; Detamore, M.S. The bioactivity of agarose-PEGDA interpenetrating network hydrogels with covalently immobilized RGD peptides and physically entrapped aggrecan. Biomaterials 2014, 35, 3558–3570. [Google Scholar] [CrossRef] [Green Version]
- Kreimendahl, F.; Köpf, M.; Thiebes, A.L.; Duarte Campos, D.F.; Blaeser, A.; Schmitz-Rode, T.; Apel, C.; Jockenhoevel, S.; Fischer, H. Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type I Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng. Part C Methods 2017, 23, 604–615. [Google Scholar] [CrossRef]
- Rao, R.R.; Peterson, A.W.; Ceccarelli, J.; Putnam, A.J.; Stegemann, J.P. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, 253–264. [Google Scholar] [CrossRef]
- Rao, R.R.; Ceccarelli, J.; Vigen, M.L.; Gudur, M.; Singh, R.; Deng, C.X.; Putnam, A.J.; Stegemann, J.P. Effects of hydroxyapatite on endothelial network formation in collagen/fibrin composite hydrogels in vitro and in vivo. Acta Biomater. 2014, 10, 3091–3097. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, T.A.; Lee, T.G.; Shon, H.K.; Moon, D.W.; Kumar, S. Microscale mechanisms of agarose-induced disruption of collagen remodeling. Biomaterials 2011, 32, 5633–5642. [Google Scholar] [CrossRef] [Green Version]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Skold, M.; Umino, T.; Zhu, Y.K.; Romberger, D.J.; Spurzem, J.R.; Rennard, S.I. Endothelial cell-mediated type I collagen gel contraction is regulated by hemin. J. Lab. Clin. Med. 2000, 136, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Critser, P.J.; Whittington, C.; Kuske, J.L.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 2011, 95, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked Collagen Hydrogel Matrix Resisting Contraction To Facilitate Full-Thickness Skin Equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Tortora, G.J. Applications to Health to Accompany Principles of Human Anatomy, 8th ed.; Benjamin/Cummings Science Pub.: Menlo Park, CA, USA, 1999; p. 60. [Google Scholar]
- LaValley, D.J.; Zanotelli, M.R.; Bordeleau, F.; Wang, W.; Schwager, S.C.; Reinhart-King, C.A. Matrix Stiffness Enhances VEGFR-2 Internalization, Signaling, and Proliferation in Endothelial Cells. Converg. Sci. Phys. Oncol. 2017, 3. [Google Scholar] [CrossRef]
- Peterson, A.W.; Caldwell, D.J.; Rioja, A.Y.; Rao, R.R.; Putnam, A.J.; Stegemann, J.P. Vasculogenesis and Angiogenesis in Modular Collagen-Fibrin Microtissues. Biomater. Sci. 2014, 2, 1497–1508. [Google Scholar] [CrossRef]
- Whisler, J.A.; Chen, M.B.; Kamm, R.D. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng. Part C Methods 2014, 20, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cossío, S.; León-Mateos, A.; Sampedro, F.G.; Oreja, M.T. Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast. Reconstr. Surg. 2007, 120, 1161–1169. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Piattelli, A. Lip augmentation with a new filler (agarose gel): A 3-year follow-up study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e11–e15. [Google Scholar] [CrossRef]
- Karapantzou, C.; Jakob, M.; Kinney, B.; Vandeputte, J.; Vale, J.P.; Canis, M. The use of algeness in the face and neck: A safe, alternative filler for cosmetics and reconstruction. Ann. Transl. Med. 2020, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Lux, M.; Andree, B.; Horvath, T.; Nosko, A.; Manikowski, D.; Hilfiker-Kleiner, D.; Haverich, A.; Hilfiker, A. In vitro maturation of large-scale cardiac patches based on a perfusable starter matrix by cyclic mechanical stimulation. Acta Biomater. 2016, 30, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Dudziak, S.; Hagemann, R.; Barcikowski, S.; Fliess, M.; Israelowitz, M.; Kracht, D.; Kuhbier, J.W.; Radtke, C.; Reimers, K.; et al. Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actuator-mediated mechanical stress. PLoS ONE 2012, 7, e51264. [Google Scholar] [CrossRef] [Green Version]
- Vukadinovic-Nikolic, Z.; Andree, B.; Dorfman, S.E.; Pflaum, M.; Horvath, T.; Lux, M.; Venturini, L.; Bar, A.; Kensah, G.; Lara, A.R.; et al. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa. Tissue Eng. Part A 2014, 20, 799–809. [Google Scholar] [CrossRef] [PubMed]





| MatrigelTM/rCOL/Agarose | hCOL/Agarose | Significance | |
|---|---|---|---|
| Mean Diameter (µm) | 7.2 ± 2.2 | 7.7 ± 2.6 | ns |
| Number of nodes | 137.6 ± 20.2 | 102.0 ± 11.2 | ns |
| Total branching length (µm) | 15,300.0 ± 1248.9 | 13,366.6 ± 1222.0 | ns |
| Network area (µm2) | (3.4 ± 0.45) × 105 | (3.2 ± 0.2) × 105 | ns |
| Network Volume (µm3) | (6.7 ± 1.2) × 105 | (6.9 ± 0.9) × 105 | ns |
| Number of segments | 250.3 ± 34.2 | 182.3 ± 17.0 | * |
| MatrigelTM/rCOL | hCOL | Significance | |
|---|---|---|---|
| Mean Diameter (µm) | 9.3 ± 2.4 | 12.2 ± 3.3 | ns |
| Number of nodes | 113.0 ± 19.3 | 81.7 ± 4.0 | ns |
| Total branching length (µm) | 10,747.0 ± 938.9 | 8357.3 ± 526.6 | * |
| Network area (µm2) | (3.2 ± 0.3) × 105 | (3.2 ± 0.1) × 105 | ns |
| Network Volume (µm3) | (7.7 ± 1.0) × 105 | (10.4 ± 0.8) × 105 | * |
| Number of segments | 207.3 ± 34.6 | 146.3 ± 4.2 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichanti, H.; Sladic, S.; Kalies, S.; Haverich, A.; Andrée, B.; Hilfiker, A. Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels 2020, 6, 27. https://doi.org/10.3390/gels6030027
Ichanti H, Sladic S, Kalies S, Haverich A, Andrée B, Hilfiker A. Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels. 2020; 6(3):27. https://doi.org/10.3390/gels6030027
Chicago/Turabian StyleIchanti, Houda, Sanja Sladic, Stefan Kalies, Axel Haverich, Birgit Andrée, and Andres Hilfiker. 2020. "Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels" Gels 6, no. 3: 27. https://doi.org/10.3390/gels6030027
APA StyleIchanti, H., Sladic, S., Kalies, S., Haverich, A., Andrée, B., & Hilfiker, A. (2020). Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels, 6(3), 27. https://doi.org/10.3390/gels6030027