Microgel Particles with Distinct Morphologies and Common Chemical Compositions: A Unified Description of the Responsivity to Temperature and Osmotic Stress
Abstract
1. Introduction
2. Results and Discussion
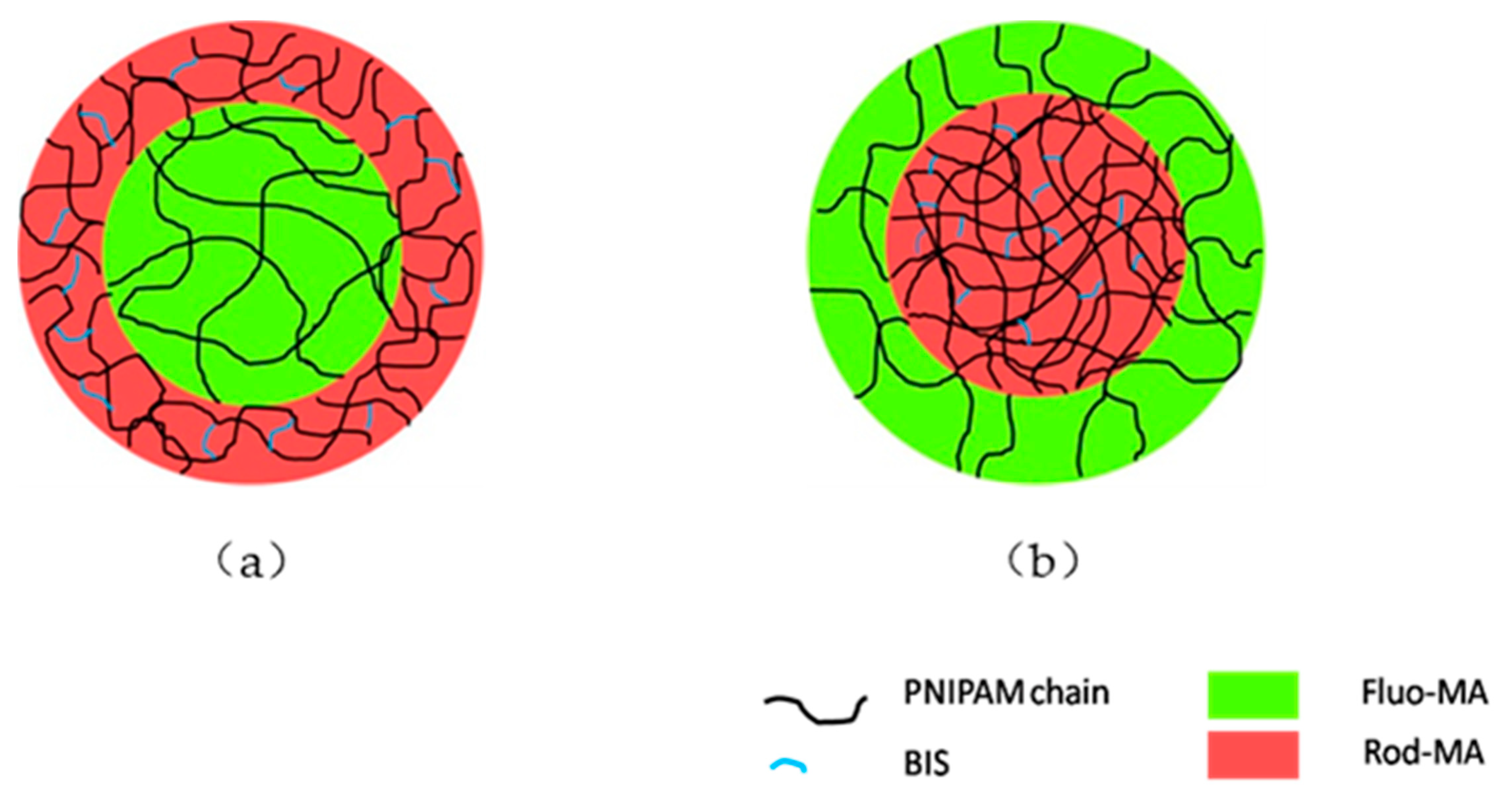
2.1. Morphological Characterization of PNIPAM Microgels
2.2. Determination of the Maximum Swelling Degree of PNIPAM Microgels
2.3. Dependence of PNIPAM Microgel Size on Temperature and Osmotic Stress
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PNIPAM Microgels with Un-Crosslinked Core and Crosslinked Shell (CS)
4.3. Preparation of PNIPAM Microgels with Crosslinked Core and Un-Crosslinked Shell (DC)
4.4. Purification of PNIPAM Microgels
4.5. Preparation of Fluorescent PNIPAM Microgels
4.6. Characterization of PNIPAM Microgels by Optical, Confocal Microscopy
4.7. FITC Labeling of PEG (FITC-PEG)
4.8. Determination of the Equilibrium Water Content of the PNIPAM Microgels: Maximum Swelling Degree
4.9. Dynamic Light Scattering (DLS)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Functional microgels: Recent advances in their biomedical applications. Small 2018, 14, 1801724. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and microgels: From model colloids to applications, recent developments, and future trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nieves, A.; Wyss, H.; Mattsson, J.; Weitz, D.A. Microgel Suspensions: Fundamentals and Applications; Wiley-VCH Verlag: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Virtanen, O.; Mourran, A.; Pinard, P.; Richtering, W. Persulfate initiated ultra-low cross-linked poly (N-isopropylacrylamide) microgels possess an unusual inverted cross-linking structure. Soft Matter 2016, 12, 3919–3928. [Google Scholar] [CrossRef]
- Mueller, E.; Alsop, R.J.; Scotti, A.; Bleuel, M.; Rheinstädter, M.C.; Richtering, W.; Hoare, T. Dynamically cross-linked self-assembled thermoresponsive microgels with homogeneous internal structures. Langmuir 2018, 34, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Gilányi, T.; Meszaros, R.; Filipcsei, G.; Zrinyi, M. Effect of cross-link density on the internal structure of poly (N-isopropylacrylamide) microgels. J. Phys. Chem. B 2001, 105, 9071–9076. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Sierra-Martín, B.; Laporte, Y.; South, A.B.; Lyon, L.A.; Fernández-Nieves, A. Bulk modulus of poly(N-isopropylacrylamide) microgels through the swelling transition. Phys. Rev. E 2011, 84, 011406. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Frederick, J.A.; Laporte, Y.; Markou, G.; Lietor-Santos, J.J.; Fernandez-Nieves, A. Determination of the bulk modulus of microgel particles. Colloid Polym. Sci. 2011, 289, 721–728. [Google Scholar] [CrossRef]
- Guo, M.; Wyss, H.M. Micromechanics of Soft Particles. Macromol. Mater. Eng. 2011, 296, 223–229. [Google Scholar] [CrossRef]
- Cerroni, B.; Pasale, S.K.; Mateescu, A.; Domenici, F.; Oddo, L.; Bordi, F.; Paradossi, G. Temperature-Tunable Nanoparticles for Selective Biointerface. Biomacromolecules 2015, 16, 1753–1760. [Google Scholar] [CrossRef]
- Pasale, S.K.; Cerroni, B.; Ghugare, S.; Paradossi, G. Multiresponsive Hyaluronan-p(NiPAAm) “Click”-Linked Hydrogels. Macromol. Biosci. 2014, 14, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 241–256. [Google Scholar] [CrossRef]
- Su, W.; Zhao, K.; Wei, J.; Ngai, T. Dielectric Relaxations of poly(N-isopropylacrylamide) Microgels Near the Volume Phase Transition Temperature: Impact of Cross-Linking Density Distribution on the Volume Phase Transition. Soft Matter 2014, 10, 8711–8723. [Google Scholar] [CrossRef]
- Gelissen, A.P.H.; Oppermann, A.; Caumanns, T.; Hebbeker, P.; Turnhoff, S.K.; Tiwari, R.; Eisold, S.; Simon, U.; Lu, Y.; Mayer, J.; et al. 3D Structures of Responsive Nanocompartmentalized Microgels. Nano Lett. 2016, 16, 7295–7301. [Google Scholar] [CrossRef]
- Siemes, E.; Nevskyi, O.; Sysoiev, D.; Turnhoff, S.K.; Oppermann, A.; Huhn, T.; Richtering, W.; Wöll, D. Nanoscopic Visualization of Cross-Linking Density in Polymer Networks with Diarylethene Photoswitches. Angew. Chem. Int. Ed. Engl. 2018, 57, 12280–12284. [Google Scholar] [CrossRef]
- Karanastasis, A.A.; Zhang, Y.; Kenath, G.S.; Lessard, M.D.; Bewersdorf, J.; Ullal, C.K. 3D mapping of nanoscale crosslink heterogeneities in microgels. Mater. Horiz. 2018, 5, 1130–1136. [Google Scholar] [CrossRef]
- Scheffold, F. Pathways and challenges towards a complete characterization of microgels. Nat. Commun. 2020, 11, 4315. [Google Scholar] [CrossRef]
- Alvarez, L.H.; Eisold, S.; Gumerov, R.S.; Strauch, M.; Rudov, A.A.; Lenssen, P.; Merhof, D.; Potemkin, I.I.; Simon, U.; Wöll, D. Deformation of Microgels at Solid–Liquid Interfaces Visualized in Three-Dimension. Nano Lett. 2019, 19, 8862–8867. [Google Scholar] [CrossRef]
- Domenici, F.; Brasili, F.; Oddo, L.; Cerroni, B.; Bedini, A.; Bordi, F.; Paradossi, G. Long-term physical evolution of an elastomeric ultrasound contrast microbubble. J. Colloid Interf. Sci. 2019, 540, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Garini, Y.; Vermolen, B.J.; Young, I.T. From micro to nano: Recent advances in high-resolution microscopy. Curr. Opin. Biotechnol. 2005, 16, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods, D.R.; McPhee, W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 1994, 272, 467–477. [Google Scholar] [CrossRef]
- Stieger, M.; Richtering, W.; Pedersen, J.S.; Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 2004, 120, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Kesenci, K.; Piskin, E. Production of poly[(ethylene glycol dimethacrylate)-co-acrylamide] based hydrogel beads by suspension copolymerization. Macromol. Chem. Phys. 1998, 199, 385–391. [Google Scholar] [CrossRef]
- Shukla, P.G.; Rajagopalan, N.; Bhaskar, C.; Sivaram, S. Cross-linked starch-urea formaldehyde (St-UF) as a hydrophilic matrix for encapsulation: Studied is swelling and release of carbofuran. J. Control. Release 1991, 15, 153–166. [Google Scholar] [CrossRef]
- Murat Elçin, Y. Encapsulation of urease enzyme in xanthan-alginate spheres. Biomaterials 1995, 16, 1157–1161. [Google Scholar] [CrossRef]
- Atkins, T.W.; McCallion, R.L.; Tighe, B.J. Incorporation and release of fluorescein isothiocyanate-linked dextrans from a bead-formed macroporous hydrophilic matrix with potential for sustained release. Biomaterials 1993, 14, 16–20. [Google Scholar] [CrossRef]
- Wang, N.; Wu, X.S. A novel approach to stabilization of protein drugs in poly(lactic-co-glycolic acid) microspheres using agarose hydrogel. Int. J. Pharm. 1998, 166, 1–14. [Google Scholar] [CrossRef]
- Stenekes, R.J.; Hennink, W.E. Equilibrium Water Content of Microspheres Based on Cross-Linked Dextran. Int. J. Pharm. 1999, 189, 131–135. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E. Proton Fluctuations and Water Diffusion in Dextran Chemical Hydrogels Studied by Incoherent Elastic and Quasielastic Neutron Scattering. Carbohydr. Res. 2005, 340, 921–927. [Google Scholar] [CrossRef]
- Yanwei Ding, Y.; Zhang, G. Collapse and Aggregation of Poly(N-isopropylacrylamide) Chains in Aqueous Solutions Crowded by Polyethylene Glycol. J. Phys. Chem. C 2007, 111, 5309–5312. [Google Scholar] [CrossRef]
- Aangenendt, F.J.; Mattsson, J.; Ellenbroek, W.G.; Wyss, H.M. Mechanics from Calorimetry: Probing the Elasticity of Responsive Hydrogels. Phys. Rev. Appl. 2017, 8, 014003. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Paradossi, G.; Chiessi, E. Tacticity-Dependent Interchain Interactions of Poly(N-Isopropylacrylamide) in Water: Toward the Molecular Dynamics Simulation of a Thermoresponsive Microgel. Gels 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Chiessi, E.; Paradossi, G. Influence of Tacticity on Hydrophobicity of Poly(N-isopropylacrylamide): A Single Chain Molecular Dynamics Simulation Study. J. Phys. Chem. B 2016, 120, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.C.; Richtering, W. Does Flory–Rehner Theory Quantitatively Describe the Swelling of Thermoresponsive Microgels? Soft Matter 2017, 13, 8271–8280. [Google Scholar] [CrossRef]
- Routh, A.F.; Fernandez-Nieves, A.; Bradley, M.; Vincent, B. Effect of Added Free Polymer on the Swelling of Neutral Microgel Particles: A Thermodynamic Approach. J. Phys. Chem. B 2006, 110, 12721–12727. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Osmotic de-swelling of polystyrene microgel particles. Colloid Polym Sci. 1997, 275, 9–17. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Thermal and osmotic deswelling of poly(NIPAM) microgel particles. J. Chem. Soc. Faraday Trans. 1996, 92, 3385–3389. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Fernández-Nieves, A.; Grillo, I.; López-Cabarcos, E. Structural modifications in the swelling of inhomogeneous microgels by light and neutron scattering. Phys. Rev. E 2002, 66, 051803. [Google Scholar] [CrossRef]
- Sbeih, S.; Mohanty, P.S.; Morrow, M.R.; Yethiraj, A. Structural parameters of soft PNIPAM microgel particles as a function of crosslink density. J. Colloid Interf. Sci. 2019, 552, 781–793. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- ImageJ Image Processing and Analysis in Java. Available online: https://imagej.net/Fiji.html#Downloads (accessed on 11 September 2020).
- Sim, S.L.; He, T.; Tscheliessnig, A.; Mueller, M.; Tan, R.B.H.; Jungbauer, A. Branched Polyethylene Glycol for Protein Precipitation. Biotechnol. Bioeng. 2012, 109, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Rubinson, K.A.; Meuse, C.W. Deep hydration: Poly(ethylene glycol) Mw 2000–8000 Da probed by vibrational spectrometry and small-angle neutron scattering and assignment of ΔG° to individual water layers. Polymer 2013, 54, 709–723. [Google Scholar] [CrossRef]









| CS_05 | CS_01 | DC_05 | DC_01 | |
|---|---|---|---|---|
| % H2O (w/w) | 98.9 ± 3.0 | 98.6 ± 3.0 | 98.9 ± 3.0 | 98.7 ± 3.0 |
| ϕ | 0.010 ± 0.002 | 0.013 ± 0.003 | 0.010 ± 0.002 | 0.012 ± 0.002 |
| Microgel | A | θ (K) | C | D | f | ϕ0 | ϕcollapsed | ϕ (298 K)Π=0 |
|---|---|---|---|---|---|---|---|---|
| CS_05 | −4.5 | 303.6 | 0.16 | 0.15 | 0.005 | 0.019 | 0.50 | 0.015 |
| CS_01 | −4.5 | 303.6 | 0.16 | 0.15 | 0.005 | 0.019 | 0.35 | 0.015 |
| DC_05 1 | −12 | 304.6 | 0.10 | 0.10 | 0.010 | 0.008 | 0.99 | 0.0075 |
| DC_05 2 | −0.88 | 303.6 | 0.313 | 0.333 | 0.003 | 0.019 | - 3 | 0.0075 |
| DC_01 | –0.88 | 303.6 | 0.313 | 0.333 | 0.003 | 0.019 | 0.80 | 0.023 |
| PEG % (w/v) | π (Atm) | Viscosity (cP) |
|---|---|---|
| 0 | 0 | 0.89 ± 0.01 |
| 0.8 | 0.05 | 1.04 ± 0.05 |
| 2 | 0.13 | 1.23 ± 0.06 |
| 2.7 | 0.17 | 1.33 ± 0.06 |
| 3.3 | 0.20 | 1.43 ± 0.07 |
| 4.3 | 0.26 | 1.63 ± 0.08 |
| 5.8 | 0.36 | 1.89 ± 0.09 * |
| 7.3 | 0.45 | 2.25 ± 0.11 |
| 9 | 0.55 | 2.70 ± 0.14 |
| 10 | 0.62 | 2.89 ± 0.14 |
| 12.3 | 0.75 | 3.49 ± 0.17 * |
| 16.4 | 1 | 5.12 ± 0.26 |
| 19.2 | 1.2 | 6.24 ± 0.31 * |
| 29.5 | 1.8 | 9.70 ± 0.49 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruscito, A.; Chiessi, E.; Toumia, Y.; Oddo, L.; Domenici, F.; Paradossi, G. Microgel Particles with Distinct Morphologies and Common Chemical Compositions: A Unified Description of the Responsivity to Temperature and Osmotic Stress. Gels 2020, 6, 34. https://doi.org/10.3390/gels6040034
Ruscito A, Chiessi E, Toumia Y, Oddo L, Domenici F, Paradossi G. Microgel Particles with Distinct Morphologies and Common Chemical Compositions: A Unified Description of the Responsivity to Temperature and Osmotic Stress. Gels. 2020; 6(4):34. https://doi.org/10.3390/gels6040034
Chicago/Turabian StyleRuscito, Andrea, Ester Chiessi, Yosra Toumia, Letizia Oddo, Fabio Domenici, and Gaio Paradossi. 2020. "Microgel Particles with Distinct Morphologies and Common Chemical Compositions: A Unified Description of the Responsivity to Temperature and Osmotic Stress" Gels 6, no. 4: 34. https://doi.org/10.3390/gels6040034
APA StyleRuscito, A., Chiessi, E., Toumia, Y., Oddo, L., Domenici, F., & Paradossi, G. (2020). Microgel Particles with Distinct Morphologies and Common Chemical Compositions: A Unified Description of the Responsivity to Temperature and Osmotic Stress. Gels, 6(4), 34. https://doi.org/10.3390/gels6040034






