Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Physical Characterization of Microemulsions
2.2. Solubility of Minoxidil
2.3. Characterization of the Gels
2.4. Stability
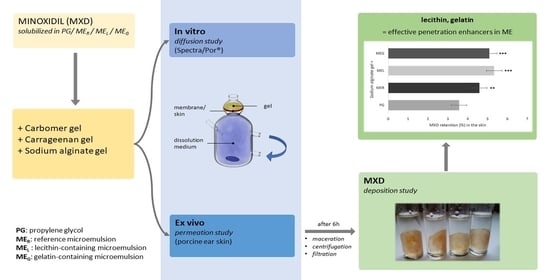
2.5. The In Vitro Diffusion Study
2.6. The Ex Vivo Permeation Study
2.7. Drug Release Kinetics
2.8. Drug Deposition in the Skin
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Microemulsions
4.3. Physical Characterization of the Microemulsions
4.4. Solubility of Minoxidil
4.5. Preparation and Characterization of the Gels
4.6. Preparation of the Drug-Loaded Gels
4.7. Stability
4.8. In Vitro Diffusion Study
4.9. Ex Vivo Permeation Study
4.10. Drug-Release Kinetics
4.11. Drug Deposition in the Skin
4.12. Data Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and Its Use in Hair Disorders: A Review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Braun-Falco, O.; Plewig, G.; Wolff, H.H. Ochorenia vlasov. In Dermatológia a Venerológia, 1st ed.; Osveta: Martin, Slovakia, 2001; p. 905. ISBN 80-8063-080-1. [Google Scholar]
- Yum, S.; Jeong, S.; Kistm, D.; Lee, S.; Kim, W.; Yoo, J.-W.; Kim, J.-A.; Kwon, O.S.; Kim, D.-D.; Min, D.S.; et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. Int. J. Mol. Sci. 2017, 19, 53. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of Hair Loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.; Tosti, A. Oral Minoxidil Treatment for Hair Loss: A Review of Efficacy and Safety. J. Am. Acad. Dermatol. 2020, 84, 737–746. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of Action on Hair Growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Goren, A.; Naccarato, T. Minoxidil in the Treatment of Androgenetic Alopecia. Dermatol. Ther. 2018, 31, e12686. [Google Scholar] [CrossRef]
- Choi, N.; Shin, S.; Song, S.; Sung, J.-H. Minoxidil Promotes Hair Growth through Stimulation of Growth Factor Release from Adipose-Derived Stem Cells. IJMS 2018, 19, 691. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M. Management of Hair Loss Diseases. Dermatol. Sin. 2010, 28, 139–145. [Google Scholar] [CrossRef]
- Tully, A.S.; Schwartzenberger, J.; Studdiford, J. Androgenic Alopecia. J. Men’s Health 2010, 7, 270–277. [Google Scholar] [CrossRef]
- Gowardhane, A.P.; Kadam, N.V.; Dutta, S. Review on Enhancement of Solubilization Process. Am. J. Drug Discov. Dev. 2014, 4, 134–152. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Satrawala, Y.; Rajput, M.S. Physical and Chemical Penetration Enhancers in Transdermal Drug Delivery System. Asian J. Pharm. (AJP) Free Full Text Artic. Asian J. Pharm. 2014, 4. [Google Scholar] [CrossRef]
- Mehta, D.P.; Rathod, H.J.; Shah, D.P.; Shah, C.N. A Review on Microemulsion Based Gel: A Recent Approach for Topical Drug Delivery System. Res. J. Pharm. Technol. 2015, 8, 118. [Google Scholar] [CrossRef]
- Sahle, F.F.; Metz, H.; Wohlrab, J.; Neubert, R.H.H. Lecithin-Based Microemulsions for Targeted Delivery of Ceramide AP into the Stratum Corneum: Formulation, Characterizations, and in Vitro Release and Penetration Studies. Pharm. Res. 2013, 30, 538–551. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of Microemulsions in Dermal and Transdermal Drug Delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef]
- Singh, V.; Veerma, R.; Singh, M.; Javed, A.; Sharma, H. Topical Non Steroidal Anti Inflammatory Drug (NSAIDs Microemulsions: Rationale, Review and Future Prospective. Asian J. Pharm. 2013, 7, 1. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Boonme, P. Lipid-Based Colloidal Systems (Nanoparticles, Microemulsions) for Drug Delivery to the Skin: Materials and End-Product Formulations. J. Drug Deliv. Sci. Technol. 2011, 21, 43–54. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dhachinamoorth, D.; Saravanan, R.; Gopal, U.K. Microemulsions as carrier for novel drug delivery: A review. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 37–45. [Google Scholar]
- Maitra, M.; Goyal, A.K.; Rath, G. A Novel Approach for Follicular Delivery of Minoxidil for Treatment of Alopecia. J. Drug Deliv. Sci. Technol. 2017, 41, 113–123. [Google Scholar] [CrossRef]
- Salim, S.; Kamalasanan, K. Controlled drug delivery for alopecia: A review. J. Control. Release 2020, 325, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Barbareschi, M.; Vescovi, V.; Starace, M.; Piraccini, B.M.; Milani, M. Propylene Glycol Free 5% Minoxidil Lotion Formulation: Cosmetic Acceptability, Local Tolerability, Clinical Efficacy and in-Vitro Skin Absorption Evaluations. G. Ital. Dermatol. Venereol. 2020, 155. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the Skin Barrier and Its Modulation by Vesicular Formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Azeem, A.; Khan, Z.I.; Aqil, M.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Microemulsions as a Surrogate Carrier for Dermal Drug Delivery. Drug Dev. Ind. Pharm. 2009, 35, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Ramli, S.; Chyi, K.T.; Zainuddin, N.; Mokhtar, W.N.A.W.; Abdul Rahman, I. The Influence of Surfactant/Co-Surfactant Hydrophilic-Lipophilic Balance on the Formation of Limonene-Based Microemulsion as Vitamin C Carrier. JSM 2019, 48, 1035–1042. [Google Scholar] [CrossRef]
- Nollet, M.; Boulghobra, H.; Calligaro, E.; Rodier, J.-D. An Efficient Method to Determine the Hydrophile-Lipophile Balance of Surfactants Using the Phase Inversion Temperature Deviation of CiEj/n-Octane/Water Emulsions. Int. J. Cosmet. Sci. 2019, 41, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation Utilizing the HLB Concept for the Development of Moisturizing Cream and Lotion: In-Vitro Characterization and Stability Evaluation. Cosmetics 2020, 7, 43. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Z.; Ying, X. Calculation of Hydrophile–Lipophile Balance for Polyethoxylated Surfactants by Group Contribution Method. J. Colloid Interface Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- Niazi, S.K. The Scope of Preformulation Studies. In Handbook of Preformulation, 1st ed.; Niazi, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 30. ISBN 9780429163388. [Google Scholar]
- Council of Europe. Minoxidil. In European Pharmacopoeia, Suppl. 8.6., 8th ed.; EDQM: Strasbourg, France, 2016; p. 2780. [Google Scholar]
- Gad, S.C. Pharmaceutical Manufacturing Handbook: Production and Processes; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 1370. ISBN 978-0-470-25958-0. [Google Scholar]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An in Vitro Model for Human Skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Nada, A. Comparative Ex Vivo and in Vitro Permeation Kinetics of Tocopherol in Liquid Formulations. Asian J. Pharm. (AJP) Free Full Text Artic. Asian J. Pharm. 2018, 12. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Špaglová, M.; Čuchorová, M.; Bartoníková, K.; Šimunková, V. Chemical penetration enhancers in topical application and their synergistic combination. Chem. Listy 2020, 114, 530–536. [Google Scholar]
- Boonme, P.; Kaewbanjong, J.; Amnuaikit, T.; Andreani, T.; M Silva, A.; B Souto, E. Microemulsion and Microemulsion-Based Gels for Topical Antifungal Therapy with Phytochemicals. CPD 2016, 22, 4257–4263. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.; Roberts, M.; Grice, J. Minoxidil Skin Delivery from Nanoemulsion Formulations Containing Eucalyptol or Oleic Acid: Enhanced Diffusivity and Follicular Targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef]
- Cardoso, S.A.; Barradas, T.N. Developing Formulations for Drug Follicular Targeting: Nanoemulsions Loaded with Minoxidil and Clove Oil. J. Drug Deliv. Sci. Technol. 2020, 59, 101908. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Handa, V.; Kathuria, H. Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery. Medicines 2018, 5, 103. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS Pharm. Sci. Tech. 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Bao, L.; Gong, L.; Guo, M.; Liu, T.; Shi, A.; Zong, H.; Xu, X.; Chen, H.; Gao, X.; Li, Y. Randomized Trial of Electrodynamic Microneedle Combined with 5% Minoxidil Topical Solution for the Treatment of Chinese Male Androgenetic Alopecia. J. Cosmet. Laser Ther. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Sharma, A.; Goren, A.; Dhurat, R.; Agrawal, S.; Sinclair, R.; Trüeb, R.M.; Vañó-Galván, S.; Chen, G.; Tan, Y.; Kovacevic, M.; et al. Tretinoin Enhances Minoxidil Response in Androgenetic Alopecia Patients by Upregulating Follicular Sulfotransferase Enzymes. Dermatol. Ther. 2019, 32. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira-Silva, M.; Guerra, C.; Costa, D.; Peixoto, D.; Pereira, I.; Pita, I.; Ribeiro, A.J.; Veiga, F. Topical Minoxidil-Loaded Nanotechnology Strategies for Alopecia. Cosmetics 2020, 7, 21. [Google Scholar] [CrossRef]
- Tricarico, D.; Maqoud, F.; Curci, A.; Camerino, G.; Zizzo, N.; Denora, N.; Cutrignelli, A.; Laquintana, V.; Lopalco, A.; la Forgia, F.; et al. Characterization of Minoxidil/Hydroxypropyl-β-Cyclodextrin Inclusion Complex in Aqueous Alginate Gel Useful for Alopecia Management: Efficacy Evaluation in Male Rat. Eur. J. Pharm. Biopharm. 2018, 122, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Sakr, F.M.; Gado, A.; Mohammed, H.; Ismail, A.A.N. Preparation and Evaluation of a Multimodal Minoxidil Microemulsion versus Minoxidil Alone in the Treatment of Androgenic Alopecia of Mixed Etiology: A Pilot Study. DDDT 2013, 413. [Google Scholar] [CrossRef]
- Fiume, Z. Final Report on the Safety Assessment of Lecithin and Hydrogenated Lecithin. Int. J. Toxicol. 2001, 20 (Suppl. 1), 21–45. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes Can Both Enhance or Reduce Drugs Penetration through the Skin. Sci. Rep. 2018, 8, 13253. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, G.M.M.E.; Williams, A.C.; Barry, B.W. Can Drug-Bearing Liposomes Penetrate Intact Skin? J. Pharm. Pharmacol. 2006, 58, 415–429. [Google Scholar] [CrossRef]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichoń, M.A.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Effects of Lecithin-Based Nanoemulsions on Skin: Short-Time Cytotoxicity MTT and BrdU Studies, Skin Penetration of Surfactants and Additives and the Delivery of Curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef]
- Talaat, S.M.; Elnaggar, Y.S.R.; Abdalla, O.Y. Lecithin Microemulsion Lipogels Versus Conventional Gels for Skin Targeting of Terconazole: In Vitro, Ex Vivo, and In Vivo Investigation. AAPS Pharm. Sci. Tech. 2019, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.-Y.; Cheng, Y.-L.; Acosta, E. Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery. Pharmaceutics 2012, 4, 104–129. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Han, F.; Yao, H.; Li, S. Gelatin-stabilised Microemulsion-based Organogels Facilitates Percutaneous Penetration of Cyclosporin a In Vitro and Dermal Pharmacokinetics In Vivo. J. Pharm. Sci. 2007, 96, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, Invasomes, and Liposomes: A Skin Penetration Study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R. Lipozómy v liekoch. In Moderné Lieky vo Farmaceutickej Technológii; Žabka, M., Ed.; Slovak Academic Press: Bratislava, Slovakia, 1999; p. 239. ISBN 80-88908-43-4. [Google Scholar]
- Tas, C.; Ozkan, Y.; Okyar, A.; Savaser, A. In Vitro and Ex Vivo Permeation Studies of Etodolac from Hydrophilic Gels and Effect of Terpenes as Enhancers. Drug Deliv. 2007, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chow, P.S.; Macbeath, C. Microemulsion Composed of Combination of Skin Beneficial Oils as Vehicle: Development of Resveratrol-Loaded Microemulsion Based Formulations for Skin Care Applications. Colloids Surf. B Biointerfaces 2020, 194, 111161. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Špaglová, M.; Žabka, M.; Čuchorová, M.; Starýchová, L.; Vitková, M. Vplyv kosolventov na liberáciu liečiva indometacínu z karbopolových gélov s obsahom mikroemulzie. Chem. Listy 2014, 108, 615–619. [Google Scholar]
- Špaglová, M.; Čuchorová, M.; Šimunková, V.; Matúšová, D.; Čierna, M.; Starýchová, L.; Bauerová, K. Possibilities of the Microemulsion Use as Indomethacin Solubilizer and Its Effect on in Vitro and Ex Vivo Drug Permeation from Dermal Gels in Comparison with Transcutol®. Drug Dev. Ind. Pharm. 2020, 46, 1468–1476. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric Nanoparticles Containing Diazepam: Preparation, Optimization, Characterization, in-Vitro Drug Release and Release Kinetic Study. Nano Converg. 2016, 3, 3. [Google Scholar] [CrossRef]






| MER (%, w/w) | MEL (%, w/w) | MEG (%, w/w) | |
|---|---|---|---|
| Polysorbate 80 | 26.50 | 26.50 | 26.50 |
| Isopropyl alcohol | 26.50 | 26.50 | 26.50 |
| Isopropyl myristate | 16.00 | 15.25 | 16.00 |
| Water | 31.00 | 31.00 | 30.25 |
| Lecithin | - | 0.75 | - |
| Gelatin | - | - | 0.75 |
| MER | MEG | MEL | |
|---|---|---|---|
| Size (nm) | 127.6 ± 2.6 | 158.3 ± 3.2 | 148.0 ± 3.0 |
| Polydispersity index | 0.279 ± 0.006 | 0.283 ± 0.006 | 0.244 ± 0.005 |
| Viscosity (mPa·s) | 22.44 ± 0.07 | 26.69 ± 0.02 | 22.15 ± 0.05 |
| Density (g·cm−3) | 0.936 ± 0.001 | 0.940 ± 0.001 | 0.939 ± 0.001 |
| pH | 6.65 ± 0.01 | 6.09 ± 0.01 | 6.21 ± 0.00 |
| Conductivity (μS·cm−1) | 28.00 ± 0.58 | 17.93 ± 0.69 | 16.83 ± 0.71 |
| Surface tension (mN·m−1) | 28.26 ± 0.02 | 27.98 ± 0.02 | 27.79 ± 0.03 |
| Solvent | Solubility (mg·mL−1) |
|---|---|
| water | 0.247 |
| Isopropyl alcohol | 0.367 |
| Polysorbate 80 | 1.098 |
| MER | 1.377 |
| MEL | 1.364 |
| MEG | 1.214 |
| Gel and Solubilizer | JSS (μg·cm−2·h−1) | CP (10−5 In Vitro; 10−7 Ex Vivo) | ER |
|---|---|---|---|
| In Vitro | |||
| CRB + PG | 0.619 | 2.58 | - |
| CRB + MER | 0.768 | 3.20 | 1.24 |
| CRB + MEL | 0.252 | 1.05 | 0.41 |
| CRB + MEG | 0.365 | 1.52 | 0.59 |
| CRG + PG | 0.877 | 3.66 | - |
| CRG + MER | 0.635 | 2.65 | 0.96 |
| CRG + MEL | 0.449 | 1.87 | 0.96 |
| CRG + MEG | 0.403 | 1.68 | 0.60 |
| ALG + PG | 1.678 | 6.99 | - |
| ALG + MER | 1.606 | 6.69 | 0.72 |
| ALG + MEL | 1.614 | 6.73 | 0.51 |
| ALG + MEG | 1.013 | 4.22 | 0.46 |
| Ex Vivo | |||
| ALG + PG | 0.012 | 5.00 | - |
| ALG + MER | 0.182 | 7.58 | 1.52 |
| ALG + MEL | 0.182 | 7.58 | 1.52 |
| ALG + MEG | 0.012 | 5.00 | 1.00 |
| Gel & Solubilizer | R2 (Zero-Order) | R2 (First-Order) | R2 (Higuchi) |
|---|---|---|---|
| In Vitro | |||
| CRB + PG | 0.9879 | 0.8932 | 0.9829 |
| CRB + MER | 0.9790 | 0.9076 | 0.9527 |
| CRB + MEL | 0.9083 | 0.7447 | 0.9841 |
| CRB + MEG | 0.9675 | 0.8965 | 0.9775 |
| In Vitro | |||
| CRG + PG | 0.9968 | 0.8930 | 0.9662 |
| CRG + MER | 0.9819 | 0.8152 | 0.9076 |
| CRG + MEL | * 0.9655 | 0.8813 | 0.9685 |
| CRG + MEG | 0.9569 | 0.8355 | * 0.9554 |
| In Vitro | |||
| ALG + PG | 0.9980 | 0.8786 | 0.9471 |
| ALG + MER | 0.9943 | 0.8721 | 0.9342 |
| ALG + MEL | 0.9800 | 0.8733 | 0.9494 |
| ALG + MEG | 0.9940 | 0.7281 | 0.9749 |
| Ex Vivo | |||
| ALG + PG | 0.9738 | 0.9862 | 0.8744 |
| ALG + MER | 0.9835 | 0.9773 | 0.9108 |
| ALG + MEL | 0.9846 | 0.8277 | 0.9754 |
| ALG + MEG | 0.9733 | 0.9877 | 0.8768 |
| CRB (%, w/w) | ALG (%, w/w) | CRG (%, w/w) | |
|---|---|---|---|
| Carbomer | 1.0 | - | - |
| Sodium alginate | - | 4.0 | - |
| Carrageenan | - | - | 4.0 |
| NaOH (10% sol., w/w) | 4.0 | - | - |
| water | 97.0 | 96.0 | 96.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špaglová, M.; Čuchorová, M.; Čierna, M.; Poništ, S.; Bauerová, K. Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels. Gels 2021, 7, 26. https://doi.org/10.3390/gels7010026
Špaglová M, Čuchorová M, Čierna M, Poništ S, Bauerová K. Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels. Gels. 2021; 7(1):26. https://doi.org/10.3390/gels7010026
Chicago/Turabian StyleŠpaglová, Miroslava, Mária Čuchorová, Martina Čierna, Silvester Poništ, and Katarína Bauerová. 2021. "Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels" Gels 7, no. 1: 26. https://doi.org/10.3390/gels7010026
APA StyleŠpaglová, M., Čuchorová, M., Čierna, M., Poništ, S., & Bauerová, K. (2021). Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels. Gels, 7(1), 26. https://doi.org/10.3390/gels7010026