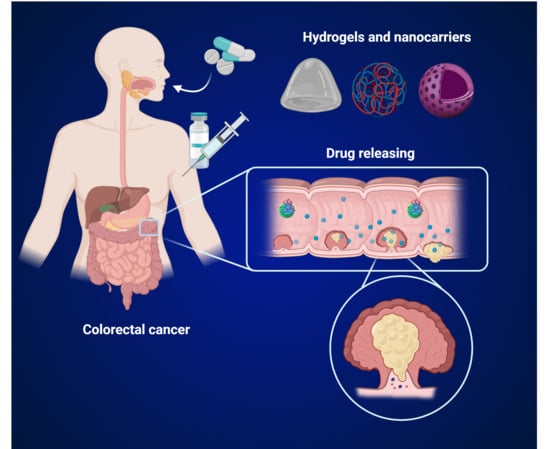
Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms
Abstract
:1. Introduction
2. New Treatments for Liver Colorectal Cancer Metastases
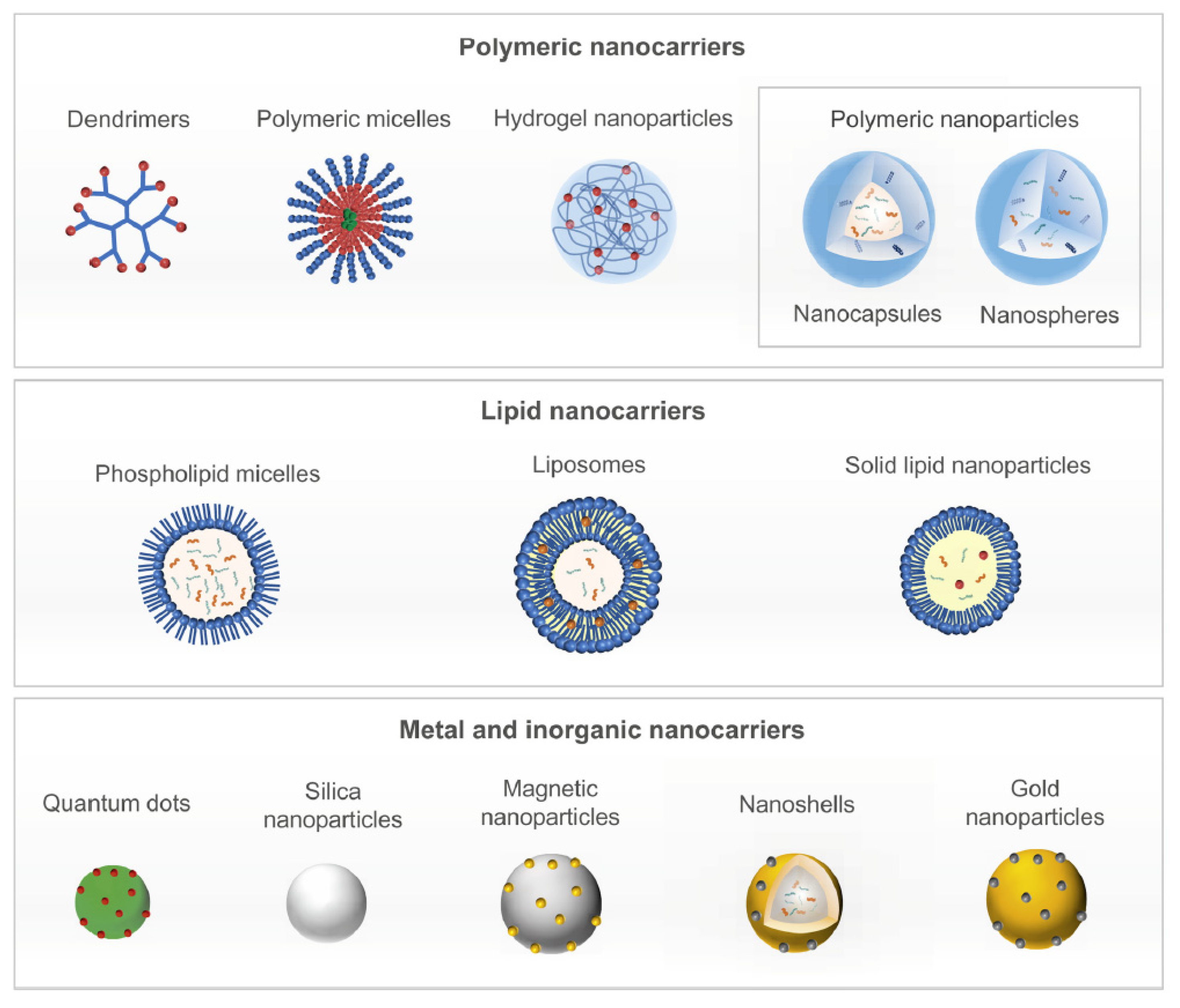
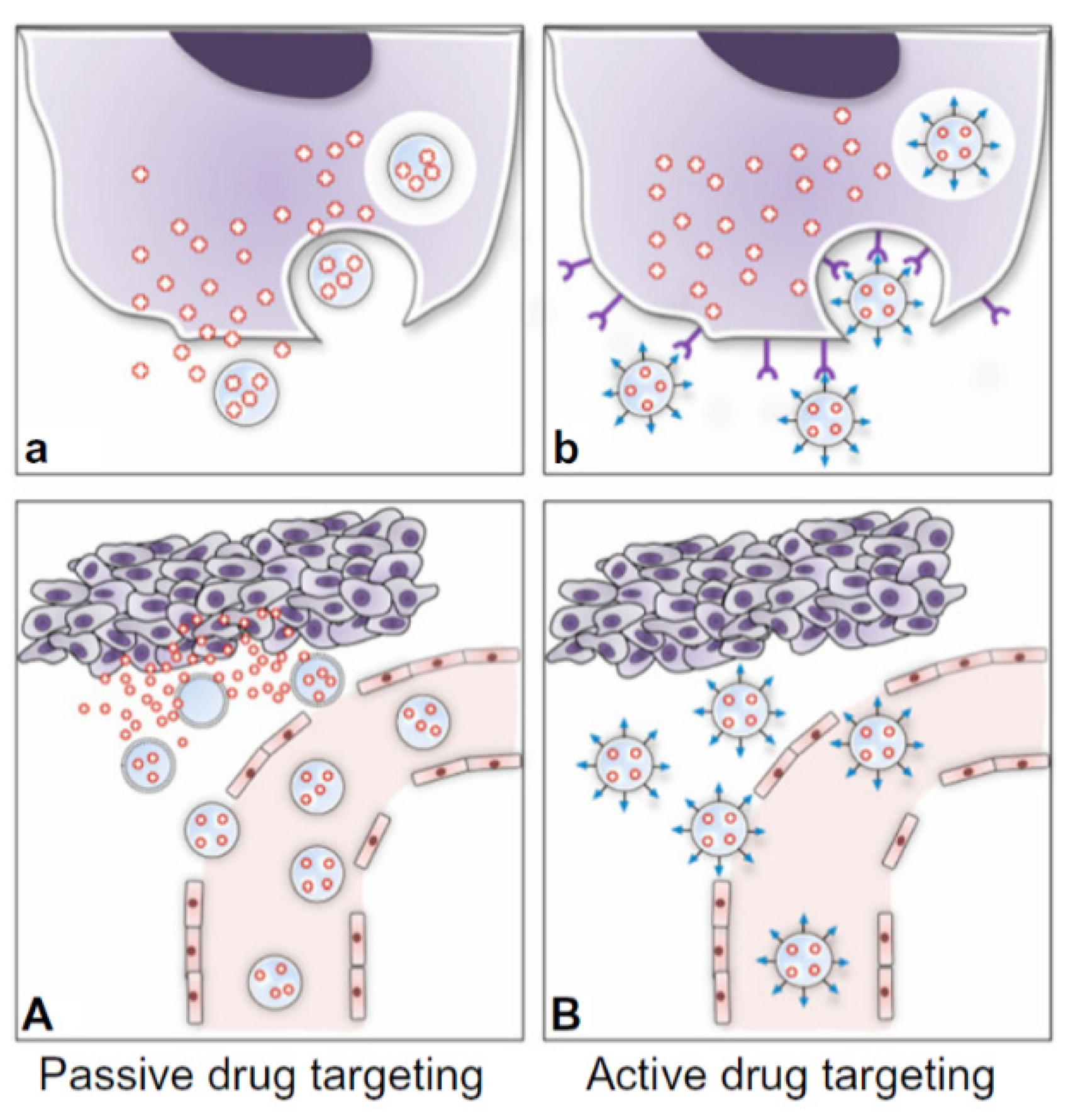
2.1. Delivery Technologies for Combination Therapies
- Other biomaterials and cell-based platforms.
2.2. Hydrogels
2.3. Nanoparticles
- Biodegradable and biocompatible;
- Capable of effective homing with most of the therapeutic agent localized within the target site;
- Optimal properties design for superior drug-loading, circulation, half-life and sustained drug release across infrequent administration times;
- Affordable, cost-effective scale-up for commercialization.
2.4. Biomaterials for the Treatment of CRC and Liver Metastases
2.4.1. PEG/PLA
2.4.2. PEG/PCL
2.4.3. Chitin
2.4.4. Chitosan
2.4.5. Alginate
2.4.6. Hyaluronic Acid
3. Challenges and New Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fauci, A.; Hauser Jameson, S.L.; Kasper, J.L.; Longo, D.L.; Loscalzo, D.L. Harrison’s Principles of Internal Medicine, 19th ed.; McGraw Hill Education Medical: New York, NY, USA, 2015. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Perkins, J.A. (Eds.) Robbins Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Jaferian, S.; Negahdari, B.; Eatemadi, A. Colon Cancer Targeting Using Conjugates Biomaterial 5-Flurouracil. Biomed. Pharmacother. 2016, 84, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Price, T. Biologic Therapies in the Metastatic Colorectal Cancer Treatment Continuum—Applying Current Evidence to Clinical Practice. Cancer Treat. Rev. 2012, 38, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Ramsay, R.G.; Narasimhan, V.; Heriot, A.G.; De Wever, O. Targeting the Tumor Microenvironment in Colorectal Peritoneal Metastases. Trends Cancer 2020, 6, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Gulbake, A. Insight to Drug Delivery Aspects for Colorectal Cancer. WJG 2016, 22, 582. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.S.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the Human Tumor Microenvironment: Colon Tumor-Derived Extracellular Matrix Promotes Angiogenesis and Tumor Cell Growth. Biomaterials 2017, 116, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Nii, T.; Makino, K.; Tabata, Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Burdett, E.; Kasper, F.K.; Mikos, A.; Ludwig, J.A. Engineering tumors: A tissue engineering perspective in cancer biology. Tissue Eng. Part B 2010, 16, 351–359. [Google Scholar] [CrossRef]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502.e15. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, F.; Cai, Y.; Chen, Y.; Wei, L.; Liu, Z.; Yuan, W. Local Antitumor Effects of Intratumoral Delivery of RlL-2 Loaded Sustained-Release Dextran/PLGA–PLA Core/Shell Microspheres. Int. J. Pharm. 2013, 450, 235–240. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable Hydrogel-Based Drug Delivery Systems for Local Cancer Therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.E.B.; Anwer, K.; Mowafy, H.A.; El-Bagory, I.M.; Alsarra, I.A. Optimization of 5-Flurouracil Solid-Lipid Nanoparticles: A Preliminary Study to Treat Colon Cancer. Int. J. Med. Sci. 2010, 7, 398–408. [Google Scholar] [PubMed] [Green Version]
- Choi, K.Y.; Jeon, E.J.; Yoon, H.Y.; Lee, B.S.; Na, J.H.; Min, K.H.; Kim, S.Y.; Myung, S.-J.; Lee, S.; Chen, X.; et al. Theranostic Nanoparticles Based on PEGylated Hyaluronic Acid for the Diagnosis, Therapy and Monitoring of Colon Cancer. Biomaterials 2012, 33, 6186–6193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, A.I.; Carreira, B.; Peres, C.; Moura, L.I.F.; Conniot, J.; Fourniols, T.; Scomparin, A.; Martínez-Barriocanal, Á.; Arango, D.; Conde, J.P.; et al. Nanotechnology Is an Important Strategy for Combinational Innovative Chemo-Immunotherapies against Colorectal Cancer. J. Control. Release 2019, 307, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Amidi, M.; Ossendorp, F.; Hennink, W.E. Polymeric Microparticles for Sustained and Local Delivery of AntiCD40 and AntiCTLA-4 in Immunotherapy of Cancer. Biomaterials 2015, 61, 33–40. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [Green Version]
- Madl, C.M.; Katz, L.M.; Heilshorn, S.C. Bio-Orthogonally Crosslinked, Engineered Protein Hydrogels with Tunable Mechanics and Biochemistry for Cell Encapsulation. Adv. Funct. Mater. 2016, 26, 3612–3620. [Google Scholar] [CrossRef] [Green Version]
- Bryant, S.J.; Vernerey, F.J. Programmable Hydrogels for Cell Encapsulation and Neo-Tissue Growth to Enable Personalized Tissue Engineering. Adv. Healthc. Mater. 2018, 7, 1700605. [Google Scholar] [CrossRef] [Green Version]
- Sechi, M.; Sanna, V.; Pala, N. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer Nanomedicine: From Targeted Delivery to Combination Therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Capretto, L.; Carugo, D.; Mazzitelli, S.; Nastruzzi, C.; Zhang, X. Microfluidic and Lab-on-a-Chip Preparation Routes for Organic Nanoparticles and Vesicular Systems for Nanomedicine Applications. Adv. Drug Deliv. Rev. 2013, 65, 1496–1532. [Google Scholar] [CrossRef]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic Platform for Combinatorial Synthesis and Optimization of Targeted Nanoparticles for Cancer Therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef] [Green Version]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Keefe, A.J.; Jiang, S. Poly(Zwitterionic)Protein Conjugates Offer Increased Stability without Sacrificing Binding Affinity or Bioactivity. Nat. Chem. 2012, 4, 59–63. [Google Scholar] [CrossRef]
- Yuan, Y.-Y.; Mao, C.-Q.; Du, X.-J.; Du, J.-Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA Nanoparticles Containing Various Anticancer Agents and Tumour Delivery by EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of Nanomaterials and Biological Systems: Implications to Personalized Nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Roy, K. Engineering Nanoparticles to Overcome Barriers to Immunotherapy: Toy and Roy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil ®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Chow, E.K.-H.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of Nanoparticles to Diagnostics and Therapeutics in Colorectal Cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted Nanoparticles for Colorectal Cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Rychahou, P.; Haque, F.; Shu, Y.; Zaytseva, Y.; Weiss, H.L.; Lee, E.Y.; Mustain, W.; Valentino, J.; Guo, P.; Evers, B.M. Delivery of RNA Nanoparticles into Colorectal Cancer Metastases Following Systemic Administration. ACS Nano 2015, 9, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable Block Copolymers as Injectable Drug-Delivery Systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Y.-L.; Qu, Y.; Liao, J.-F.; Chu, B.-Y.; Zhang, H.-P.; Luo, F.; Qian, Z.-Y. Synthesis, Characterization and Application of Reversible PDLLA-PEG-PDLLA Copolymer Thermogels in Vitro and in Vivo. Sci. Rep. 2016, 6, 19077. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.H.; Hong, G.B.; Wang, Y.; Cheng, D.; Zhou, J.X.; Shuai, X.T. Effect of PEG-PDLLA Polymeric Nanovesicles Loaded with Doxorubicin and Hematoporphyrin Monomethyl Ether on Human Hepatocellular Carcinoma HepG2 Cells in Vitro. Int. J. Nanomed. 2013, 8, 4613. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Sun, Q.; Zhou, J.; Cheng, D.; Hong, G. Folate-Functionalized Polymeric Micelles Based on Biodegradable PEG-PDLLA as a Hepatic Carcinoma-Targeting Delivery System. Asian Pac. J. Cancer Prev. 2011, 12, 1995–1999. [Google Scholar] [PubMed]
- Phan, Q.T.; Le, M.H.; Le, T.T.H.; Tran, T.H.H.; Xuan, P.N.; Ha, P.T. Characteristics and Cytotoxicity of Folate-Modified Curcumin-Loaded PLA-PEG Micellar Nano Systems with Various PLA: PEG Ratios. Int. J. Pharm. 2016, 507, 32–40. [Google Scholar] [CrossRef]
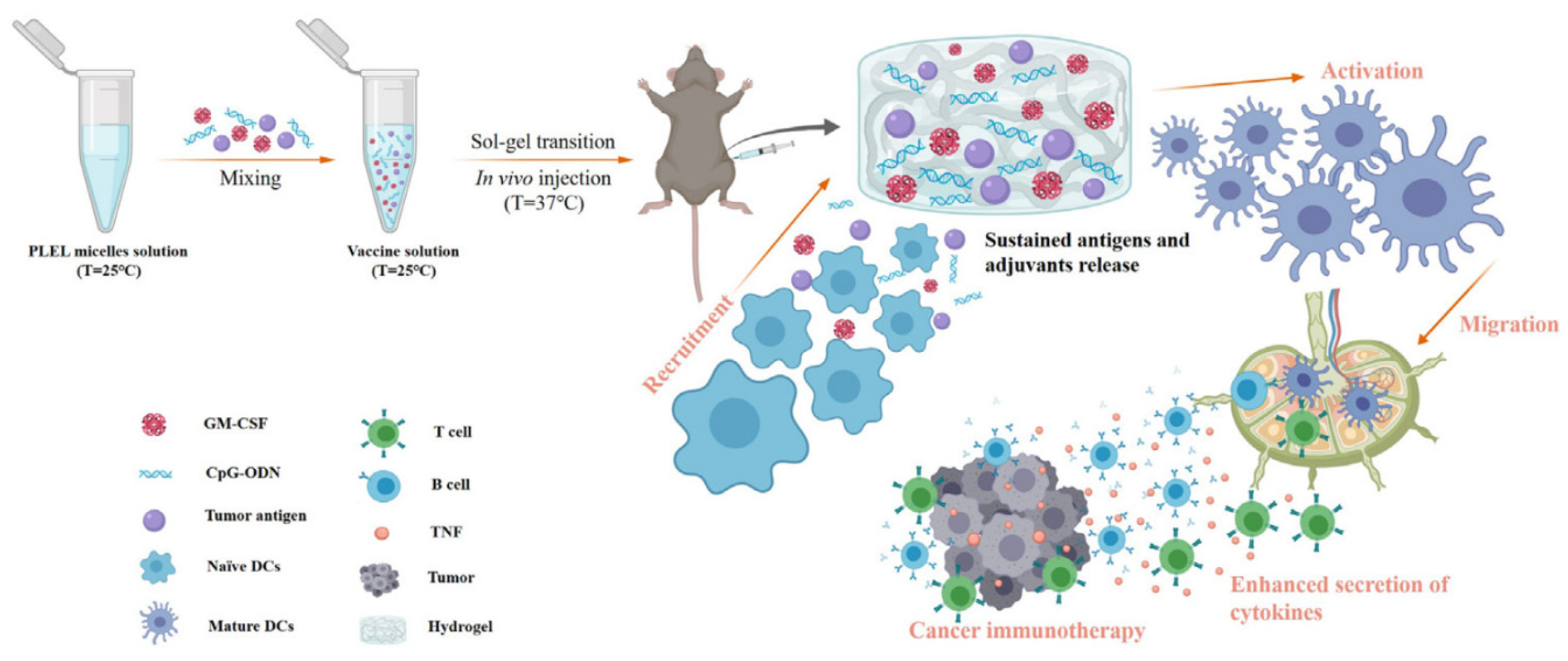
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A Biodegradable Thermosensitive Hydrogel Vaccine for Cancer Immunotherapy. Appl. Mater. Today 2020, 19, 100608. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Yang, B.; Qi, X.R.; Guo, G.; Gu, Y.C.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Biodegradable in Situ Gel-Forming Controlled Drug Delivery System Based on Thermosensitive PCL–PEG–PCL Hydrogel: Part 1—Synthesis, Characterization, and Acute Toxicity Evaluation. J. Pharm. Sci. 2009, 98, 4684–4694. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable Poly(ε-Caprolactone)-Poly(Ethylene Glycol) Block Copolymers: Characterization and Their Use as Drug Carriers for a Controlled Delivery System. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Gong, C.; Shi, S.; Dong, P.; Kan, B.; Gou, M.; Wang, X.; Li, X.; Luo, F.; Zhao, X.; Wei, Y. Synthesis and Characterization of PEG-PCL-PEG Thermosensitive Hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef]
- Gong, C.; Wang, C.; Wang, Y.; Wu, Q.; Zhang, D.; Luo, F.; Qian, Z. Efficient Inhibition of Colorectal Peritoneal Carcinomatosis by Drug Loaded Micelles in Thermosensitive Hydrogel Composites. Nanoscale 2012, 4, 3095. [Google Scholar] [CrossRef]
- Ma, G.; Miao, B.; Song, C. Thermosensitive PCL-PEG-PCL Hydrogels: Synthesis, Characterization, and Delivery of Proteins. J. Appl. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Gong, C.; Shi, S.; Wu, L.; Gou, M.; Yin, Q.; Guo, Q.; Dong, P.; Zhang, F.; Luo, F.; Zhao, X.; et al. Biodegradable in Situ Gel-Forming Controlled Drug Delivery System Based on Thermosensitive PCL–PEG–PCL Hydrogel. Part 2: Sol–Gel–Sol Transition and Drug Delivery Behavior. Acta Biomater. 2009, 5, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, C.; Yang, L.; Wu, Q.; Shi, S.; Shi, H.; Qian, Z.; Wei, Y. 5-FU-Hydrogel Inhibits Colorectal Peritoneal Carcinomatosis and Tumor Growth in Mice. BMC Cancer 2010, 10, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wei, Y.; Fang, G.; Hong, D.; An, L.; Jiao, T.; Shi, Y.; Zang, A. Colorectal cancer combination therapy using drug and gene co-delivered, targeted poly(ethylene glycol)-ε-poly(caprolactone) nanocarriers. Drug Des. Dev. Ther. 2018, 12, 3171–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, M.; Zheng, X.; Men, K.; Zhang, J.; Zheng, L.; Wang, X.; Luo, F.; Zhao, Y.; Zhao, X.; Wei, Y.; et al. Poly(ε-Caprolactone)/Poly(Ethylene Glycol)/Poly(ε-Caprolactone) Nanoparticles: Preparation, Characterization, and Application in Doxorubicin Delivery. J. Phys. Chem. B 2009, 113, 12928–12933. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xu, X.; Song, J.; Fu, S.; Gou, M.; Luo, F.; Qian, Z. Preparation of Camptothecin-Loaded PCEC Microspheres for the Treatment of Colorectal Peritoneal Carcinomatosis and Tumor Growth in Mice. Cancer Lett. 2011, 312, 189–196. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved Anti-Colorectal Carcinomatosis Effect of Tannic Acid Co-Loaded with Oxaliplatin in Nanoparticles Encapsulated in Thermosensitive Hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Rejinold N, S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Rangasamy, J. Multifunctional Chitin Nanogels for Simultaneous Drug Delivery, Bioimaging, and Biosensing. ACS Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of Chitin Hydrogel Under Mild Conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Tamura, H.; Furuike, T.; Nair, S.V.; Jayakumar, R. Biomedical Applications of Chitin Hydrogel Membranes and Scaffolds. Carbohydr. Polym. 2011, 84, 820–824. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Nair, A.; Sabitha, M.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, Characterization and in Vitro Cytocompatibility Studies of Chitin Nanogels for Biomedical Applications. Carbohydr. Polym. 2012, 87, 943–949. [Google Scholar] [CrossRef]
- Vishnu Priya, M.; Sabitha, M.; Jayakumar, R. Colloidal Chitin Nanogels: A Plethora of Applications under One Shell. Carbohydr. Polym. 2016, 136, 609–617. [Google Scholar] [CrossRef]
- Ashwinkumar, N.; Maya, S.; Jayakumar, R. Redox-Responsive Cystamine Conjugated Chitin–Hyaluronic Acid Composite Nanogels. RSC Adv. 2014, 4, 49547–49555. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nair, A.; Rejinold, N.S.; Maya, S.; Nair, S.V. Doxorubicin-Loaded PH-Responsive Chitin Nanogels for Drug Delivery to Cancer Cells. Carbohydr. Polym. 2012, 87, 2352–2356. [Google Scholar] [CrossRef]
- Arunraj, T.R.; Sanoj Rejinold, N.; Ashwin Kumar, N.; Jayakumar, R. Bio-Responsive Chitin-Poly(l-Lactic Acid) Composite Nanogels for Liver Cancer. Colloids Surf. B Biointerfaces 2014, 113, 394–402. [Google Scholar] [CrossRef]
- Smitha, K.T.; Anitha, A.; Furuike, T.; Tamura, H.; Nair, S.V.; Jayakumar, R. In Vitro Evaluation of Paclitaxel Loaded Amorphous Chitin Nanoparticles for Colon Cancer Drug Delivery. Colloids Surf. B Biointerfaces 2013, 104, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1600262. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J.-C. Characterization of Thermosensitive Chitosan Gels for the Sustained Delivery of Drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Depani, B.P.; Naik, A.A.; Nair, H.A. Preparation and Evaluation of Chitosan Based Thermoreversible Gels for Intraperitoneal Delivery of 5-Fluorouracil (5-FU). Acta Pharm. 2013, 63, 479–491. [Google Scholar] [CrossRef] [Green Version]
- López-León, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical Characterization of Chitosan Nanoparticles: Electrokinetic and Stability Behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, L.; Gu, X.; Zhang, B.; Hu, X.; Zhang, J.; Chen, J.; Wang, Y.; Chen, C.; Gao, B.; et al. Prevention of Colorectal Cancer Liver Metastasis by Exploiting Liver Immunity via Chitosan-TPP/Nanoparticles Formulated with IL-12. Biomaterials 2012, 33, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. Mannosylated Chitosan Nanoparticle-Based Cytokine Gene Therapy Suppressed Cancer Growth in BALB/c Mice Bearing CT-26 Carcinoma Cells. Mol. Cancer Ther. 2006, 5, 1723–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siahmansouri, H.; Somi, M.H.; Babaloo, Z.; Baradaran, B.; Jadidi-Niaragh, F.; Atyabi, F.; Mohammadi, H.; Ahmadi, M.; Yousefi, M. Effects of HMGA2 SiRNA and Doxorubicin Dual Delivery by Chitosan Nanoparticles on Cytotoxicity and Gene Expression of HT-29 Colorectal Cancer Cell Line. J Pharm. Pharm. 2016, 68, 1119–1130. [Google Scholar] [CrossRef]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-Coated Cinnamon/Oregano-Loaded Solid Lipid Nanoparticles to Augment 5-Fluorouracil Cytotoxicity for Colorectal Cancer: Extract Standardization, Nanoparticle Optimization, and Cytotoxicity Evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel Alginate-Enclosed Chitosan–Calcium Phosphate-Loaded Iron-Saturated Bovine Lactoferrin Nanocarriers for Oral Delivery in Colon Cancer Therapy. Nanomedicine 2012, 7, 1521–1550. [Google Scholar] [CrossRef] [Green Version]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Shukla, R.K.; Tiwari, A. Carbohydrate Polymers: Applications and Recent Advances in Delivering Drugs to the Colon. Carbohydr. Polym. 2012, 88, 399–416. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A Colon Targeted Drug Delivery System Based on Alginate Modificated Graphene Oxide for Colorectal Liver Metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate Beads for Colon Specific Delivery of Self-Emulsifying Curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Hsu, F.-Y.; Yu, D.-S.; Huang, C.-C. Development of PH-Sensitive Pectinate/Alginate Microspheres for Colon Drug Delivery. J. Mater. Sci. Mater. Med. 2013, 24, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium Alginate-Carboxymethyl Cellulose Beads for Colon-Targeted Drug Delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Gulbake, A.; Tiwari, J.; Jain, S.K. Development of Liposomes Entrapped in Alginate Beads for the Treatment of Colorectal Cancer. Int. J. Biol. Macromol. 2016, 82, 687–695. [Google Scholar] [CrossRef]
- Ma, Y.; Coombes, A.G.A. Designing Colon-Specific Delivery Systems for Anticancer Drug-Loaded Nanoparticles: An Evaluation of Alginate Carriers: Anticancer Drug-Loaded NPs. J. Biomed. Mater. Res. 2014, 102, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Abbaszad Rafi, A.; Mahkam, M. Preparation of Magnetic PH-Sensitive Microcapsules with an Alginate Base as Colon Specific Drug Delivery Systems through an Entirely Green Route. RSC Adv. 2015, 5, 4628–4638. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin Potentiates Antitumor Activity of 5-Fluorouracil in a 3D Alginate Tumor Microenvironment of Colorectal Cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef] [Green Version]
- Ivanovska, J.; Zehnder, T.; Lennert, P.; Sarker, B.; Boccaccini, A.R.; Hartmann, A.; Schneider-Stock, R.; Detsch, R. Biofabrication of 3D Alginate-Based Hydrogel for Cancer Research: Comparison of Cell Spreading, Viability, and Adhesion Characteristics of Colorectal HCT116 Tumor Cells. Tissue Eng. Part C Methods 2016, 22, 708–715. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Y.; Wang, H.; Deng, F.; Zhang, Y.; Zhao, S.; Ma, X.; Wu, H.; Sun, G. Novel Decellularized Liver Matrix-Alginate Hybrid Gel Beads for the 3D Culture of Hepatocellular Carcinoma Cells. Int. J. Biol. Macromol. 2018, 109, 1154–1163. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Liu, Y.; Li, N.; Guo, X.; Wang, S.; Sun, G.; Wang, W.; Ma, X. Encapsulated Human Hepatocellular Carcinoma Cells by Alginate Gel Beads as an in Vitro Metastasis Model. Exp. Cell Res. 2013, 319, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.W. Practical Aspects of Hyaluronan Based Medical Products; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Stern, R.; Asari, A.; Sugahara, K. Hyaluronan Fragments: An Information-Rich System. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.-A.; Kong, J.-H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target Specific and Long-Acting Delivery of Protein, Peptide, and Nucleotide Therapeutics Using Hyaluronic Acid Derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.A.J.; Vreuls, C.P.H.; Winstanley, A.; Jansen, R.L.H.; van Bijnen, A.A.; Dello, S.A.W.G.; Bemelmans, M.H.; Dejong, C.H.C.; Driessen, A.; Olde Damink, S.W.M. Hyaluronic Acid as a Marker of Hepatic Sinusoidal Obstruction Syndrome Secondary to Oxaliplatin-Based Chemotherapy in Patients with Colorectal Liver Metastases. Ann. Surg. Oncol. 2013, 20, 1462–1469. [Google Scholar] [CrossRef]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.H.; Ryu, J.H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Jian, Y.-S.; Chen, C.-W.; Lin, C.-A.; Yu, H.-P.; Lin, H.-Y.; Liao, M.-Y.; Wu, S.-H.; Lin, Y.-F.; Lai, P.-S. Hyaluronic Acid–Nimesulide Conjugates as Anticancer Drugs against CD44-Overexpressing HT-29 Colorectal Cancer in vitro and in vivo. Int. J. Nanomed. 2017, 12, 2315–2333. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and Development of Ligand-Appended Polysaccharidic Nanoparticles for the Delivery of Oxaliplatin in Colorectal Cancer. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 179–190. [Google Scholar] [CrossRef]
- Xu, K.; Lee, F.; Gao, S.J.; Chung, J.E.; Yano, H.; Kurisawa, M. Injectable Hyaluronic Acid-Tyramine Hydrogels Incorporating Interferon-A2a for Liver Cancer Therapy. J. Control. Release 2013, 166, 203–210. [Google Scholar] [CrossRef]
- Luo, J.; Wu, Z.; Lu, Y.; Xiong, K.; Wen, Q.; Zhao, L.; Wang, B.; Gui, Y.; Fu, S. Intraperitoneal Administration of Biocompatible Hyaluronic Acid Hydrogel Containing Multi-Chemotherapeutic Agents for Treatment of Colorectal Peritoneal Carcinomatosis. Int. J. Biol. Macromol. 2020, 152, 718–726. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable Thermosensitive Hydrogel Containing Hyaluronic Acid and Chitosan as a Barrier for Prevention of Postoperative Peritoneal Adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Oxaliplatin-Loaded Chemically Cross-Linked Hydrogels for Prevention of Postoperative Abdominal Adhesion and Colorectal Cancer Therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.D.; Grande-Allen, K.J. Review. Hyaluronan: A Powerful Tissue Engineering Tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [PubMed]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust Alginate/Hyaluronic Acid Thiol–Yne Click-Hydrogel Scaffolds with Superior Mechanical Performance and Stability for Load-Bearing Soft Tissue Engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended Release of Perioperative Immunotherapy Prevents Tumor Recurrence and Eliminates Metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]

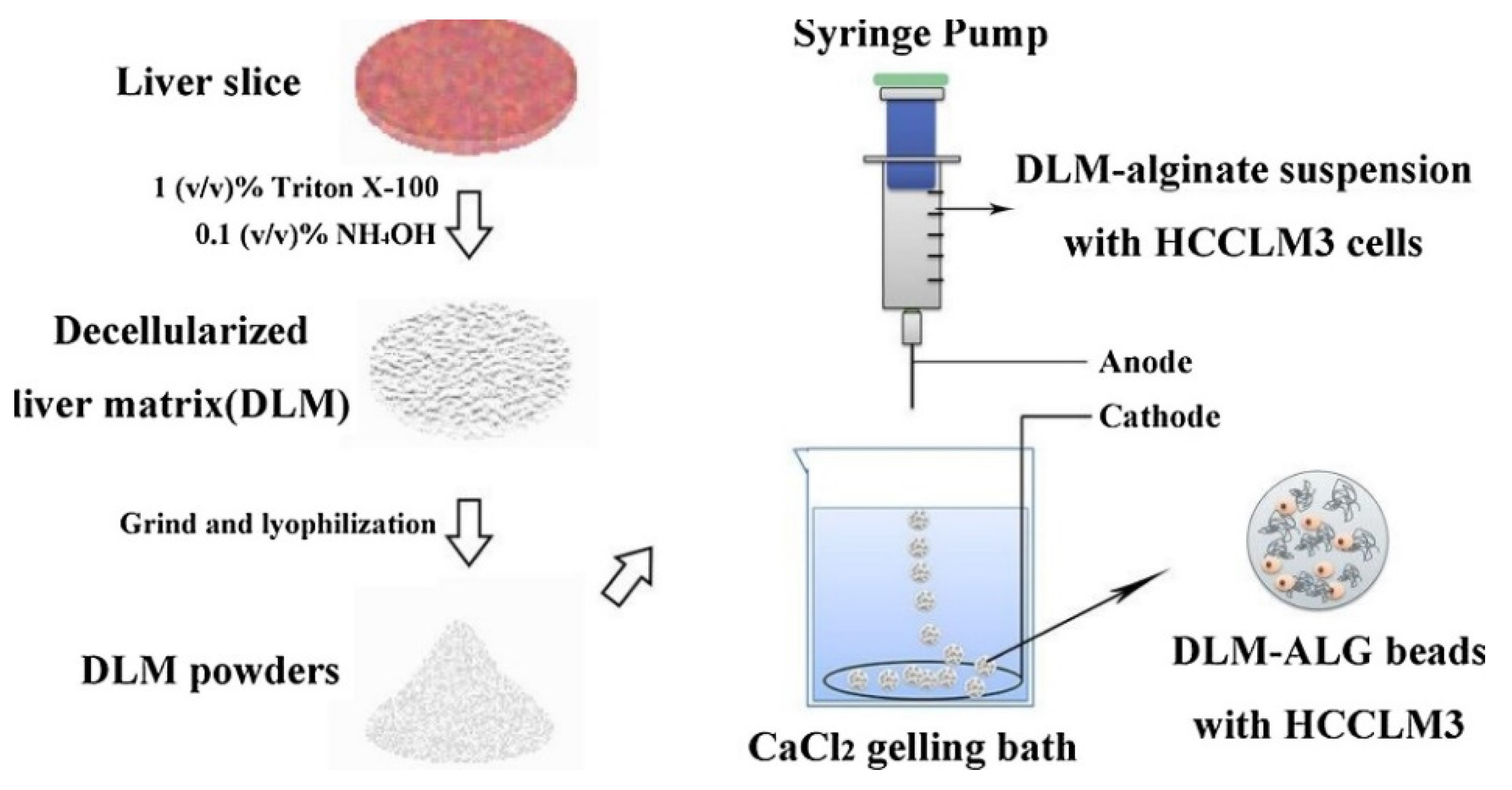
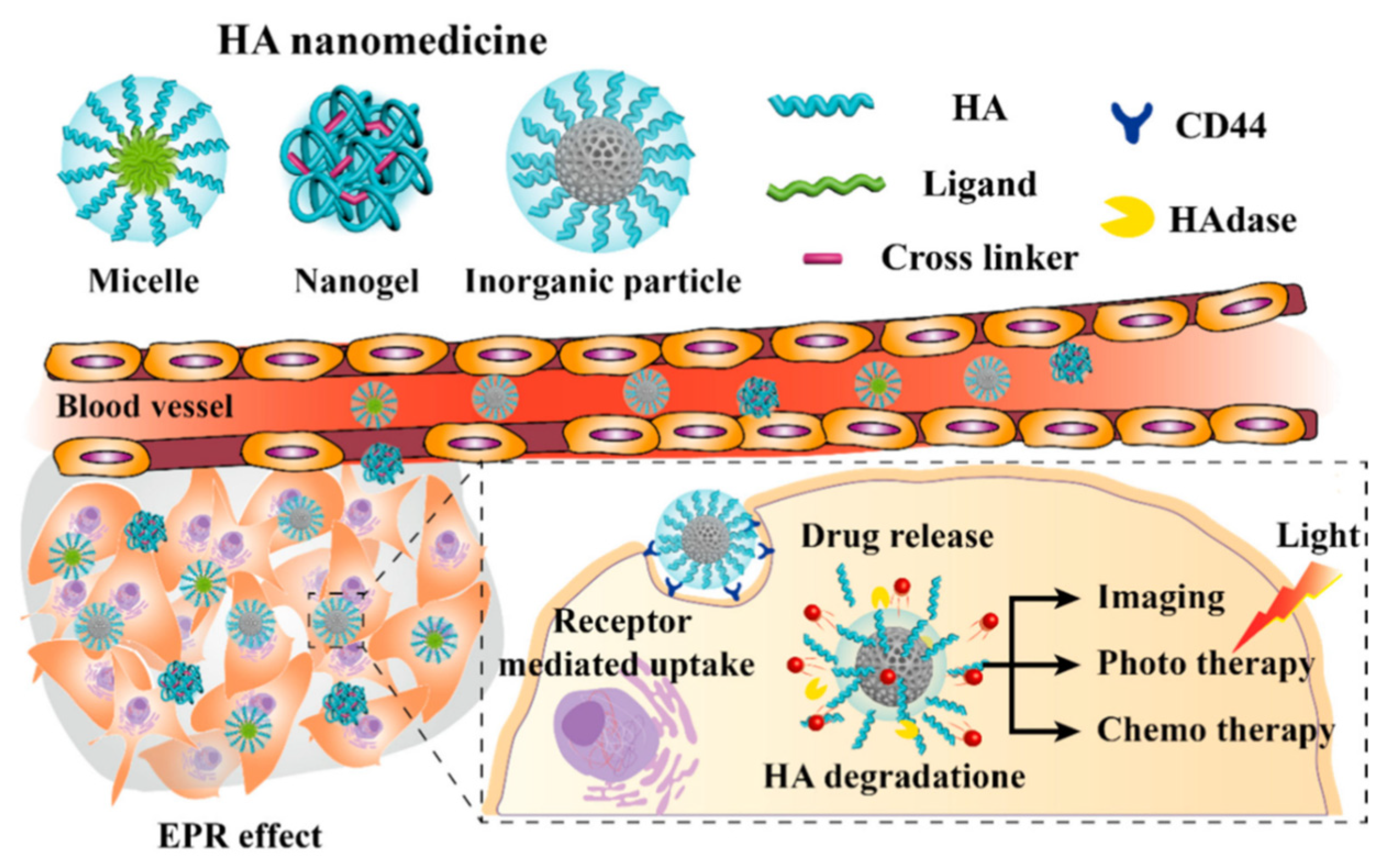
| Material | Formulation | Cargo | Remarks | Ref. |
|---|---|---|---|---|
| PEG/PLA | Injectable thermosensitive hydrogel made of micelles | Cytokines and Toll-like receptor agonists | Hydrogel undergoes sol–gel transition at physiological conditions entrapping the immune and chemotherapeutic cargo. Minimal cytotoxicity and hemolysis. Cargo release through swelling diffusion in 7 days and hydrogel disappearance in 10 weeks. Empty hydrogel prevents intraperitoneal post-surgery adhesion, and the vaccine-loaded one improves HCC survival by 20%. | [42,43,44,45,46,47] |
| Nanovesicles | Doxorubicin HMME Curcumin | Injected in blood vessels, they can reach the target thanks to the EPR effect or active targeting with Fol. Satisfactory liver CRC metastases targeting hydrophilic and light-sensible hydrophobic chemotherapies. | ||
| PEG/PCL | Nanoparticles | 5-fluorouracil and DNA | PEG–PCL polyplexes constructed by electrostatic interactions, injectable in blood vessels. Combined and effective gene and chemotherapy, with synergistic effects against CRC. Optimal efficiency encapsulation, 80% drug release in 72 h and poor MPS recognition thanks to PEGylation. | [48,49,50,51,52,53,54,55,56,57,58] |
| Doxorubicin | NPs injectable in blood vessels, good results against CRC thanks to passive targeting and DOX slow release. | |||
| PCEC hydrogel | 5-fluorouracil and paclitaxel | Injectable in situ, sol–gel transition occurs at body temperature. Applied mainly for inhibiting CRC spreading into the abdominal cavity, hindering peritoneal carcinomatosis (CRPC). | ||
| PCEC microspheres | Camptothecin | Hydrogel layer to protect the cargo from hydrolysis. Weekly abdominal injections are needed. Main application: counteracting CRPC. | ||
| PCL injectable thermosensitive hydrogel + NPs | Oxaliplatin and tannic acid | Hybrid solution: PCL scaffold with NPs encapsulated. The system undergoes sol–gel transition at physiological conditions, shielding the toxic effects of its cargo. Assessed for CRPC therapy, but under study for liver CRC metastases. | ||
| CHITIN | Nanogels | Doxorubicin | Chitin can be coupled with hyaluronic acid or PLA to obtain injectable nanogels for intravenous therapy. Technique useful for both CRC and HCC foci: the drug is released in endosomes or lysosomes by pH-controlled hydrolysis. | [59,60,61,62,63,64,65,66,67,68,69,70] |
| Nanoparticles | Paclitaxel Honokiol | Injectable systems whose integrity is provided by complexation with TPP. Passive targeting can be substituted by the active one binding EGCG to chitin. PTX-loaded NPs are more active against CRC, while honokiol ones are effective against HCC. | ||
| CHITOSAN | CS/β-GP injectable thermosensitive hydrogel | 5-fluorouracil 5-fluorouracil and cisplatin | Reversible hydrogel suitable for intraperitoneal, abdominal or intratumoral injection that shields the toxicity of the cargo. The combined therapy is very efficient in hindering CRC metastatic spreading to the liver. | [71,72,73,74,75,76,77,78,79,80] |
| CS/TPP nanoparticles | Interleukin-12 | Immunotherapy is injected into blood vessels. NPs reach the hepatic tumor site through passive targeting, and the release of the cytokine is triggered by pH. It is the most effective treatment for liver CRC metastases among the chitosan alternatives. | ||
| ALGINATE | Microparticles | 5-fluorouracil | Suitable for oral administration and colon targeted delivery, thanks to pH-sensitivity. Graphene oxide-based sodium alginate functionalized microparticles for the specific treatment of liver CRC metastasis. | [81,82,83,84,85,86,87,88,89,90,91,92,93] |
| Beads or microcapsules | Curcumin Cisplatin 5-fluorouracil Oxaliplatin Indomethacin Naproxen | Suitable for oral administration and colon targeted delivery, thanks to pH-sensitivity. The main counter-cation used is Ca2+, alginate can be complexed with carboxymethyl cellulose, pectinate or chitosan; the system can be coated with Eudragit®, encapsulate liposomes or magnetic NPs for external control of the position. Active targeting of CRC cells is achievable by conjugating folic acid. | ||
| 3D hydrogel matrices | Colon or liver cancer cells | Alginate 3D scaffolds can efficiently mimic the TME and allow the possibility to screen the effects of new chemotherapies, limiting animal-based experiments. | ||
| Biodegradable hydrogel implant | Immunostimulatory compounds | Alginate scaffold-loading antibodies, cytokines, interferons or immune system cells can be placed into CRC resection site during surgery to prevent cancer recurrence and distal metastases, to the liver, for instance. | ||
| HYALURONIC ACID | Nanogels and nanoparticles | Irinotecan Nimesulide | Nanogels are suitable for intravenous administration. HA can be used alone or paired with PEG, conjugating dyes an early primary CRC and metastases diagnosis can be achieved thanks to the CD44 active targeting promoted by HA specificity. | [16,94,95,96,97,98,99,100,101,102,103,104,105,106,107] |
| Oxaliplatin | HA-CS/TPP beads coated with Eudragit, suitable for oral administration and colon targeting. | |||
| Injectable thermosensitive hydrogels | IFN-α2a 5-fluorouracil, cisplatin, paclitaxel Oxaliplatin | Administration in situ thanks to the gelling property of the material. One week to one-month degradation can be achieved by tuning rheologic properties. In addition to chemotherapy and peritoneal adhesions prevention, immunotherapy is the future application of HA hydrogels for CRC treatment and metastases hindrance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maspes, A.; Pizzetti, F.; Rossetti, A.; Makvandi, P.; Sitia, G.; Rossi, F. Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms. Gels 2021, 7, 6. https://doi.org/10.3390/gels7010006
Maspes A, Pizzetti F, Rossetti A, Makvandi P, Sitia G, Rossi F. Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms. Gels. 2021; 7(1):6. https://doi.org/10.3390/gels7010006
Chicago/Turabian StyleMaspes, Anna, Fabio Pizzetti, Arianna Rossetti, Pooyan Makvandi, Giovanni Sitia, and Filippo Rossi. 2021. "Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms" Gels 7, no. 1: 6. https://doi.org/10.3390/gels7010006
APA StyleMaspes, A., Pizzetti, F., Rossetti, A., Makvandi, P., Sitia, G., & Rossi, F. (2021). Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms. Gels, 7(1), 6. https://doi.org/10.3390/gels7010006