Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update
Abstract
:1. Introduction
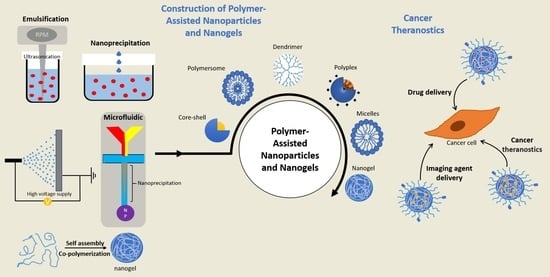
2. Types of Polymer-Based Nanoparticles and Nanogels
2.1. Formulation of Polymeric Nanoparticles and Nanogels
2.1.1. Core–Shell Nanoparticles
2.1.2. Dendrimer/Hyperbranched Polymer
2.1.3. Polymersome
2.1.4. Polyplex
2.1.5. Polymeric Micelles
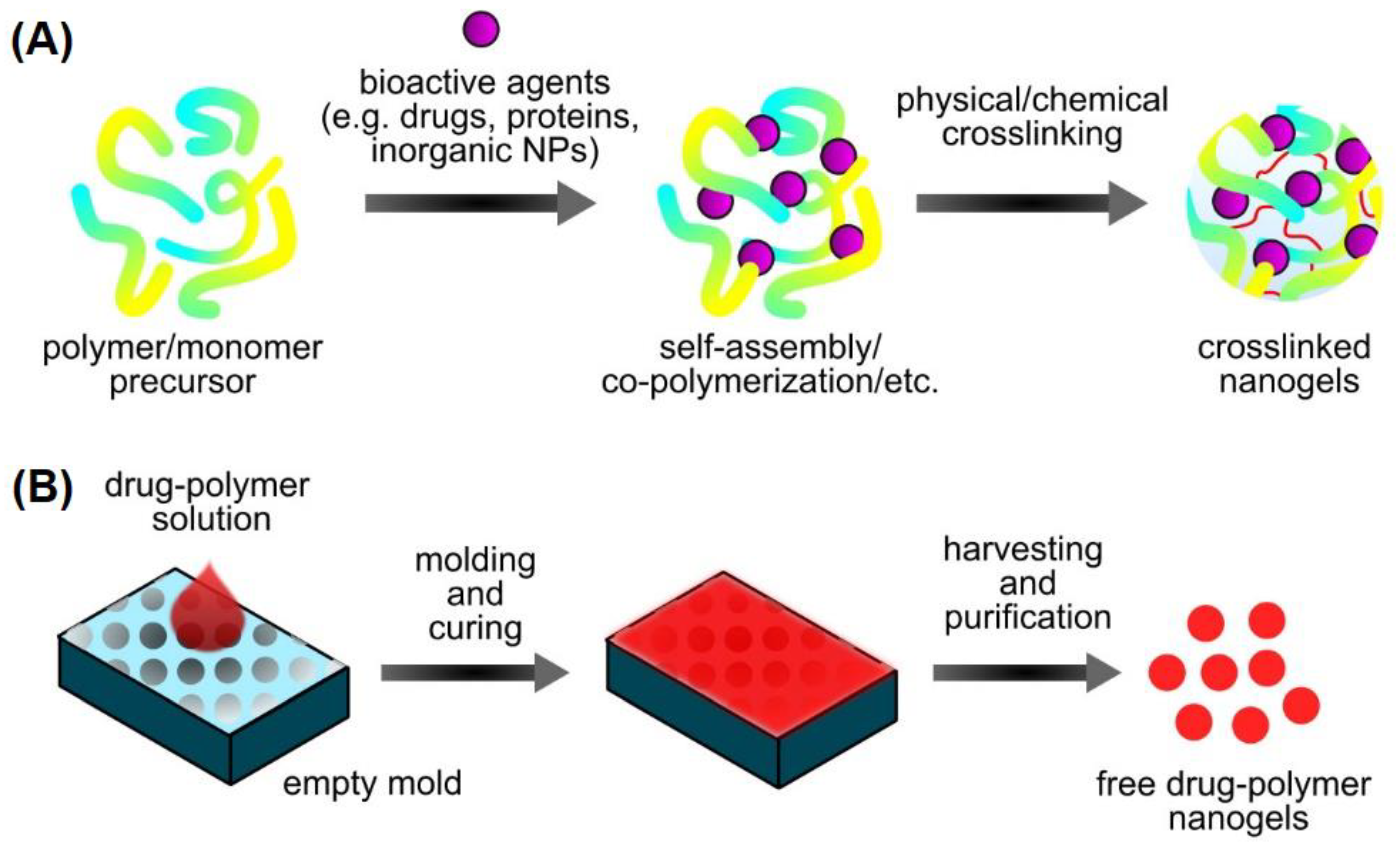
2.1.6. Nanogels
2.2. Fabrication Strategies of Polymeric Nanoparticles
2.2.1. Emulsification
2.2.2. Nanoprecipitation
2.2.3. Electrospraying
2.2.4. Microfluidic Technology
2.2.5. Preparation of Nanogels
2.3. Types of Polymers for Polymeric Nanoparticles Formation
2.3.1. Natural Polymers
2.3.2. Synthetic Polymers
2.3.3. Peptide-Polymer Conjugates
3. Functionally Active Nanoparticles for Cancer Therapy
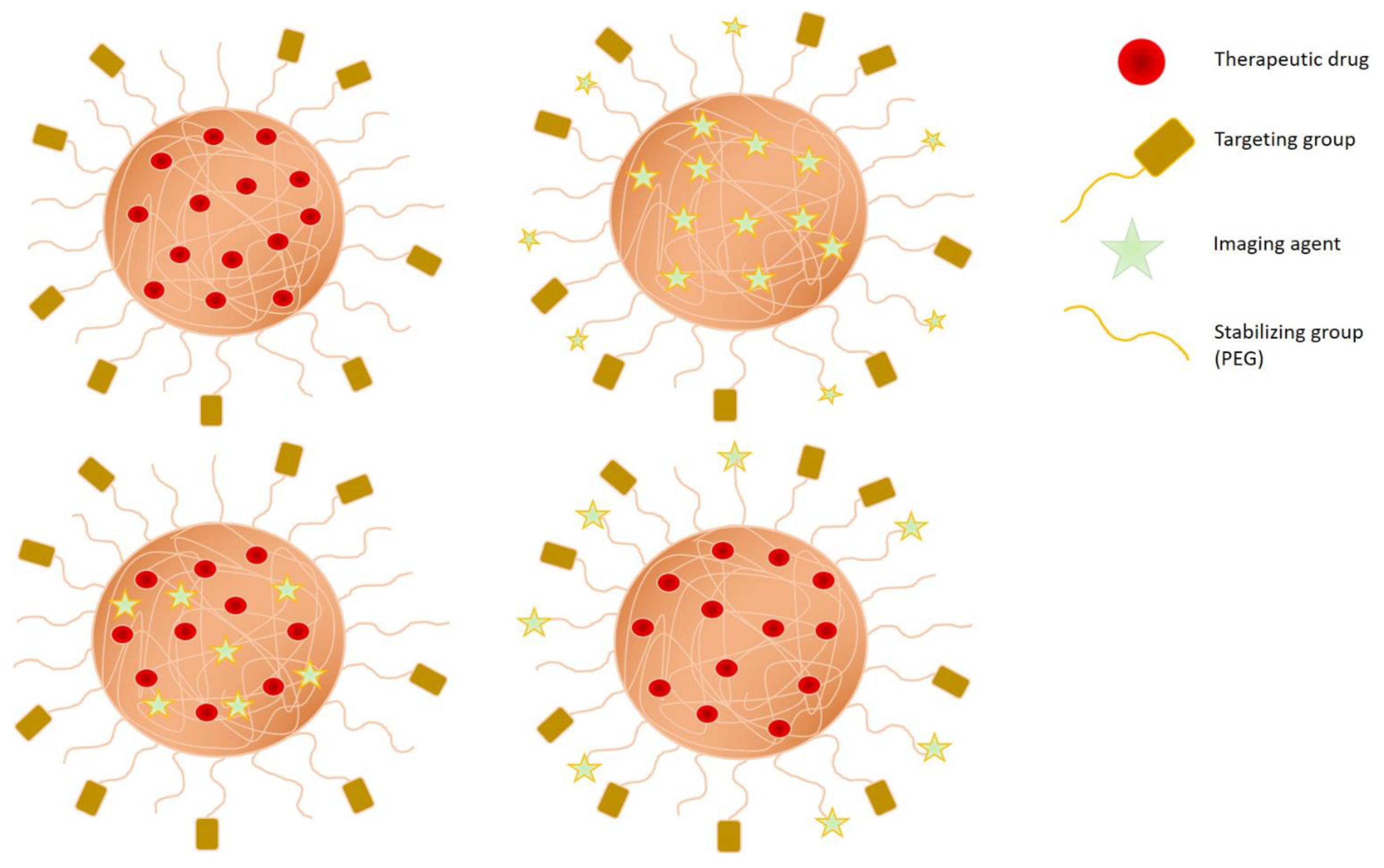
3.1. Specific Feature of Polymers for Cancer Therapy
3.2. Engineering Strategies of Nanogels for Cancer Therapy
3.3. Polymeric Nanomaterials Targeting Various Cancers
| Nanoparticle | Polymer | Function of Polymer | Cancer Type | Year | Status | Reference |
|---|---|---|---|---|---|---|
| Magnetite nanoparticles | Magnetic nanoparticles, coated with antibodies | The coating of the magnetic nanoparticles consist of antibodies such as epithelial cell adhesion molecule (EpCAM) and CD52 as markers to attempt to remove tumor cells from the blood. | prostate, colon, lung or pancreatic cancer and lymphoma or leukemia | 2017–current | Recruiting | [147] |
| Cetuximab nanoparticles | Ethylcellulose, decorated with somatostatin analogue | Release drug at only above pH 6.8. Holds Cetuximab at pH 1.5. | Colon Cancer | 2018–current | Recruiting | [148] |
| Immuno-tethered lipoplex nanoparticle (ILN) biochip | Immuno-tethered lipoplex | To monitor treatment response and to detect relapse in patient. | B-cell lymphoma | 2018–current | Recruiting | [149] |
| Gadolinium-chelated polysiloxane based nanoparticles | Gadolinium-chelated polysiloxane | Has great theronostic properties by radiosensitization and diagnosis by multimodal imaging. | Brain metastases | 2019–current | Recruiting | [150] |
| Quantum dots nanoparticles | Cds/ZnS core–shell type quantum dots with carboxylic acid, decorated with veldoreotide | Using veldoreotide as a somatostatin analog to deliver anticancer drugs to target and for bioimaging of the cancer cells. | Breast Cancer | 2019–current | Recruiting | [151] |
| PLGA nanoparticle | PLGA | PLGA nanoparticles are minimal in toxicity and is used for drug delivery for anti-tumor immune response. | New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1) positive cancers | 2021–current | Recruiting | [152] |
| Ultrasmall Superparamagentic Iron Oxide nanoparticles (USPION) | Ferrotran | To detect lymph node metastases of solid tumors with the assistance of MRI. | Pancreatic Cancer | 2017–2021 | Recruiting | [153] |
| Superparamagentic Iron Oxide nanoparticles (SPION) | Iron Oxide | To trace delayed sentinel lymph node dissection. | Breast Cancer | 2020–current | Recruiting | [154] |
| Superparamagentic Iron Oxide nanoparticles (SPION) | Iron Oxide | Increase the safety of liver after stereotactic body radiotherapy by assisting in the detection and the avoidance of high levels of radiation. | Hepatocellular carcinomas | 2020–current | Recruiting | [155] |
| Hafnium Oxide-containing nanoparticles NBTXR3 | Hafnium Oxide | To target cancer cells for destruction through radiation therapy. | Pancreatic Cancer | 2020–current | Recruiting | [156] |
| Hafnium Oxide-containing nanoparticles NBTXR3 | Hafnium Oxide | To improve sensitivity of tumor cells to radiation therapy. | Recurrent/Metastatic Head and Neck Squamous Cell Cancer | 2021–current | Recruiting | [158] |
| Hafnium Oxide-containing nanoparticles NBTXR3 | Hafnium Oxide | Using NBTXR3 to improve the effectiveness of radiation therapy. | Head and Neck Squamous Cell Cancer | 2021–current | Recruiting | [157] |
| Albumin-bound Rapamycin nanoparticle (Nab-rapamycin) | Albumin | Along with pazopanib hydrochloride, it may block cell growth enxymes which in turn halts the growth of tumor cells. | Advance Nonadipocytic Soft Tissue Sarcomas | 2019–current | Recruiting | [159] |
| Albumin-bound rapamycin, temozolomide, and irinotecan nanoparticles | Albumin | To evaluate the drug response as a combinational therapy. | Solid Tumors | 2017–current | Recruiting | [160] |
| Albumin-bound Rapamycin nanoparticles (Nab-Rapamycin) | Albumin | To evaluate the effect of Nab-rapamycin alone or in combination with various drugs in patients. | Glioma and Glioblastoma | 2018–current | Recruiting | [173] |
| Paclitaxel Albumin-bound nanoparticles (Nab-Paclitaxel) | Paclitaxel Albumin-bound combining with gemcitabine, and cisplastin with high dose of Ascorbic Acid | To improve survival of paclitaxel albumin-bound nanoparticle with gemcitabine comparing to only gemcitabine. | Pancreatic cancer | 2017–current | Recruiting | [161] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | As combinational drug therapy with cisplastin and gemcitabine. | Pacreatic Adenocarcinoma | 2019–current | Recruiting | [162] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | Nab-paclitaxel is able to stop tumor growth by killing, arrest cell division, or by preventing it from metastasis. | Metastatic Pancreatic Cancer | 2020–current | Recruiting | [163] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | Nab-paclitaxel is able to stop tumor growth by killing, arrest cell division, or by preventing it from metastasis. | Metastatic Pancreatic Cancer | 2020–current | Recruiting | [164] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | As combinational drug therapy. | Pancreatic Cancer | 2020–current | Recruiting | [165] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | As combinational drug therapy. | Triple Negative Breat Cancer | 2018–current | Recruiting | [166] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | Nab-paclitaxel is able to stop tumor growth by killing, arrest cell division, or by preventing it from metastasis. | Triple Negative Breast Cancer | 2020–current | Recruiting | [174] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | Nab-paclitaxel is able to stop tumor growth by killing, arrest cell division, or by preventing it from metastasis. | Biliary Tract Cancer | 2018–current | Recruiting | [167] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | Combinining nab-paclitaxel, gemcitabine, and cisplastin to halt growth of tumor cells. | Liver Bile Duct Cancer | 2018–current | Recruiting | [168] |
| Nab-paclitaxel/Rituximab-coated nanoparticle | Albumin | Combining Nab-paclitaxel to halt growth by killing or stopping the groth of tumor cells, while using rituximab may affec the growth and spreading of tumor cells. | Reccurent or refraxtory B-cell non-Hodgkin lymphoma | 2019–current | Recruiting | [169] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | To evaluate the drug response as a combinational therapy. | Non-squamous non-small cell lung cancer (NSCLS) | 2020–current | Recruiting | [170] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | As combinational drug therapy with cisplastin and capecitabine | Esophageal cancer | 2020–current | Recruiting | [171] |
| Albumin-bound Paclitaxel nanoparticle (Nab-paclitaxel) | Albumin | As combinational drug therapy with cisplastin and sinitilimab. | Esophageal cancer | 2020–current | Recruiting | [172] |
4. Mechanism of Anticancer Action
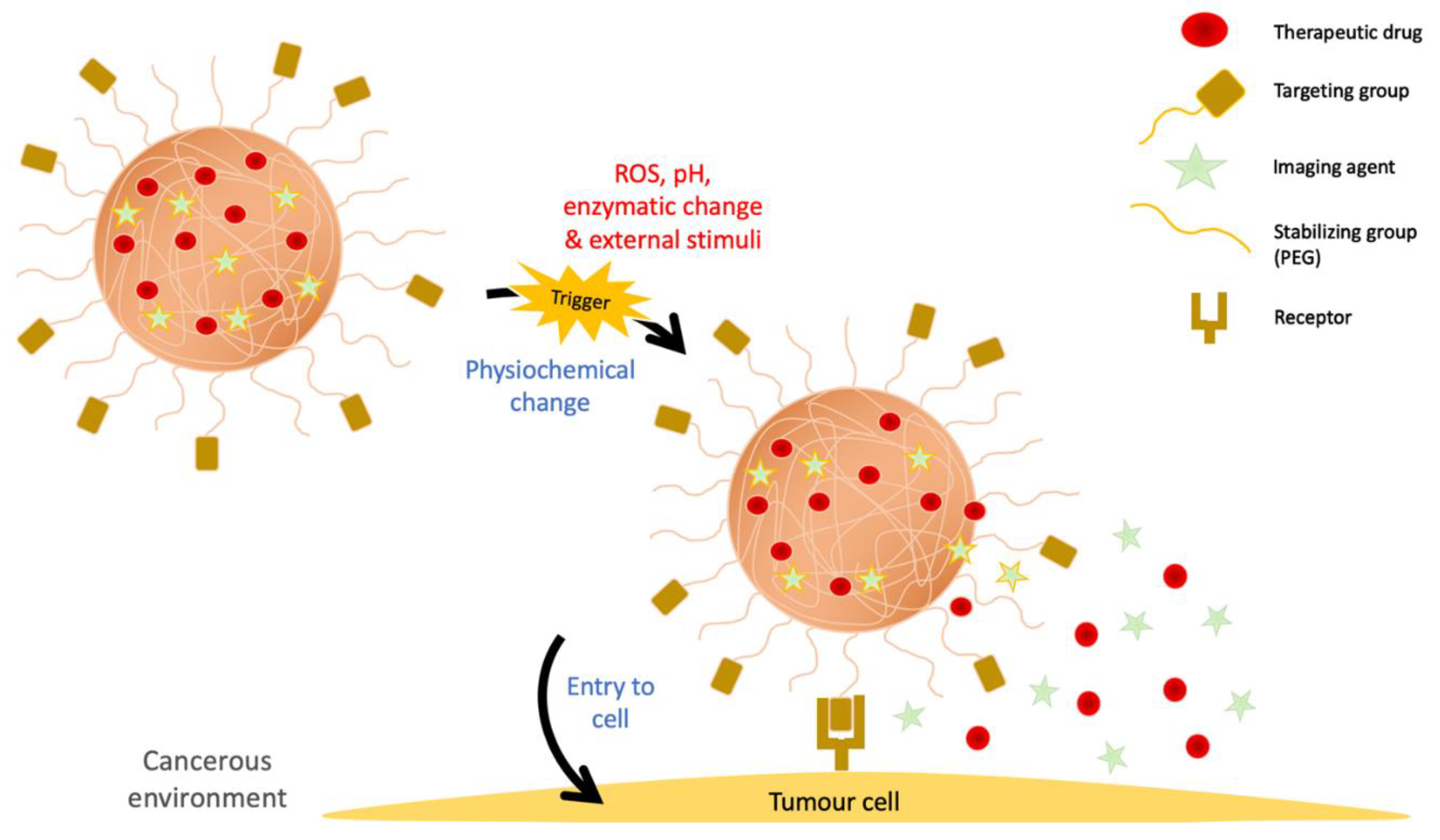
4.1. Targeting
4.2. Cellular Uptake
4.3. Drug Release
5. Limitation and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Carr, D.F.; Turner, R.M.; Pirmohamed, M. Pharmacogenomics of anticancer drugs: Personalising the choice and dose to manage drug response. Br. J. Clin. Pharmacol. 2021, 87, 237–255. [Google Scholar] [CrossRef]
- Ismail, N.A.; Shameli, K.; Wong, M.M.T.; Teow, S.Y.; Chew, J.; Sukri, S.N.A.M. Antibacterial and cytotoxic effect of honey mediated copper nanoparticles synthesized using ultrasonic assistance. Mater. Sci. Eng. C 2019, 104, 109899. [Google Scholar] [CrossRef]
- Mohamad Sukri, S.N.A.; Shameli, K.; Wong, M.M.T.; Teow, S.Y.; Chew, J.; Ismail, N.A. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J. Mol. Struct. 2019, 1189, 57–65. [Google Scholar] [CrossRef]
- Teow, S.Y.; Wong, M.M.T.; Yap, H.Y.; Peh, S.C.; Shameli, K. Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPs). Molecules 2018, 23, 1366. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.X.; Shameli, K.; Mohamad, S.E.; Yew, Y.P.; Mohamed Isa, E.D.; Yap, H.-Y.; Lim, W.L.; Teow, S.-Y. Bio-mediated synthesis and characterisation of silver nanocarrier, and its potent anticancer action. Nanomaterials 2019, 9, 1423. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.-Y.; Peh, S.-C.; Mohamad, S.E.; Mohd Taib, S.H. Green fabrication of biologically active magnetic core-shell Fe3O4/Au nanoparticles and their potential anticancer effect. Mater. Sci. Eng. C 2019, 96, 51–57. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica granatum fruit peel extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.B.; Nagao, Y.; Teow, S.-Y.; Lee, K.X.; Mohamed Isa, E.D. Potential anticancer activity of protocatechuic acid loaded in montmorillonite/Fe3O4 nanocomposites stabilized by seaweed Kappaphycus alvarezii. Int. J. Pharm. 2019, 572, 118743. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.; Lee, K.X.; Teow, S.-Y. Green Synthesized montmorillonite/carrageenan/Fe3O4 nanocomposites for pH-responsive release of protocatechuic acid and its anticancer activity. Int. J. Mol. Sci. 2020, 21, 4851. [Google Scholar] [CrossRef]
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of metal nanoparticle–hydrogel composites in tissue regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Colazo, J.; Berg, D.; Mugo, S.M.; Serpe, M.J. Multiresponsive nanogels for targeted anticancer drug delivery. Mol. Pharm. 2017, 14, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D. Polymers and polymer nanocomposites for cancer therapy. Appl. Sci. 2019, 9, 3899. [Google Scholar] [CrossRef] [Green Version]
- Appelbe, O.K.; Kim, B.-K.; Rymut, N.; Wang, J.; Kron, S.J.; Yeo, Y. Radiation-enhanced delivery of plasmid DNA to tumors utilizing a novel PEI polyplex. Cancer Gene Ther. 2018, 25, 196–206. [Google Scholar] [CrossRef]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-silk core–shell nanoparticles as potential carriers for targeted delivery of curcumin into human breast cancer cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, R.; Huang, J.; Luo, M.; Chen, X.; Kang, Y.; Wu, J. Albumin enhances PTX delivery ability of dextran NPs and therapeutic efficacy of PTX for colorectal cancer. J. Mater. Chem. B 2019, 7, 3537–3545. [Google Scholar] [CrossRef]
- Lian, H.; Du, Y.; Chen, X.; Duan, L.; Gao, G.; Xiao, C.; Zhuang, X. Core cross-linked poly(ethylene glycol)-graft-Dextran nanoparticles for reduction and pH dual responsive intracellular drug delivery. J. Colloid Interface Sci. 2017, 496, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, X.; Li, H.; Deng, P.; Li, H.; He, T.; Rong, J.; Zhao, J.; Liu, Z. A core/shell stabilized polysaccharide-based nanoparticle with intracellular environment-sensitive drug delivery for breast cancer therapy. J. Mater. Chem. B 2018, 6, 6646–6659. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of long-circulating zwitterionic cross-linked micelles for active-targeted drug delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Wang, T.; Yeh, C.; Kuan, C.; Wang, L.; Chen, L.; Wu, H.; Sun, J. Tailored design of multifunctional and programmable pH-responsive self-assembling polypeptides as drug delivery nanocarrier for cancer therapy. Acta Biomater. 2017, 58, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D. A dual targeting dendrimer-mediated siRNA delivery system for effective gene silencing in cancer therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooja, D.; Sistla, R.; Kulhari, H. Dendrimer-drug conjugates: Synthesis strategies, stability and application in anticancer drug delivery. In Design of Nanostructures for Theranostics Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–303. [Google Scholar]
- Jiang, G.; Liu, S.; Yu, T.; Wu, R.; Ren, Y.; van der Mei, H.C.; Liu, J.; Busscher, H.J. PAMAM dendrimers with dual-conjugated vancomycin and Ag-nanoparticles do not induce bacterial resistance and kill vancomycin-resistant Staphylococci. Acta Biomater. 2021, 123, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-sensitive and amphiphilic PEGylated dendrimer-paclitaxel prodrug-based nanoparticles for enhanced stability and anticancer efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pérez, S.A.; del Pilar Ramos-Godínez, M.; Ramón-Gallegos, E. Glycosylated one-step PAMAM dendrimers loaded with methotrexate for target therapy in breast cancer cells MDA-MB-231. J. Drug Deliv. Sci. Technol. 2020, 58, 101769. [Google Scholar] [CrossRef]
- Xiong, Z.; Alves, C.S.; Wang, J.; Li, A.; Liu, J.; Shen, M.; Rodrigues, J.; Tomás, H.; Shi, X. Zwitterion-functionalized dendrimer-entrapped gold nanoparticles for serum-enhanced gene delivery to inhibit cancer cell metastasis. Acta Biomater. 2019, 99, 320–329. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K.; et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, L.; Chen, S.; Gao, J.; Zhao, P.; Du, J. A superparamagnetic polymersome with extremely high T 2 relaxivity for MRI and cancer-targeted drug delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef]
- Kocere, A.; Resseguier, J.; Wohlmann, J.; Skjeldal, F.M.; Khan, S.; Speth, M.; Dal, N.-J.K.; Ng, M.Y.W.; Alonso-Rodriguez, N.; Scarpa, E.; et al. Real-time imaging of polymersome nanoparticles in zebrafish embryos engrafted with melanoma cancer cells: Localization, toxicity and treatment analysis. EBioMedicine 2020, 58, 102902. [Google Scholar] [CrossRef]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic polymersome nanoreactors with tumor-specific activable cascade reactions for cooperative cancer therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Lächelt, U.; Bartek, J.; Wagner, E.; Moghimi, S.M. Polyplex evolution: Understanding biology, optimizing performance. Mol. Ther. 2017, 25, 1476–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghaei, M.; Tekie, F.S.M.; Khoshayand, M.R.; Varshochian, R.; Hajiramezanali, M.; Kachousangi, M.J.; Dinarvand, R.; Atyabi, F. Optimization of chitosan-based polyelectrolyte nanoparticles for gene delivery, using design of experiment: In vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111036. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jin, Z.; Tan, X.; Zhang, C.; Zou, C.; Zhang, W.; Ding, J.; Das, B.C.; Severinov, K.; Hitzeroth, I.I.; et al. Hyperbranched poly(β-amino ester) based polyplex nanopaticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J. Control. Release 2020, 321, 654–668. [Google Scholar] [CrossRef]
- Lin, M.; Dai, Y.; Xia, F.; Zhang, X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111626. [Google Scholar] [CrossRef]
- Shi, H.; van Steenbergen, M.J.; Lou, B.; Liu, Y.; Hennink, W.E.; Kok, R.J. Folate decorated polymeric micelles for targeted delivery of the kinase inhibitor dactolisib to cancer cells. Int. J. Pharm. 2020, 582, 119305. [Google Scholar] [CrossRef]
- Dariva, C.G.; Figueiredo, J.P.H.; Ferreira, C.; Laranjo, M.; Botelho, M.F.; Fonseca, A.C.; Coelho, J.F.J.; Serra, A.C. Development of red-light cleavable PEG-PLA nanoparticles as delivery systems for cancer therapy. Colloids Surf. B Biointerfaces 2020, 196, 111354. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, S.; Li, Y.; He, H.; Ji, Y.; Zheng, M.; Liu, Y.; Yin, L. Co-delivery of dual chemo-drugs with precisely controlled, high drug loading polymeric micelles for synergistic anti-cancer therapy. Biomater. Sci. 2020, 8, 949–959. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Duro-Castano, A.; Sousa-Herves, A.; Armiñán, A.; Charbonnier, D.; Arroyo-Crespo, J.J.; Wedepohl, S.; Calderón, M.; Vicent, M.J. Polyglutamic acid-based crosslinked doxorubicin nanogels as an anti-metastatic treatment for triple negative breast cancer. J. Control. Release 2021, 332, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Perale, G.; Rossi, F. Nanogel functionalization: A versatile approach to meet the challenges of drug and gene delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541. [Google Scholar] [CrossRef]
- Cinay, G.E.; Erkoc, P.; Alipour, M.; Hashimoto, Y.; Sasaki, Y.; Akiyoshi, K.; Kizilel, S. Nanogel-integrated pH-responsive composite hydrogels for controlled drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Tsai, W.-B. Fabrication of photothermo-responsive drug-loaded nanogel for synergetic cancer therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, J.; Wang, Y.; Zhao, L.; Li, Y.; Liu, C. Formation of graphene oxide-hybridized nanogels for combinative anticancer therapy. Nanomed. NBM 2018, 14, 2387–2395. [Google Scholar] [CrossRef]
- Amanlou, N.; Parsa, M.; Rostamizadeh, K.; Sadighian, S.; Moghaddam, F. Enhanced cytotoxic activity of curcumin on cancer cell lines by incorporating into gold/chitosan nanogels. Mater. Chem. Phys. 2019, 226, 151–157. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Smart Core−Shell Hybrid Nanogels with Ag Nanoparticle Core for Cancer Cell Imaging and Gel Shell for pH-Regulated Drug Delivery. Chem. Mater. 2010, 22, 1966–1976. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Colucci, G.; Santamaria-Echart, A.; Silva, S.C.; Fernandes, I.P.M.; Sipoli, C.C.; Barreiro, M.F. Development of water-in-oil emulsions as delivery vehicles and testing with a natural antimicrobial extract. Molecules 2020, 25, 2105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Szczęch, M.; Szczepanowicz, K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. Nanomaterials 2020, 10, 496. [Google Scholar] [CrossRef] [Green Version]
- Ben David-Naim, M.; Grad, E.; Aizik, G.; Nordling-David, M.M.; Moshel, O.; Granot, Z.; Golomb, G. Polymeric nanoparticles of siRNA prepared by a double-emulsion solvent-diffusion technique: Physicochemical properties, toxicity, biodistribution and efficacy in a mammary carcinoma mice model. Biomaterials 2017, 145, 154–167. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Machado, A.; Lecommandoux, S.; Sandre, O.; Gu, F.; Colin, A. Controllable microfluidic production of drug-loaded plga nanoparticles using partially water-miscible mixed solvent microdroplets as a precursor. Sci. Rep. 2017, 7, 4794. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Lakshmi, B.A.; Kim, S.; Kim, J. Synthesis and characterization of acetyl curcumin-loaded core/shell liposome nanoparticles via an electrospray process for drug delivery, and theranostic applications. Eur. J. Pharm. Biopharm. 2019, 142, 518–530. [Google Scholar] [CrossRef]
- Zhang, S.; Campagne, C.; Salaün, F. Influence of solvent selection in the electrospraying; Process of polycaprolactone. Appl. Sci. 2019, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Baranov, M.; Olekhnovich, R.; Uspenskaya, M. Electrosprayed nanoparticles based on hyaluronic acid: Preparation and characterization. Technologies 2020, 8, 71. [Google Scholar] [CrossRef]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and challenges of engineering carriers for biomedical applications—A mini review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, M.; Reardon, P.J.T.; Knowles, J.C.; Browning, R.J.; Stride, E.; Pedley, R.B.; Grego, T.; Edirisinghe, M. Performance of novel high throughput multi electrospray systems for forming of polymeric micro/nanoparticles. Mater. Des. 2017, 126, 73–84. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, B.; Chen, Q.; Feng, Q.; Lin, L.; Sun, J. Point-of-care-testing of nucleic acids by microfluidics. TrAC Trends Anal. Chem. 2017, 94, 106–116. [Google Scholar] [CrossRef]
- Meng, Y.; Asghari, M.; Aslan, M.K.; Yilmaz, A.; Mateescu, B.; Stavrakis, S.; DeMello, A.J. Microfluidics for extracellular vesicle separation and mimetic synthesis: Recent advances and future perspectives. Chem. Eng. J. 2021, 404, 126110. [Google Scholar] [CrossRef]
- Nasiri, R.; Shamloo, A.; Ahadian, S.; Amirifar, L.; Akbari, J.; Goudie, M.J.; Lee, K.; Ashammakhi, N.; Dokmeci, M.R.; Di Carlo, D.; et al. Microfluidic-based approaches in targeted cell/particle separation based on physical properties: Fundamentals and applications. Small 2020, 16, 2000171. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Controlled and tunable polymer particles’ production using a single microfluidic device. Appl. Nanosci. 2018, 8, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, B.; Middha, E.; Huang, Z.; Hu, Q.; Fu, Z.; Liu, B. Microfluidics-prepared uniform conjugated polymer nanoparticles for photo-triggered immune microenvironment modulation and cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 11167–11176. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Cesteros Iturbe, L.C.; Katime, I. “Water dispersible pH-responsive chitosan nanogels modified with biocompatible crosslinking-agents”. Polymer 2012, 53, 3107–3116. [Google Scholar] [CrossRef]
- Radwan, R.R.; Ali, H.E. Radiation-synthesis of chitosan/poly (acrylic acid) nanogel for improving the antitumor potential of rutin in hepatocellular carcinoma. Drug Deliv. Transl. Res. 2021, 11, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Ditta, L.A.; Dahlgren, B.; Sabatino, M.A.; Dispenza, C.; Jonsson, M. The role of molecular oxygen in the formation of radiation-engineered multifunctional nanogels. Eur. Polym. J. 2019, 114, 164–175. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. Polymeric nano/microgels. In Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 8, pp. 845–871. [Google Scholar]
- Matusiak, M.; Kadlubowski, S.; Rosiak, J.M. Nanogels synthesized by radiation-induced intramolecular crosslinking of water-soluble polymers. Radiat. Phys. Chem. 2020, 169, 108099. [Google Scholar] [CrossRef]
- Pinelli, F.; Perale, G.; Rossi, F. Coating and functionalization strategies for nanogels and nanoparticles for selective drug delivery. Gels 2020, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Thummarati, P.; Suksiriworapong, J.; Sakchaisri, K.; Junyaprasert, V.B. Effect of chemical linkers of curcumin conjugated hyaluronic acid on nanoparticle properties and in vitro performances in various cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102323. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. pH-responsive hyaluronic acid-based nanoparticles for targeted curcumin delivery and enhanced cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Tian, L.; Qiu, Y.; Yu, Q.; Wang, X.; Guo, R.; He, Q. Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int. J. Pharm. 2020, 578, 119122. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Sharipov, M.; Huy, B.T.; Gerelkhuu, Z.; Biechele-Speziale, D.; Lee, Y.-I. Naturally modified nonionic alginate functionalized upconversion nanoparticles for the highly efficient targeted pH-responsive drug delivery and enhancement of NIR-imaging. J. Ind. Eng. Chem. 2018, 57, 424–435. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Paolì, A.; Naimo, G.D.; Mauro, L.; Amantea, D.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Self-assembling Dextran prodrug for redox- and pH-responsive co-delivery of therapeutics in cancer cells. Colloids Surf. B Biointerfaces 2020, 185, 110537. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-based drug delivery systems for remotely triggered cancer therapeutic and diagnostic applications. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Islam, M.A.; Xing, L.; Firdous, J.; Cao, W.; He, Y.-J.; Zhu, Y.; Cho, K.-H.; Li, H.-S.; Cho, C.-S. Degradable polyethylenimine-based gene carriers for cancer therapy. Top. Curr. Chem. 2017, 375, 34. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, R.J.; Liu, L.; Dong, S.; Zhang, J.; Zhang, Y.; Yao, Y.; Lu, J.; Meng, Q.; Xie, J.; et al. Multifunctional drug carrier based on PEI derivatives loaded with small interfering RNA for therapy of liver cancer. Int. J. Pharm. 2019, 564, 214–224. [Google Scholar] [CrossRef]
- Pang, S.-W.; Soon, M.L.-K.; Shameli, K.; Janarthanan, P.; Teow, S.-Y. Delivery of drug payloads to organs and organ-systems. Cell. Mol. Phytotoxicity Heavy Met. 2021, 199–224. [Google Scholar] [CrossRef]
- Chen, B.-W.; He, Y.-C.; Sung, S.-Y.; Le, T.T.H.; Hsieh, C.-L.; Chen, J.-Y.; Wei, Z.-H.; Yao, D.-J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, B.N.; Pereira, R.F.; Barrias, C.C.; Fischbach, C.; Oliveira, C.; Granja, P.L. Engineering modular half-antibody conjugated nanoparticles for targeting CD44v6-expressing cancer cells. Nanomaterials 2021, 11, 295. [Google Scholar] [CrossRef]
- Sadr, S.H.; Davaran, S.; Alizadeh, E.; Salehi, R.; Ramazani, A. PLA-based magnetic nanoparticles armed with thermo/pH responsive polymers for combination cancer chemotherapy. J. Drug Deliv. Sci. Technol. 2018, 45, 240–254. [Google Scholar] [CrossRef]
- Thauvin, C.; Widmer, J.; Mottas, I.; Hocevar, S.; Allémann, E.; Bourquin, C.; Delie, F. Development of resiquimod-loaded modified PLA-based nanoparticles for cancer immunotherapy: A kinetic study. Eur. J. Pharm. Biopharm. 2019, 139, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Guo, Z.; Wang, T.; Lin, L.; Chen, J.; Tian, H.; Chen, X. A pH-responsive detachable PEG shielding strategy for gene delivery system in cancer therapy. Biomacromolecules 2017, 18, 1342–1349. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121. [Google Scholar] [CrossRef]
- Razzaq, S.; Rauf, A.; Raza, A.; Tabish, T.A.; Rauf-ul-Hassan, M.; Shahnaz, G. Papain decorated multi-functional polymeric micelles for the targeted intracellular delivery of paclitaxel. Polym. Adv. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Simonson, A.W.; Lawanprasert, A.; Goralski, T.D.; Keiler, K.C.; Medina, S.H. Bioresponsive peptide-polysaccharide nanogels—A versatile delivery system to augment the utility of bioactive cargo. Nanomed. NBM 2019, 17, 391–400. [Google Scholar] [CrossRef]
- Clegg, J.R.; Sun, J.A.; Gu, J.; Venkataraman, A.K.; Peppas, N.A. Peptide conjugation enhances the cellular co-localization, but not endosomal escape, of modular poly (acrylamide-co-methacrylic acid) nanogels. J. Control. Release 2021, 329, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, A.D.; O’Connor, D.; Domling, A.; Goda, S.K. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed. Pharmacother. 2019, 113, 108750. [Google Scholar] [CrossRef] [PubMed]
- Massi, L.; Najer, A.; Chapman, R.; Spicer, C.D.; Nele, V.; Che, J.; Booth, M.A.; Doutch, J.J.; Stevens, M.M. Tuneable peptide cross-linked nanogels for enzyme-triggered protein delivery. J. Mater. Chem. B 2020, 8, 8894–8907. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Yu, Y.; He, C.; Tan, L.; Shen, Y.-M. RGD peptide-decorated micelles assembled from polymer–paclitaxel conjugates towards gastric cancer therapy. Colloids Surf. B 2019, 180, 58–67. [Google Scholar] [CrossRef]
- Ul Ahad, I.; Bartnik, A.; Fiedorowicz, H.; Kostecki, J.; Korczyc, B.; Ciach, T.; Brabazon, D. Surface modification of polymers for biocompatibility via exposure to extreme ultraviolet radiation. J. Biomed. Mater. Res. Part A 2013, 102, 3298–3310. [Google Scholar] [CrossRef] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, P.J.; Arun, K.J.; Navas, A.A.; Irene, J. Biomedical applications of polymers -An overview. Curr. Trends Biomed. Eng. Biosci. 2018, 15, 44–45. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Sadiku, E.R.; Jamiru, T.; Hamam, A.; Kupolatin, W.K. Applications of polymers in the biomedical field. Curr. Trends Biomed. Eng. Biosci. 2017, 4, 102–104. [Google Scholar]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- de las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Bioactive Polymers. In Synthetic Biodegradable Polymer Scaffolds; Birkhäuser Boston: Boston, MA, USA, 1997; pp. 83–95. [Google Scholar]
- Espinosa-Cano, E.; Palao-Suay, R.; Aguilar, M.R.; Vázquez, B.; Román, J.S. Polymeric nanoparticles for cancer therapy and bioimaging. Nanooncology 2018, 137–172. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Wang, K.; Qin, H.; Zhao, X.; Chen, W.; Xu, L.; Cao, W.; Guo, H. Internal cross-linked polymeric nanoparticles with dual sensitivity for combination therapy of muscle-invasive bladder cancer. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Esnaashari, S.S.; Muhammadnejad, S.; Amanpour, S.; Amani, A. A combinational approach towards treatment of breast cancer: An analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech 2020, 21, 21. [Google Scholar] [CrossRef]
- Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized polyethylene glycolylated polymer–lipid hybrid nanoparticles as a potential breast cancer treatment. Pharmaceutics 2020, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cortes, M.V.; Laz-Ruiz, J.A.; Diaz-Mochon, J.J.; Sanchez-Martin, R.M. Characterization and therapeutic effect of a pH stimuli responsive polymeric nanoformulation for controlled drug release. Polymers 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-Fluorouracil and Chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef]
- Zheng, W.; Li, M.; Lin, Y.; Zhan, X. Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed. Pharmacother. 2018, 108, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, L.; Dadashpour, M.; Sadeghzadeh, H.; Mahdavi, M.; Zarghami, N. Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, F.; Asadi, A.; Afsharpour, M.; Jamadi, R.H. In vitro characterization and evaluation of the cytotoxicity effects of nisin and nisin-loaded PLA-PEG-PLA nanoparticles on gastrointestinal (AGS and KYSE-30), hepatic (HepG2) and blood (K562) cancer cell lines. AAPS PharmSciTech 2018, 19, 1554–1566. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, X.; Chen, Y.; Lee, R.J.; Wang, J.; Yao, J.; Zhang, Y.; Zhang, C.; Wang, K.; Yu, B. Anticancer activity of polymeric nanoparticles containing linoleic acid-SN38 (LA-SN38) conjugate in a murine model of colorectal cancer. Colloids Surf. B 2019, 181, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol. Rep. 2019, 42, 2087–2096. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.F.; Santos, J.F.; Mattos, R.R.; Barros, E.G.O.; Nasciutti, L.E.; Cabral, L.M.; de Sousa, V.P. Characterization and in vitro antitumor activity of polymeric nanoparticles loaded with uncaria tomentosa extract. An. Acad. Bras. Cienc. 2020, 92, 1–16. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Sailaja, A.K.; Amareshwar, P.; Chakravarty, P. Chitosan nanoparticles as a drug delivery system. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 474–484. [Google Scholar]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Masson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-loaded photosensitizer-chitosan nanoparticles for combinatorial chemo- and photodynamic-therapy of cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Kansom, T.; Sajomsang, W.; Saeeng, R.; Charoensuksai, P.; Opanasopit, P.; Tonglairoum, P. Apoptosis induction and antimigratory activity of andrographolide analog (3a.1)-incorporated self-assembled nanoparticles in cancer cells. AAPS PharmSciTech 2018, 19, 3123–3133. [Google Scholar] [CrossRef]
- Zu, M.; Ma, L.; Zhang, X.; Xie, D.; Kang, Y.; Xiao, B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy. Colloids Surf. B 2019, 177, 399–406. [Google Scholar] [CrossRef]
- Faris, T.M.; Harisa, G.I.; Alanazi, F.K.; Samy, A.M.; Nasr, F.A. Developed simvastatin chitosan nanoparticles co-crosslinked with tripolyphosphate and chondroitin sulfate for ASGPR-mediated targeted HCC delivery with enhanced oral bioavailability. Saudi Pharm. J. 2020, 28, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Racoviceanu, R.; Trandafirescu, C.; Voicu, M.; Ghiulai, R.; Borcan, F.; Dehelean, C.; Watz, C.; Aigner, Z.; Ambrus, R.; Coricovac, D.E.; et al. Solid polymeric nanoparticles of albendazole: Synthesis, physico-chemical characterization and biological activity. Molecules 2020, 25, 5130. [Google Scholar] [CrossRef] [PubMed]
- Mughees, M.; Wajid, S.; Samim, M. Cytotoxic potential of Artemisia absinthium extract loaded polymeric nanoparticles against breast cancer cells: Insight into the protein targets. Int. J. Pharm. 2020, 586, 119583. [Google Scholar] [CrossRef]
- Mamnoon, B.; Feng, L.; Froberg, J.; Choi, Y.; Sathish, V.; Mallik, S. Hypoxia-responsive, polymeric nanocarriers for targeted drug delivery to estrogen receptor-positive breast cancer cell spheroids. Mol. Pharm. 2020, 17, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Sahoo, R.K.; Nakhate, K.T.; Ajazuddin; Gupta, U. Biotinylated HPMA centered polymeric nanoparticles for Bortezomib delivery. Int. J. Pharm. 2020, 579, 119173. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vlies, A.J.; Morisaki, M.; Neng, H.I.; Hansen, E.M.; Hasegawa, U. Framboidal nanoparticles containing a curcumin-phenylboronic acid complex with antiangiogenic and anticancer activities. Bioconjug. Chem. 2019, 30, 861–870. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Zhang, X.; He, C.; Zhao, P.; Li, M.; Fan, T.; Yan, R.; Lu, Y.; Lee, R.J.; et al. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm. Sin. B 2020, 10, 1106–1121. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic acid decorated naringenin nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Han, Q.; Liu, H.; Zhu, C.; Gui, W.; Yang, X.; Li, W. Precise engineering of Gemcitabine prodrug cocktails into single polymeric nanoparticles delivery for metastatic thyroid cancer cells. Drug Deliv. 2020, 27, 1063–1072. [Google Scholar] [CrossRef]
- Ghassami, E.; Varshosaz, J.; Jahanian-Najafabadi, A.; Minaiyan, M.; Rajabi, P.; Hayati, E. Pharmacokinetics and in vitro/in vivo antitumor efficacy of aptamer-targeted ecoflex® nanoparticles for docetaxel delivery in ovarian cancer. Int. J. Nanomed. 2018, 13, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos-Silva, A.M.; de Caland, L.B.; do Nascimento, E.G.; de Oliveira, A.L.C.S.L.; de Araújo-Júnior, R.F.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Self-assembled benznidazole-loaded cationic nanoparticles containing cholesterol/sialic acid: Physicochemical properties, in vitro drug release and in vitro anticancer efficacy. Int. J. Mol. Sci. 2019, 20, 2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.T.; Lin, H.; Wang, C.S.; Chang, C.H.; Lin, A.M.Y.; Yang, J.C.H.; Lo, Y.L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Beck Schimmer, B.H. Determination of Blood Tumor Cells. Available online: https://clinicaltrials.gov/ct2/show/NCT04290923 (accessed on 8 May 2021).
- Abdellatif, A.A.H. Targeted Polymeric Nanoparticles Loaded with Cetuximab and Decorated with Somatostatin Analogue to Colon Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03774680 (accessed on 8 May 2021).
- Epperla, N. Nanochip Technology in Monitoring Treatment Response and Detecting Relapse in Participants with Diffuse Large B-Cell Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03656835 (accessed on 9 May 2021).
- Verry, C. Radiotherapy of Multiple Brain Metastases Using AGuIX® (NANORAD2). Available online: https://clinicaltrials.gov/ct2/show/NCT03818386 (accessed on 9 May 2021).
- Abdellatif, A.A.H. Topical Fluorescent Nanoparticles Conjugated Somatostatin Analog for Suppression and Bioimaging Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04138342 (accessed on 8 May 2021).
- Ottevanger, P.B. Dose Escalation Study of Immunomodulatory Nanoparticles (PRECIOUS-01). Available online: https://clinicaltrials.gov/ct2/show/NCT04751786 (accessed on 8 May 2021).
- John John Preoperative Detection of Lymph Node Metastases in Pancreatic and Periampullary Carcinoma Using USPIO MRI (NANO-PANC). Available online: https://clinicaltrials.gov/ct2/show/NCT04311047 (accessed on 10 May 2021).
- Karakatsanis, A. Delayed Sentinel Lymph Node Biopsy in Ductal Cancer in Situ (SENTINOT_2). Available online: https://clinicaltrials.gov/ct2/show/NCT04722692 (accessed on 9 May 2021).
- Kirichenko, A. Radiotherapy with Iron Oxide Nanoparticles (SPION) on MR-Linac for Primary & Metastatic Hepatic Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT04682847 (accessed on 7 May 2021).
- Koay, E.J. NBTXR3 Activated by Radiation Therapy for the Treatment of Locally Advanced or Borderline-Resectable Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04484909 (accessed on 6 May 2021).
- Phan, J. Re-irradiation With NBTXR3 in Combination with Pembrolizumab for the Treatment of Inoperable Locoregional Recurrent Head and Neck Squamous Cell Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04834349 (accessed on 10 May 2021).
- Reddy, J. NBTXR3, Radiation Therapy, and Pembrolizumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04862455 (accessed on 9 May 2021).
- Cranmer, L. Nanoparticle Albumin-Bound Rapamycin and Pazopanib Hydrochloride in Treating Patients with Advanced Nonadipocytic Soft Tissue Sarcomas. Available online: https://clinicaltrials.gov/ct2/show/NCT03660930 (accessed on 10 May 2021).
- Cramer, S.L. Nanoparticle Albumin-Bound Rapamycin, Temozolomide, and Irinotecan Hydrochloride in Treating Pediatric Patients with Recurrent or Refractory Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02975882 (accessed on 7 May 2021).
- Jameson, G.S. Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) (AA NABPLAGEM). Available online: https://clinicaltrials.gov/ct2/show/NCT03410030 (accessed on 7 May 2021).
- Jameson, G. Nab-Paclitaxel + Cisplatin + Gemcitabine in Untreated Metastatic Pancreatic Adenocarcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03915444 (accessed on 7 May 2021).
- Abushahin, L. Biologically Optimized Infusion Schedule of Gemcitabine and Nab-Paclitaxel for the Treatment of Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04115163 (accessed on 9 May 2021).
- El-Rayes, B.F. Paricalcitol and Hydroxychloroquine in Combination with Gemcitabine and Nab-Paclitaxel for the Treatment of Advanced or Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04524702 (accessed on 6 May 2021).
- Dotan, E. Comparing Two Treatment Combinations, Gemcitabine and Nab-Paclitaxel With 5-Fluorouracil, Leucovorin, and Liposomal Irinotecan for Older Patients with Pancreatic Cancer That Has Spread. Available online: https://clinicaltrials.gov/ct2/show/NCT04233866 (accessed on 9 May 2021).
- Gillanders, W.E. Testing the Addition of an Individualized Vaccine to Nab-Paclitaxel, Durvalumab and Tremelimumab and Chemotherapy in Patients with Metastatic Triple Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03606967 (accessed on 9 May 2021).
- Shroff, R. Gemcitabine Hydrochloride and Cisplatin with or without Nab-Paclitaxel in Treating Patients with Newly Diagnosed Advanced Biliary Tract Cancers. Available online: https://clinicaltrials.gov/show/NCT03768414 (accessed on 9 May 2021).
- Maithel, S. Gemcitabine, Cisplatin, and Nab-Paclitaxel Before Surgery in Patients with High-Risk Liver Bile Duct Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03579771 (accessed on 7 May 2021).
- Habermann, T. Nab-paclitaxel/Rituximab-coated Nanoparticle AR160 in Treating Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03003546 (accessed on 8 May 2021).
- Neal, J.W. Testing the Addition of the Pill Chemotherapy, Cabozantinib, to the Standard Immune Therapy Nivolumab Compared to Standard Chemotherapy for Non-small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04310007 (accessed on 6 May 2021).
- Gao, S. Neoadjuvant Chemotherapy With Nab-paclitaxel Plus Cisplatin and Capecitabine for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04390958 (accessed on 9 May 2021).
- Li, Y. Efficacy and Safety of Preoperative Sintilimab Plus Nab-paclitaxel and Cisplatin in BR-ESCC Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04548440 (accessed on 10 May 2021).
- ABI-009 (Nab-rapamycin) in Recurrent High Grade Glioma and Newly Diagnosed Glioblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03463265 (accessed on 6 May 2021).
- Damodaran, S. Nab-paclitaxel and Alpelisib for the Treatment of Anthracycline Refractory Triple Negative Breast Cancer With PIK3CA or PTEN Alterations. Available online: https://clinicaltrials.gov/ct2/show/NCT04216472 (accessed on 8 May 2021).
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Mehrgardi, M.A. Investigation of different targeting decorations effect on the radiosensitizing efficacy of albumin-stabilized gold nanoparticles for breast cancer radiation therapy. Eur. J. Pharm. Sci. 2019, 130, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Biological evaluation of redox-sensitive micelles based on hyaluronic acid-deoxycholic acid conjugates for tumor-specific delivery of paclitaxel. Int. J. Pharm. 2015, 483, 38–48. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.H.; Jeong, J.H.; Ku, S.K.; Choi, H.G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Zhu, S.; Jiang, Y.; Tang, G.; Sun, C.; Fang, C.; Shi, B.; Pei, Y. Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles. J. Control. Release 2010, 141, 22–29. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Sistla, R. Peptide conjugated polymeric nanoparticles as a carrier for targeted delivery of docetaxel. Colloids Surf. B 2014, 117, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, W.J. Polymeric nanoparticles conjugate a novel heptapeptide as an epidermal growth factor receptor-active targeting ligand for doxorubicin. Int. J. Nanomed. 2012, 7, 4749–4767. [Google Scholar]
- Kapoor, A.; Kumar, S. Cancer stem cell: A rogue responsible for tumor development and metastasis. Indian J. Cancer 2014, 51, 282–289. [Google Scholar]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H. Nanotherapeutics: From Laboratory to Clinic; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429183317. [Google Scholar]
- Eslami, P.; Rossi, F.; Fedeli, S. Hybrid nanogels: Stealth and biocompatible structures for drug delivery applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In vitro methods for assessing nanoparticle toxicity. In Methods in Molecular Biology; Springer: Cham, Switzerland, 2019; pp. 1–29. [Google Scholar]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Yang, J.; Duan, Y.; Zhang, X.; Wang, Y.; Yu, A. Modulating the cellular microenvironment with disulfide-containing nanoparticles as an auxiliary cancer treatment strategy. J. Mater. Chem. B 2016, 4, 3868–3873. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Chiang, C.S.; Lin, Y.J.; Lee, R.; Lai, Y.H.; Cheng, H.W.; Hsieh, C.H.; Shyu, W.C.; Chen, S.Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic mesoporous silica nanoparticles cloaked by red blood cell membranes: Applications in cancer therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Tian, X.; Chong, Y.; Ge, C. Understanding the nano–bio interactions and the corresponding biological responses. Front. Chem. 2020, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Chandra, S. Inorganic hybrid nanoparticles in cancer theranostics: Understanding their combinations for better clinical translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]

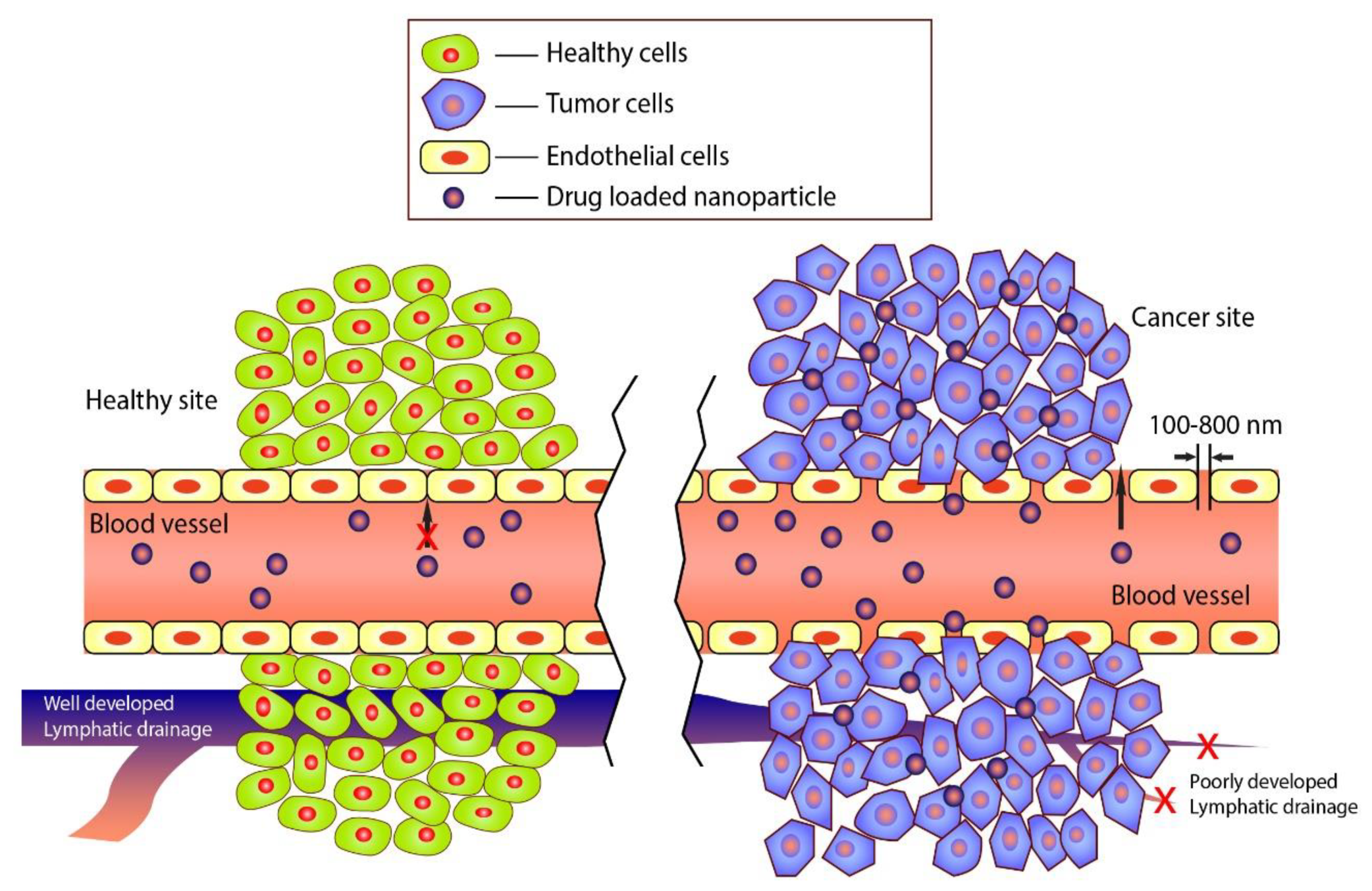
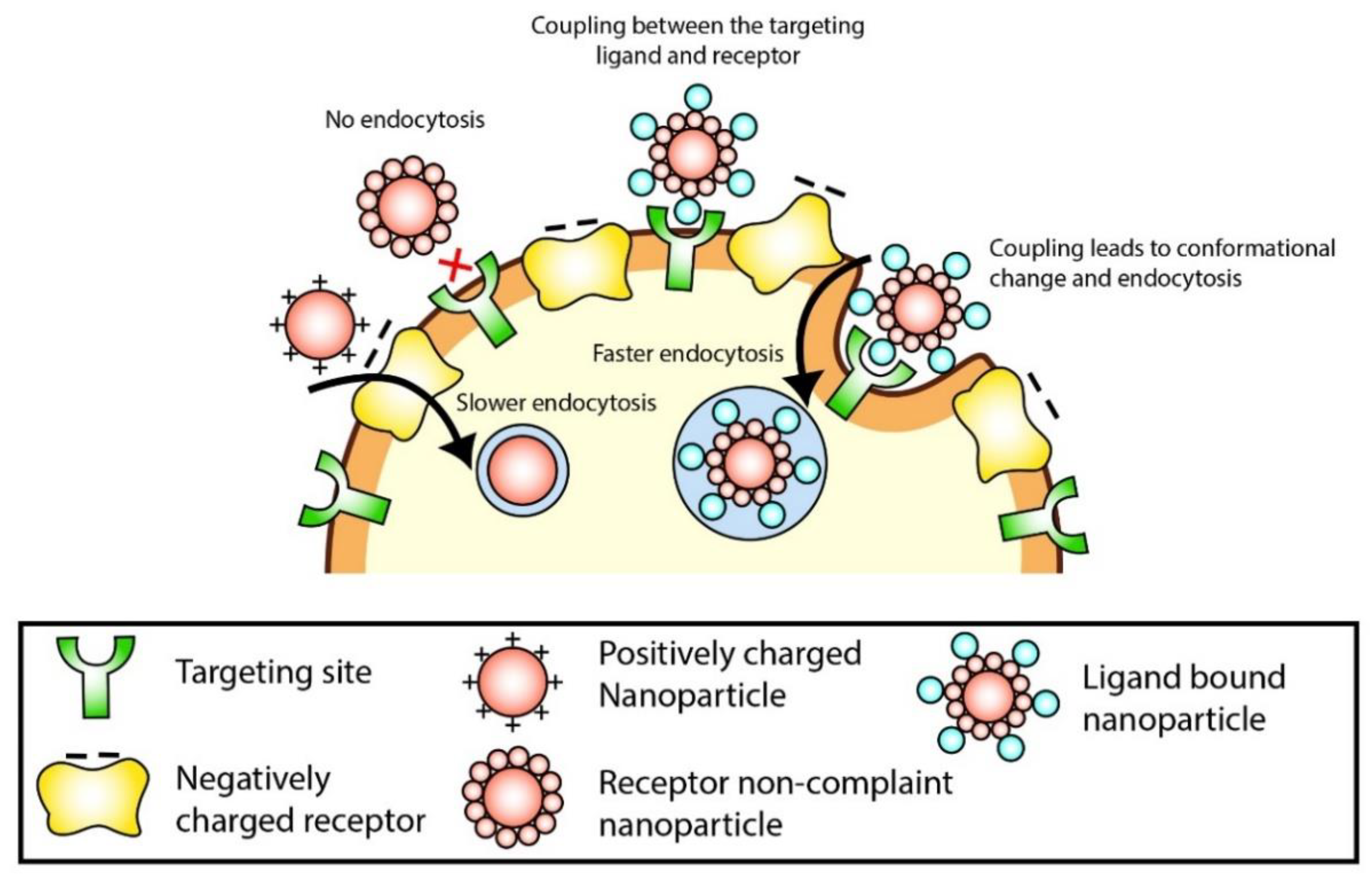
| Nanoparticle | Polymer and Additives | Function of Polymer | Drug/Anticancer Compound | Cancer Type | Tested Model | Target Action | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Doxorubicin-IR780-PEG-PCL-SS NPs (DOX IR780-PEG-PCL-SS NPs) | PEG-PCL-SS | Drug delivery | Doxorubicin | Bladder Cancer | MB49 cells (Mouse, C57BL/Icrf-a’) | NIR laser-controlled drug release and imaging guidance for chemo-photothermal synergistic therapy reduce tumor size and inhibit growth | 2020 | [116] |
| Albendazole-loaded polyurethane NPs (ABZ-polyurethane NPs) | Polyurethane | Compatible to ABZ, better drug delivery | Albendazole | Breast Cancer | MCF-7, MDA-MB-231 cells | Apoptosis, increase ABZ anticancer potency | 2020 | [135] |
| Noscapine-loaded mPEG-PLGA NPs (NOS-mPEG-PLGA-NPs) | mPEG-PLGA | Anticancer effect of Noscapine improved when encapsulated in nanoparticles compared to free form | Noscapine | Breast Cancer | 4T1 cells, 4T1 in BALB/c | Antiangiogenic, apoptotic effects | 2020 | [117] |
| mertansine (MRT) or cabazitaxel (CBZ) loaded TPC–CS NPs (MRT/CBZ-TPC-CS NPs) | Chitosan (CS) + tetraphenylchlorin (TPC) | Increase drug loading | Mertansine/Cabazitaxel | Breast Cancer | MDA-MB-231, MDA-MB-468 cells | MRT or CBZ had higher cytotoxic effect compared to free drug | 2020 | [130] |
| A. absinthium extract loaded polymeric nanoparticles (NVA-AA) | NIPAAM-VP-AA | Drug delivery | Artemisia absinthium extract | Breast Cancer | MCF-7, MDA MB-231 cells | Induces cytotoxicity, inhibition of cellular proliferation, induction of apoptosis | 2020 | [136] |
| Bortezomib (BTZ) loaded PNPs of HPLA-BT NPs | HPLA-BT | Drug delivery, higher drug load | Bortezomib | Breast Cancer | MCF-7 cells | Higher cytotoxic effects of DL (drug loaded) -HPLA-BT PNPs and significant anticancer activity | 2020 | [138] |
| Anastrozole loaded PEGylated polymer–lipid hybrid nanoparticles (ANZ -PLNPs) | PEG and lipid | stable encapsulated system with a high percentage of entrapment efficiency | Anastrozole | Breast Cancer | MCF-7 cells | Induction of apoptosis | 2020 | [118] |
| Estradiol-conjugated hypoxia-responsive polymeric nanoparticles encapsulating doxorubicin | PLA17000-PEG2000-Estradiol | targeted delivery into the hypoxic niches of estrogen-receptor-positive breast cancer microtumors | Doxorubicin | Breast Cancer | MCF7 cells | Higher cytotoxicity of targeted polymersomes in hypoxia compared to in normoxia | 2020 | [137] |
| quercetin loaded chitosan nanoparticles (QCT-CS NPs) | Chitosan | Better drug delivery, enhanced encapsulation efficiency and sustained release property | Quercetin | Breast Cancer | MDA-MB-468 cells | Cytotoxicity, decrease tumor growth | 2018 | [131] |
| Lung Cancer | A549 cells | |||||||
| DOX-loaded PEGylated therapeutic nanosystem for pH-sensitive release | PEG | releasing the drug in a controlled manner at acidic pH, increasing efficacy compared to doxorubicin in solution | Doxorubicin | Breast Cancer | MDA-MB-231 cells | Better anti-tumor activity, inhibits cell proliferation | 2020 | [119] |
| Lung Cancer | A549, H520 cells | |||||||
| 3A.1-loaded pH-sensitive chitosan nanoparticles | naphthyl-grafted succinyl chitosan (NSC), octyl-grafted succinyl chitosan (OSC), and benzyl-grafted succinyl chitosan (BSC) | delivering anticancer drugs to the targeted colon cancer sites | Andrographolide analog | Colon Cancer | HT-29 cells | significantly lower IC50 than free drug and promotes apoptosis | 2018 | [132] |
| Linoleic acid conjugated SN38 (LA-SN38)-loaded NPs (EBNPs) | PEO-PBO diblock copolymer | EBNPs had high drug loading efficiency and entrapment efficiency for LA-SN38, release behaviour of EBNPs was slow and sustained | Linoleic acid conjugated SN38 | Colon Cancer | HCT-116, HT-29 cells | Growth inhibitory effects, EBNPs promotes the uptake in cancer cells. EBNPs had prolonged blood circulation time. | 2019 | [124] |
| Cur-loaded phenylboronic acid-containing framboidal nanoparticles | PBAAM, PEGAM, MBAM | Improved chemical stability of Cur and sustained release under physiological conditions | Curcumin | Colon Cancer | HT-29 cells | Antiangiogenic, reduced tumor weight | 2019 | [139] |
| Chondroitin sulphate functionalized campththecin-loaded polymeric nanoparticles (CS-CPT-NPs) | Chitosan | Targeted drug delivery | Campththecin | Colon Cancer | CT-26 cells (Mouse, BALB/c) | significantly improved the anti-colon cancer activities, promote apoptosis effects | 2019 | [133] |
| Afatinib or miR- loaded polylactic-co-glycolic acid surrounded by PEG-lipids (shell modified with ligand R and pH-sensitive CPP H) nanoparticles (Afatinib or miR-loaded PLGA NPs) | PLGA | Protect Afatinib and miR, improve drug delivery | Afatinib/miR | Colon Cancer | Caco-2 cells | pH-responsive characteristics to increase the sensitivity of colon cancer cells to afatinib. | 2019 | [146] |
| 5-FU-Chrysin-loaded PLGA-PEG-PLGA nanoparticles (5FU-Chrysin-PLGA-PEG-PLGA NPs) | PLGA-PEG-PLGA | Improve the functional delivery efficacy of 5-FU and Chrysin in cancer | 5-FU, Chrysin | Colon Cancer | HT-29 cells | Apoptosis, growth inhibitory effects | 2020 | [120] |
| Simvastatin (SV) chitosan nanoparticles co-crosslinked with tripolyphosphate and chondroitin sulfate (SVSChSNPs) | Chitosan co-crosslinked with tripolyphosphate and chondroitin sulfate | Control the release pattern of SV. Particle size and positive surface charge of NPs enhances the accumulation of SV in intracellular compartments. | Simvastatin | Hepatic Cancer | HepG2 cells | enhanced the cytotoxicity of SV against HepG2 cells owing to its enhanced cellular uptake. ChS improved oral bioavailability | 2020 | [134] |
| Naringenin-loaded Hyaluronic acid (HA) decorated PCL NPs (NAR-HA@CH-PCL-NP) | PCL | Drug delivery | Naringenin | Lung cancer | A549 cells | Cytotoxic effect and active targeting of NAR-HA@CH-PCL-NP. Further treatment with NAR-HA@CH-PCL-NP was found effective in tumor growth inhibitory effect against urethane-induced lung cancer in rat | 2018 | [142] |
| EGFR-targeted LPNs loaded with CDDP and DOX | EGF-PEG-DSPE | Target drug delivery, faster release of DOX from LPNs than CDDP. | Doxorubicin | Lung Cancer | A549 cells | Improved anticancer activity with lower toxicity. Drug-loaded LPNs improved cytotoxicity | 2019 | [125] |
| platinum–curcumin complexes loaded into pH and redox dual-responsive nanoparticles (PteCUR@PSPPN) | mPEG-SS-PBAE-PLGA | control intracellular release, synergistic anticancer effects | Platinum–curcumin | Lung Cancer | A549 cells | Synergistic anticancer effects, enhanced anti-metastatic activity | 2019 | [140] |
| sorafenib (SF)-loaded cationically-modified polymeric nanoparticles (NPs) | PLGA | aerosolization efficiency for pulmonary delivery | Sorafenib | Lung Cancer | A549 cells | enhanced cell migration inhibition, reduction in cell survival, inhibition in the formation of colonies | 2020 | [141] |
| Uncaria tomentosa extract (UT)-PLGA & UTPCL | PCL and PLGA | Better drug delivery—UT-PLGA nanoparticles showed higher drug loading | Uncaria tomentosa extract | Prostate Cancer | LNCaP, DU145 cells | UT-PLGA showed higher cytotoxicity towards DU145 cells, UTPCL showed higher cytotoxicity against LNCaP cells | 2019 | [127] |
| Gemcitabine (GEM) NPs conjugated with linoleic acid (GEM NPs) | Linoleic acid | high drug-load, controlled release, improved intracellular uptake | Gemcitabine | Thyroid Cancer | B-CPAP, FTC-133 cells | Enhanced cytotoxic activity, induces apoptosis | 2020 | [143] |
| Ecoflex® NPs loaded with DTX (DTX-NPs) | PEG 6000 | Targeted drug delivery | Docetaxel | Ovarian Cancer | SKOV-3, MDA-468 cells | Increase antitumor efficacy, enhanced cellular uptake. | 2018 | [144] |
| DOX-verapamil/MPEG-PLA nanoparticles (DOX-VER-MPEG-PLA) | MPEG-PLA | co-delivery system –efficiently coencapsulate verapamil and chemotherapeutic agents. | Doxorubicin, Verapamil | Ovarian Cancer | A2780, SKOV3 cells | Tumor suppression | 2018 | [121] |
| Metformin-loaded PLGA-PEG nanoparticles (MET-PLGA-PEG NPs) | PLGA-PEG | Improve drug delivery | Metformin | Ovarian Cancer | SKOV3 cells | Increased nuclei fragmentation and amount of apoptotic cells induced by MET-NPs, enhance ani-cancer effects | 2018 | [122] |
| Curcumin (Cur)- loaded Polymeric poly(lactic-co-glycolic acid) (PLGA) nanoparticles (Cur-PLGA NPs) | PLGA | Stabilize curcumin in the presence of light, improved serum stability compared to free curcumin | Curcumin | Ovarian Cancer | SKOV3 cells | Cytotoxic effects on tumor cells upon irradiation at a low intensity inhibit tumor growth | 2019 | [128] |
| Nisin-loaded PLA-PEG-PLA nanoparticles | PLA-PEG-PLA | Better protection and sustained release for nisin | Nisin | Gastrointestinal Cancer | AGS, KYSE-30 cells | Higher cytotoxic effect in nisin-loaded NPs, increase cell growth reduction when comparing to free nisin | 2018 | [123] |
| Hepatic Cancer | Hep-G2 cells | |||||||
| Blood Cancer | K562 cells | |||||||
| Benznidazoles (BNZ)-loaded cationic polymeric nanoparticles (NPs) (BNZ-SA-Chol-PMMA NPs) | cationic polymethyl-methacrylate (PMMA) NPs | Improves drug efficacy | Benznidazoles | Colon Cancer | HT-29 cells | BNZ-NPs improved anticancer effect | 2019 | [145] |
| Cervical Cancer | HeLa cells | |||||||
| Hepatic Cancer | Hep-G2 cells |
| Advantages | Disadvantages/Limitations |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels 2021, 7, 60. https://doi.org/10.3390/gels7020060
Neerooa BNHM, Ooi L-T, Shameli K, Dahlan NA, Islam JMM, Pushpamalar J, Teow S-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels. 2021; 7(2):60. https://doi.org/10.3390/gels7020060
Chicago/Turabian StyleNeerooa, Bibi Noorheen Haleema Mooneerah, Li-Ting Ooi, Kamyar Shameli, Nuraina Anisa Dahlan, Jahid M. M. Islam, Janarthanan Pushpamalar, and Sin-Yeang Teow. 2021. "Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update" Gels 7, no. 2: 60. https://doi.org/10.3390/gels7020060
APA StyleNeerooa, B. N. H. M., Ooi, L.-T., Shameli, K., Dahlan, N. A., Islam, J. M. M., Pushpamalar, J., & Teow, S.-Y. (2021). Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels, 7(2), 60. https://doi.org/10.3390/gels7020060