Nanogels as a Versatile Drug Delivery System for Brain Cancer
Abstract
1. Introduction
2. Gliadel Wafers for Postsurgical Brain Cancer Treatment
3. Nanogel
3.1. Overview and Preparation of Nanogels
| Assembly | Reactions | Properties | References |
|---|---|---|---|
| Physical | Micellar | Self-assembly using triblock copolymers or branched polymers | [31,34] |
| Hybrid (nanoparticles suspended in hydrogels) | Nanoparticles immobilized in hydrogels | [35,41,42,43,44,45] | |
| Cross-linking | Disulfide | Cross-linked via thiol-disulfide exchange reaction, Cleaved in response to glutathione | [36,37,46] |
| Amide | High reactivity with carboxylic acids, activated esters, isocyanates and iodides | [38,47] | |
| Imine | Stable under physiological conditions and labile at acidic pH | [39,48] | |
| Photo-induced | Photo-induced cross-linking or cleavage | [40,49] |
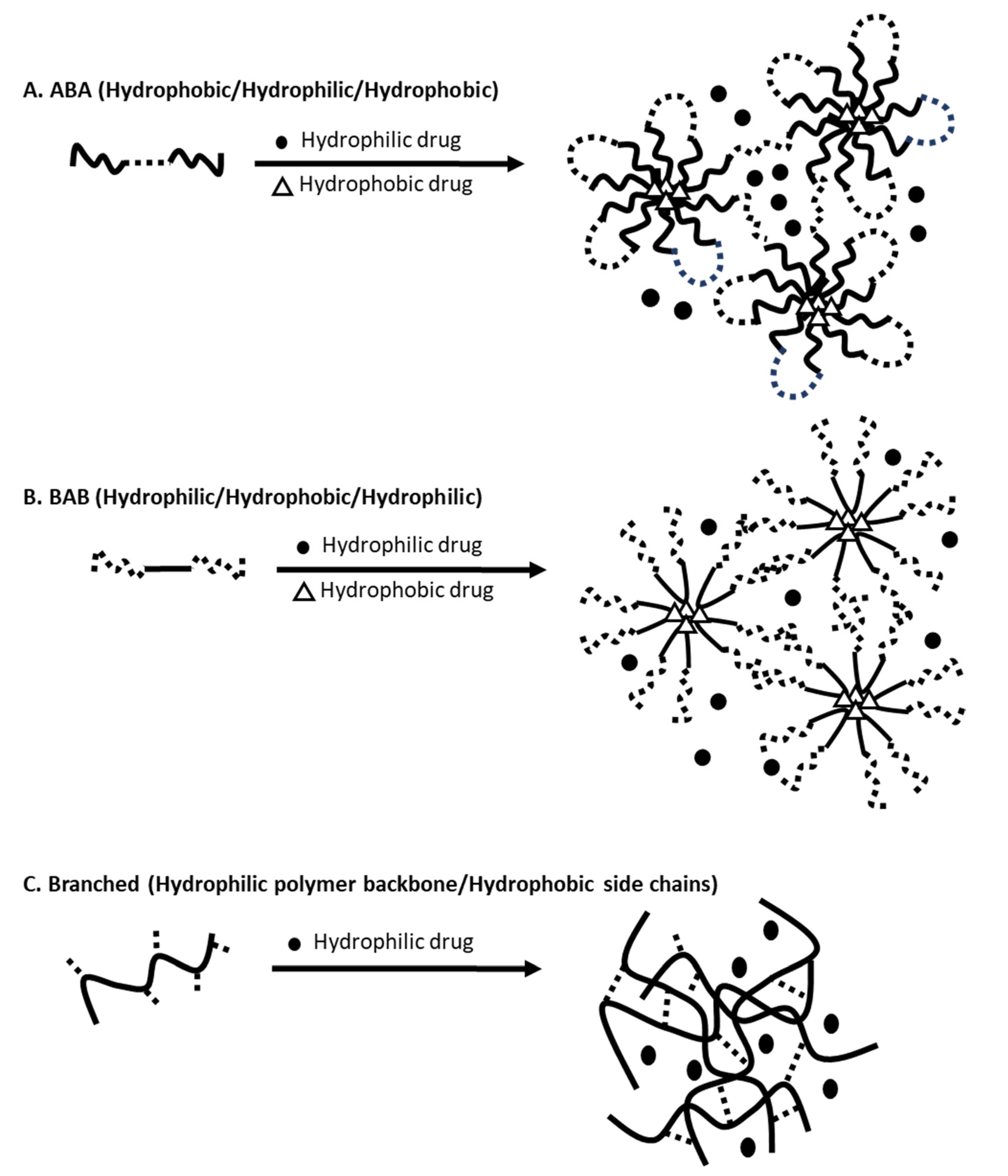
3.2. Desired Properties of Nanogels for Drug Delivery
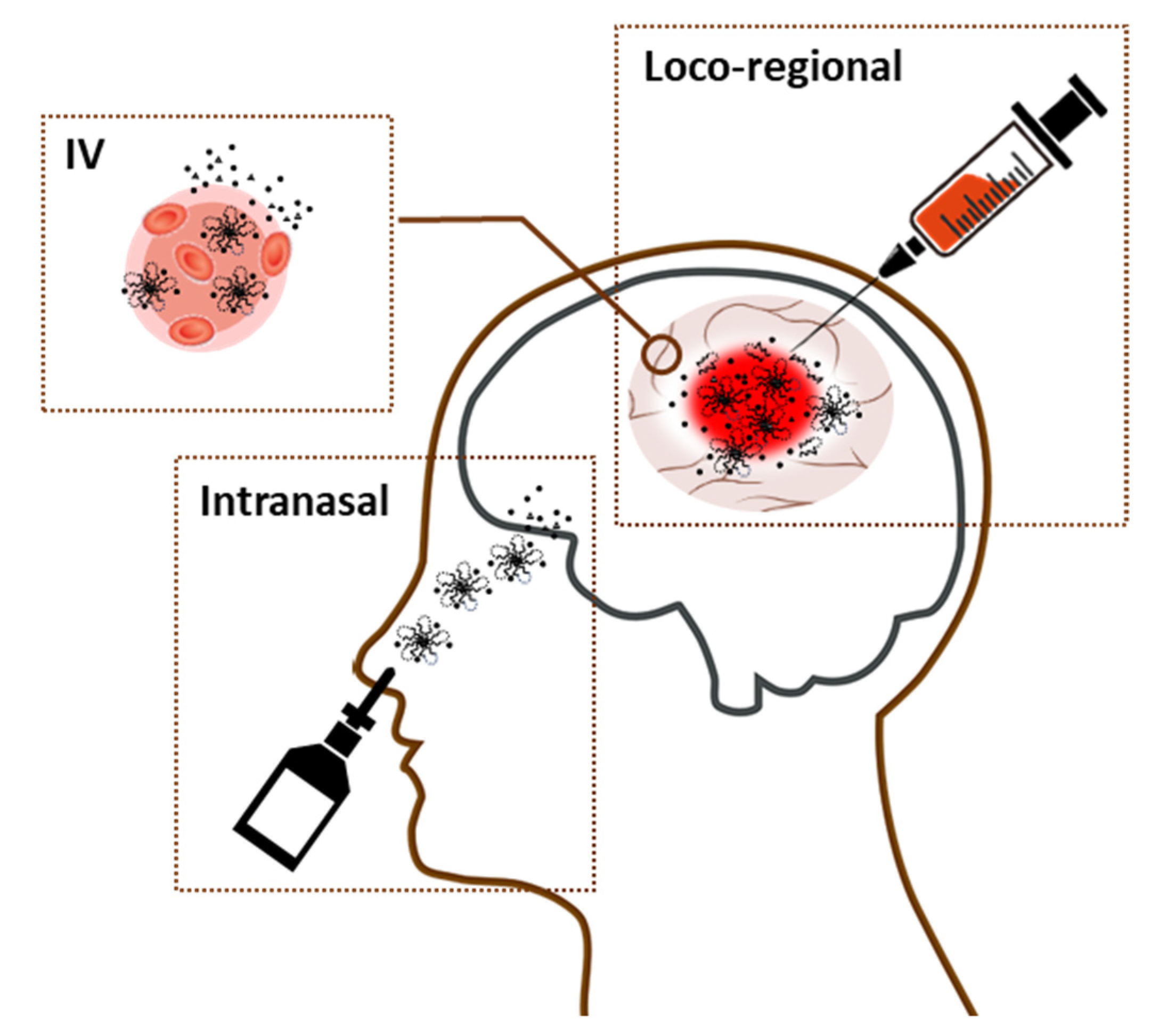
4. Nanogels That Deliver Drugs to Brain
4.1. Nanogels That Cross the BBB
4.2. Nanogel Use in Brain Cancers
5. Challenges and Prospects for Nanogel-Based Drug Delivery to Brain
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woodworth, G.F.; Dunn, G.P.; Nance, E.A.; Hanes, J.; Brem, H. Emerging Insights into Barriers to Effective Brain Tumor Therapeutics. Front. Oncol. 2014, 4, 126. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 1–12. [Google Scholar] [CrossRef]
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S.; Roselind, F.S.; et al. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef]
- Ahmed, R.; Oborski, M.J.; Hwang, M.; Lieberman, F.S.; Mountz, J.M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harbor Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Wick, W.; Winkler, F. Regimen of procarbazine, lomustine, and vincristine versus temozolomide for gliomas. Cancer 2018, 124, 2674–2676. [Google Scholar] [CrossRef]
- Lassman, A.B. Procarbazine, lomustine and vincristine or temozolomide: Which is the better regimen? CNS Oncol. 2015, 4, 341–346. [Google Scholar] [CrossRef]
- Brada, M.; Stenning, S.; Gabe, R.; Thompson, L.C.; Levy, D.; Rampling, R.; Erridge, S.; Saran, F.; Gattamaneni, R.; Hopkins, K.; et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4601–4608. [Google Scholar] [CrossRef]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Smart, D. Radiation Toxicity in the Central Nervous System: Mechanisms and Strategies for Injury Reduction. Semin. Radiat. Oncol. 2017, 27, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Pekic, S.; Miljic, D.; Popovic, V. Hypopituitarism Following Cranial Radiotherapy. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; Endotext. MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel wafers in the treatment of malignant glioma: A systematic review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef]
- Wenbin Dang, T.D.; Peter, Y.; Yong, Z.; David, N.; Charles, S.C.; Betty, T.; Henry, B. Effects of GLIADEL® wafer initial molecular weight on the erosion of wafer and release of BCNU. J. Control. Release 1996, 42, 83–92. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jaaskelainen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Esrailian, E.; Laine, L.; Chamberlain, M.C. Clinical impact of adjuvant chemotherapy in glioblastoma multiforme: A meta-analysis. CNS Drugs 2007, 21, 775–787. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Miglierini, P.; Bouchekoua, M.; Rousseau, B.; Hieu, P.D.; Malhaire, J.P.; Pradier, O. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: Efficacy and toxicity. Clin. Neurol. Neurosurg. 2012, 114, 1222–1225. [Google Scholar] [CrossRef]
- Weber, E.L.; Goebel, E.A. Cerebral edema associated with Gliadel wafers: Two case studies. Neuro-Oncology 2005, 7, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.V.; Vitaz, T.W.; Morassutti, D.J.; Doyle, M.J.; Glisson, S.D.; Hargis, J.B.; Goldsmith, G.H.; Cervera, A.; Stribinskiene, L.; New, P. A phase II study of radiation with concomitant and then sequential temozolomide (TMZ) in patients (pts) with newly diagnosed supratentorial high grade malignant glioma (MG) who have undergone surgery with carmustine (BCNU) wafer insertion. J. Clin. Oncol. 2005, 23, 1547. [Google Scholar] [CrossRef]
- Salmaggi, A.; Milanesi, I.; Silvani, A.; Gaviani, P.; Marchetti, M.; Fariselli, L.; Solero, C.L.; Maccagnano, C.; Casali, C.; Guzzetti, S.; et al. Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: Preliminary results. J. Neurosurg. 2013, 118, 821–829. [Google Scholar] [CrossRef]
- Elstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)--clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef]
- Tyler, B.; Fowers, K.D.; Li, K.W.; Recinos, V.R.; Caplan, J.M.; Hdeib, A.; Grossman, R.; Basaldella, L.; Bekelis, K.; Pradilla, G.; et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J. Neurosurg. 2010, 113, 210–217. [Google Scholar] [CrossRef]
- Torres, A.J.; Zhu, C.; Shuler, M.L.; Pannullo, S. Paclitaxel delivery to brain tumors from hydrogels: A computational study. Biotechnol. Prog. 2011, 27, 1478–1487. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control. Release Off. J. Control. Release Soc. 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Giovannini, G.; Kunc, F.; Piras, C.C.; Stranik, O.; Edwards, A.A.; Hall, A.J.; Gubala, V. Stabilizing silica nanoparticles in hydrogels: Impact on storage and polydispersity. RSC Adv. 2017, 7, 19924–19933. [Google Scholar] [CrossRef]
- Ryu, J.H.; Jiwpanich, S.; Chacko, R.; Bickerton, S.; Thayumanavan, S. Surface-functionalizable polymer nanogels with facile hydrophobic guest encapsulation capabilities. J. Am. Chem. Soc. 2010, 132, 8246–8247. [Google Scholar] [CrossRef] [PubMed]
- Elkassih, S.A.; Kos, P.; Xiong, H.; Siegwart, D.J. Degradable redox-responsive disulfide-based nanogel drug carriers via dithiol oxidation polymerization. Biomater. Sci. 2019, 7, 607–617. [Google Scholar] [CrossRef]
- Kockelmann, J.; Stickdorn, J.; Kasmi, S.; De Vrieze, J.; Pieszka, M.; Ng, D.Y.W.; David, S.A.; De Geest, B.G.; Nuhn, L. Control over Imidazoquinoline Immune Stimulation by pH-Degradable Poly(norbornene) Nanogels. Biomacromolecules 2020, 21, 2246–2257. [Google Scholar] [CrossRef]
- Liao, S.C.; Ting, C.W.; Chiang, W.H. Functionalized polymeric nanogels with pH-sensitive benzoic-imine cross-linkages designed as vehicles for indocyanine green delivery. J. Colloid Interface Sci. 2020, 561, 11–22. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive Nanogels Based on Photocontrollable Cross-Links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef]
- Levin, M.; Sonn-Segev, A.; Roichman, Y. Structural changes in nanoparticle-hydrogel composites at very low filler concentrations. J. Chem. Phys. 2019, 150, 064908. [Google Scholar] [CrossRef]
- Ayyub, O.B.; Kofinas, P. Enzyme Induced Stiffening of Nanoparticle-Hydrogel Composites with Structural Color. ACS Nano 2015, 9, 8004–8011. [Google Scholar] [CrossRef]
- Baek, K.; Liang, J.; Lim, W.T.; Zhao, H.; Kim, D.H.; Kong, H. In situ assembly of antifouling/bacterial silver nanoparticle-hydrogel composites with controlled particle release and matrix softening. ACS Appl. Mater. Interfaces 2015, 7, 15359–15367. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Takahashi, A.; Kuzuya, A. Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic. Int. J. Mol. Sci. 2018, 19, 1606. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.P.; Tan, M.B.; Tam, M.K. Application of nanogel systems in the administration of local anesthetics. Local Reg. Anesth. 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Tan, H.; Jin, H.; Mei, H.; Zhu, L.; Wei, W.; Wang, Q.; Liang, F.; Zhang, C.; Li, J.; Qu, X.; et al. PEG-urokinase nanogels with enhanced stability and controllable bioactivity. Soft Matter 2012, 8, 2644–2650. [Google Scholar] [CrossRef]
- Escobedo, H.D.; Stansbury, J.W.; Nair, D.P. Photoreactive nanogels as versatile polymer networks with tunable in situ drug release kinetics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103755. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for Pharmaceutical and Biomedical Applications and Their Fabrication Using 3D Printing Technologies. Materials 2018, 11. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Pereira, P.; Pedrosa, S.S.; Correia, A.; Lima, C.F.; Olmedo, M.P.; Gonzalez-Fernandez, A.; Vilanova, M.; Gama, F.M. Biocompatibility of a self-assembled glycol chitosan nanogel. Toxicol. In Vitro 2015, 29, 638–646. [Google Scholar] [CrossRef]
- Oishi, M.; Nagasaki, Y. Stimuli-responsive smart nanogels for cancer diagnostics and therapy. Nanomedicine 2010, 5, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D Printing of Poloxamer 407 Nanogel Discs and Their Applications in Adjuvant Ovarian Cancer Therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, S.; Yao, W.; Wang, X.; Zha, Q.; Tang, R. Hyaluronic acid nanogels prepared via ortho ester linkages show pH-triggered behavior, enhanced penetration and antitumor efficacy in 3-D tumor spheroids. J. Colloid Interface Sci. 2017, 504, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.B.; Lee, G.B.; In, I.; Park, S.Y. pH-sensitive fluorescent hyaluronic acid nanogels for tumor-targeting and controlled delivery of doxorubicin and nitric oxide. Eur. Polym. J. 2018, 101, 96–104. [Google Scholar] [CrossRef]
- Salehi, R.; Rasouli, S.; Hamishehkar, H. Smart thermo/pH responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int. J. Pharm. 2015, 487, 274–284. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, J.; Yang, K.; Cai, P.; Xiao, H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111357. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Tang, R.; Liang, K.; Achilefu, S.; Kwon, G.S.; Cho, H. Proof-of-Concept of Polymeric Sol-Gels in Multi-Drug Delivery and Intraoperative Image-Guided Surgery for Peritoneal Ovarian Cancer. Pharm. Res. 2016, 33, 2298–2306. [Google Scholar] [CrossRef]
- Su, S.; Wang, H.; Liu, X.; Wu, Y.; Nie, G. iRGD-coupled responsive fluorescent nanogel for targeted drug delivery. Biomaterials 2013, 34, 3523–3533. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Xu, W.; Sun, T.; Xiao, H.; Zhuang, X.; Chen, X. Receptor and Microenvironment Dual-Recognizable Nanogel for Targeted Chemotherapy of Highly Metastatic Malignancy. Nano Lett. 2017, 17, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Ribovski, L.; de Jong, E.; Mergel, O.; Zu, G.; Keskin, D.; van Rijn, P.; Zuhorn, I.S. Low nanogel stiffness favors nanogel transcytosis across an in vitro blood–brain barrier. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102377. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood–Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Jo, J.-I.; Yoshida, F.; Hong, Z.; Tabata, Y.; Sumiyoshi, A.; Taguchi, M.; Aoki, I. Ultra-small size gelatin nanogel as a blood brain barrier impermeable contrast agent for magnetic resonance imaging. Acta Biomater. 2021, 125, 290–299. [Google Scholar] [CrossRef]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Furtado, D.; Bjornmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Lin, F.-W.; Chen, P.-Y.; Wei, K.-C.; Huang, C.-Y.; Wang, C.-K.; Yang, H.-W. Rapid In Situ MRI Traceable Gel-forming Dual-drug Delivery for Synergistic Therapy of Brain Tumor. Theranostics 2017, 7, 2524–2536. [Google Scholar] [CrossRef] [PubMed]
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M.; et al. Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur. J. Pharm. Biopharm. 2020, 157, 108–120. [Google Scholar] [CrossRef]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderon, M.; Haag, R.; Satchi-Fainaro, R. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J. Control. Release Off. J. Control. Release Soc. 2016, 239, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Azadi, A.; Hamidi, M.; Rouini, M.R. Methotrexate-loaded chitosan nanogels as ’Trojan Horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
| Properties | Nanogels | Wafers | Nanoparticle-Based Liquid Dosage Forms |
|---|---|---|---|
| Route of administration | IV, implant, intratumoral, nasal | Implant | IV, intratumoral, nasal |
| Multi-drug delivery | Yes | Maybe (not known) | Yes |
| Delivery of hydrophobic drugs | Yes | Maybe (not known) | Yes |
| Form of drugs | Encapsulated in nanoparticles | Free form | Encapsulated in nanoparticles |
| Dose adjustment | Yes (via syringe) | Yes but manipulation needed (e.g., cutting, inserting multiple wafers) | Yes (via syringe) |
| Surface modification for targeted drug delivery | Yes | No | Yes |
| Long residence time | Yes | Yes | No |
| Controllable drug release | Yes (stimuli-responsive, diffusion followed by physical degradation) | Yes (physical erosion) | Yes (stimuli-responsive, diffusion followed by physical degradation) |
| Suitable for intratumoral injection | Yes | No | Yes |
| Available as a spray delivery system | Yes | No | Yes |
| Biocompatible and biodegradable | Yes | Yes | Yes |
| Convenience in handling | + | +++ | ++ |
| Conforming to the shape/size of the resection cavity post-surgery | Yes (intimate contacting with surrounding tissues) | No (stiff) | No (easily washed away by the interstitial fluid) |
| Properties | Nanogels |
|---|---|
| Gels | Bioadhesive, biocompatible, biodegradable, soft “tissue-like” texture, able to conform to the shape/size of the resection cavity |
| Nanoparticle size | 50–200 nm with PDI <0.7 |
| Payloads | Multiple (hydrophilic and hydrophobic) agents (therapeutics and/or diagnostics) |
| Drug release |
|
| Rheology |
|
| Modification |
|
| Gelation | Make a sol-to-gel transition rapidly by responding to stimuli |
| Retention time | Retain extended period of time to increase drug concentrations in plasma and brain tissues |
| Degradation |
|
| Administration | Exhibit the versatility in routes of administration (e.g., loco-regional, intravenous, and intranasal) |
| Storage |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawicki, B.; Schacher, T.; Cho, H. Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels 2021, 7, 63. https://doi.org/10.3390/gels7020063
Stawicki B, Schacher T, Cho H. Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels. 2021; 7(2):63. https://doi.org/10.3390/gels7020063
Chicago/Turabian StyleStawicki, Brielle, Tyler Schacher, and Hyunah Cho. 2021. "Nanogels as a Versatile Drug Delivery System for Brain Cancer" Gels 7, no. 2: 63. https://doi.org/10.3390/gels7020063
APA StyleStawicki, B., Schacher, T., & Cho, H. (2021). Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels, 7(2), 63. https://doi.org/10.3390/gels7020063







