A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering
Abstract
:1. Introduction
2. Results and Discussion
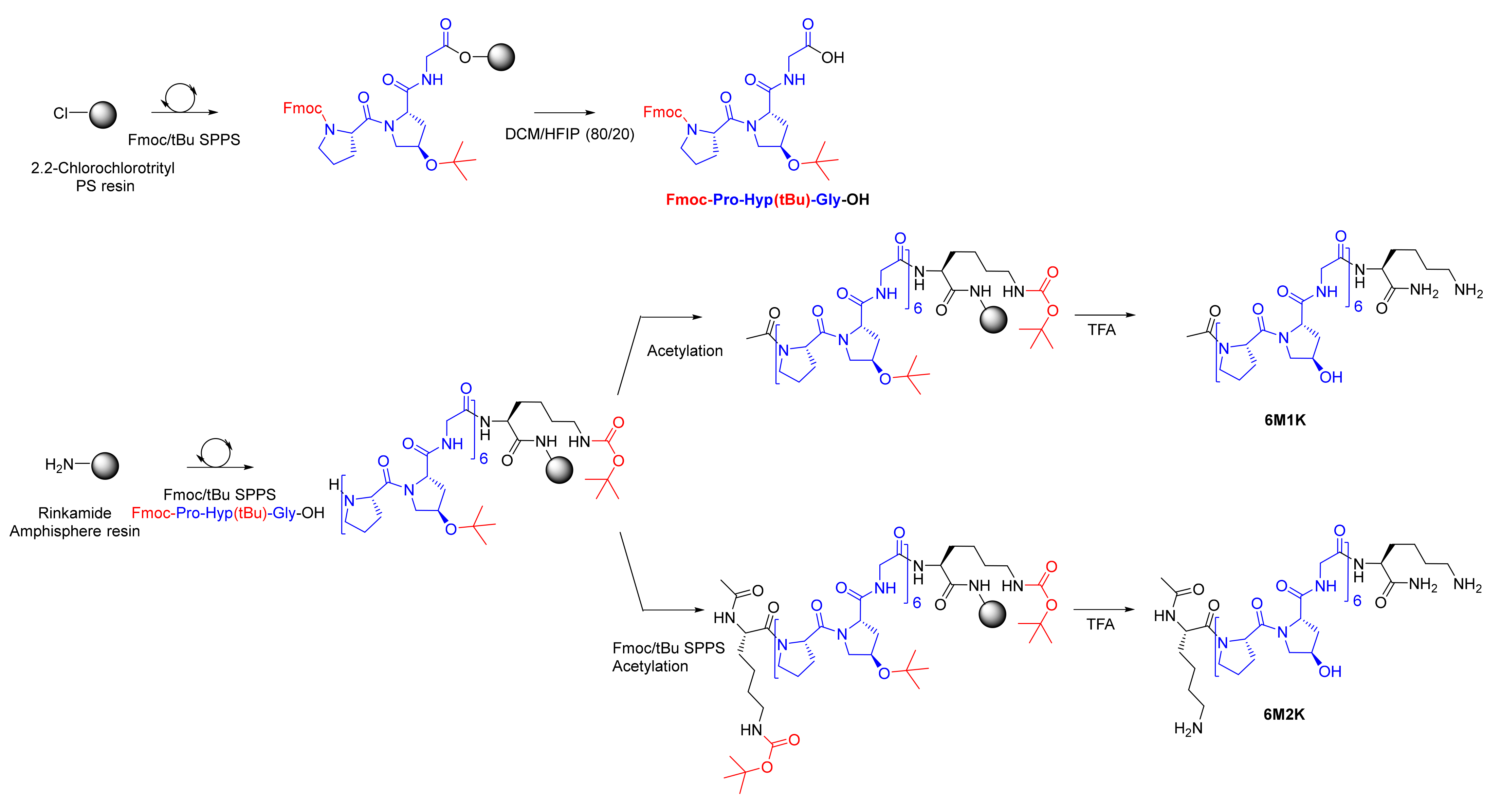
2.1. Synthesis and Characterization of the Hybrid Peptides
2.2. Hydrogel Formation, Gelation Time and the Addition of the Mono-Silylated Collagen-Like Peptide
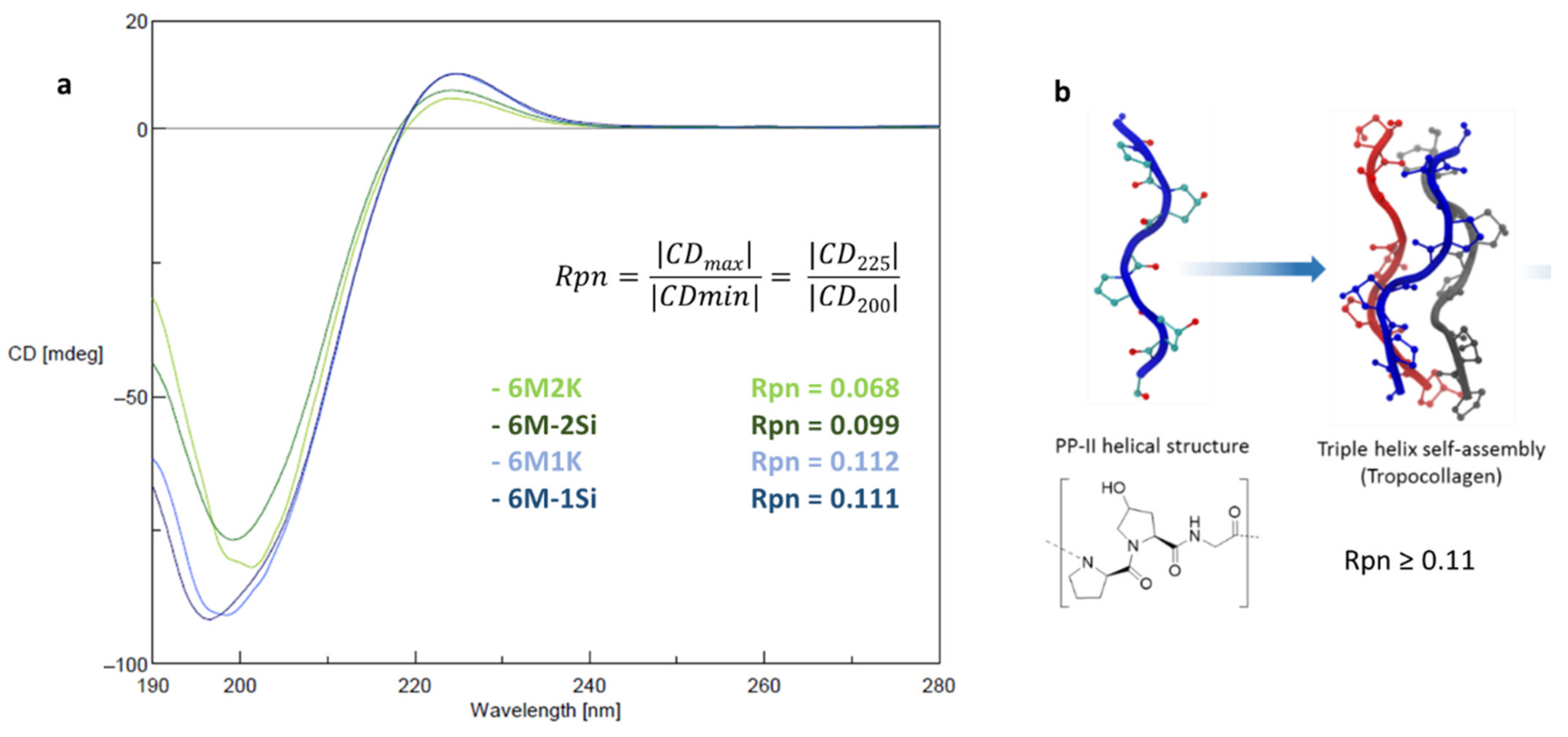
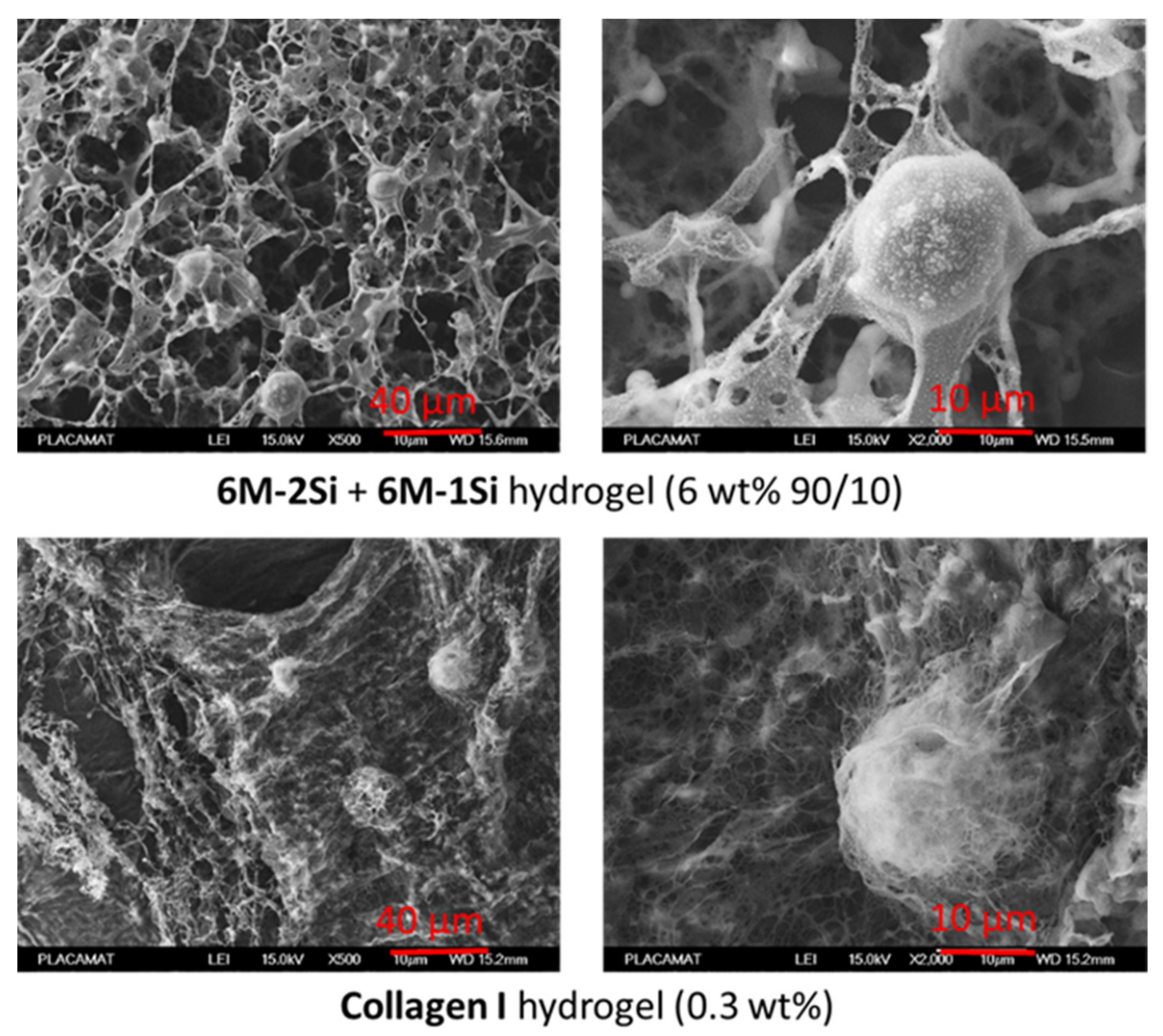
2.3. Structural and Mechanical Characterization of the Hydrogels
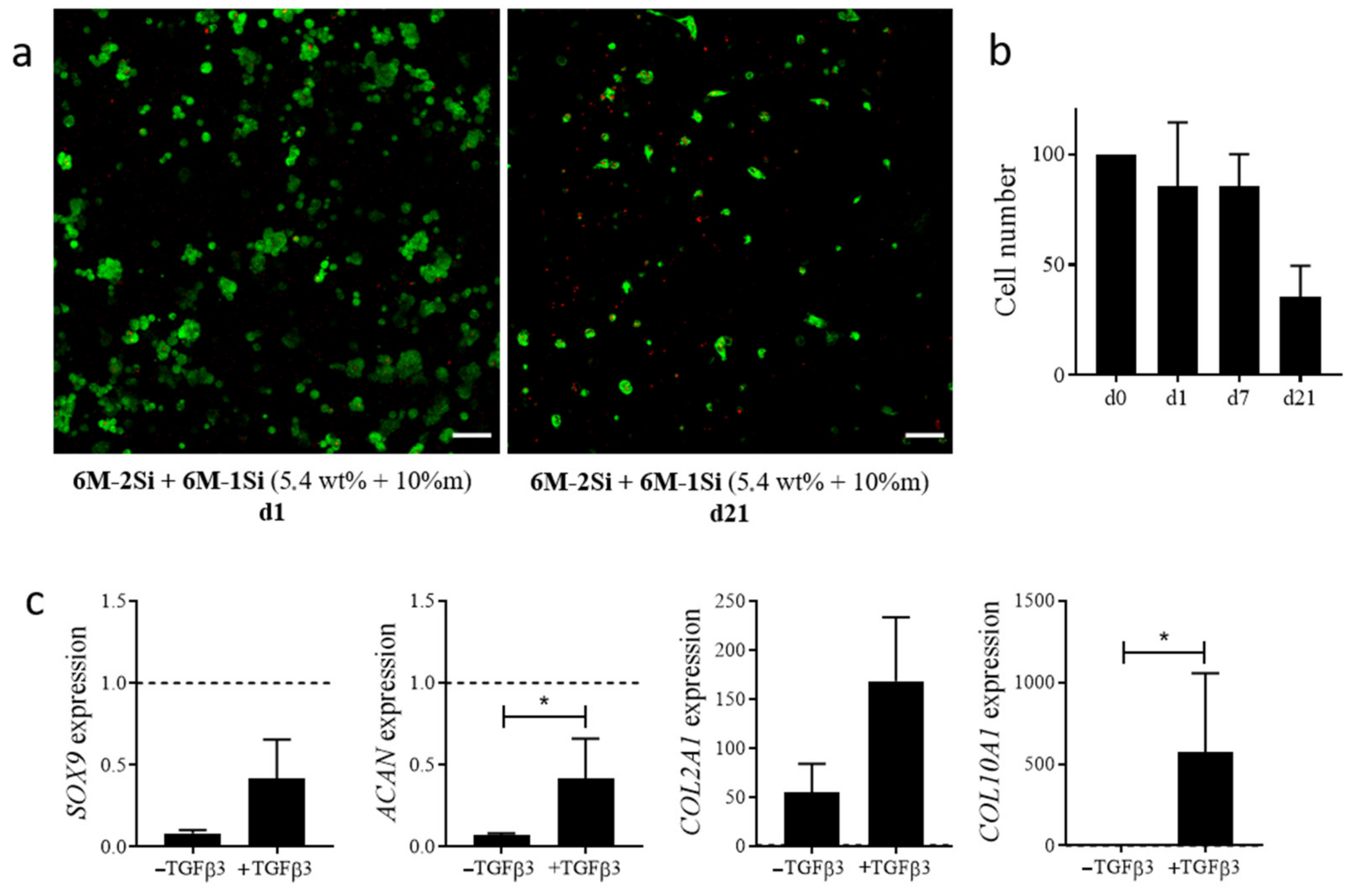
2.4. Biological Evaluation of the Hydrogels for Cell Encapsulation
3. Conclusions
4. Materials and Method
4.1. Materials
4.2. Peptide Synthesis
4.3. Peptide Silylation on the Lysine Side Chains
4.4. Circular Dichroism Analyses
4.5. Preparation of the Hybrid Hydrogels
4.6. Cryo-SEM Images
4.7. Indentation Measurements
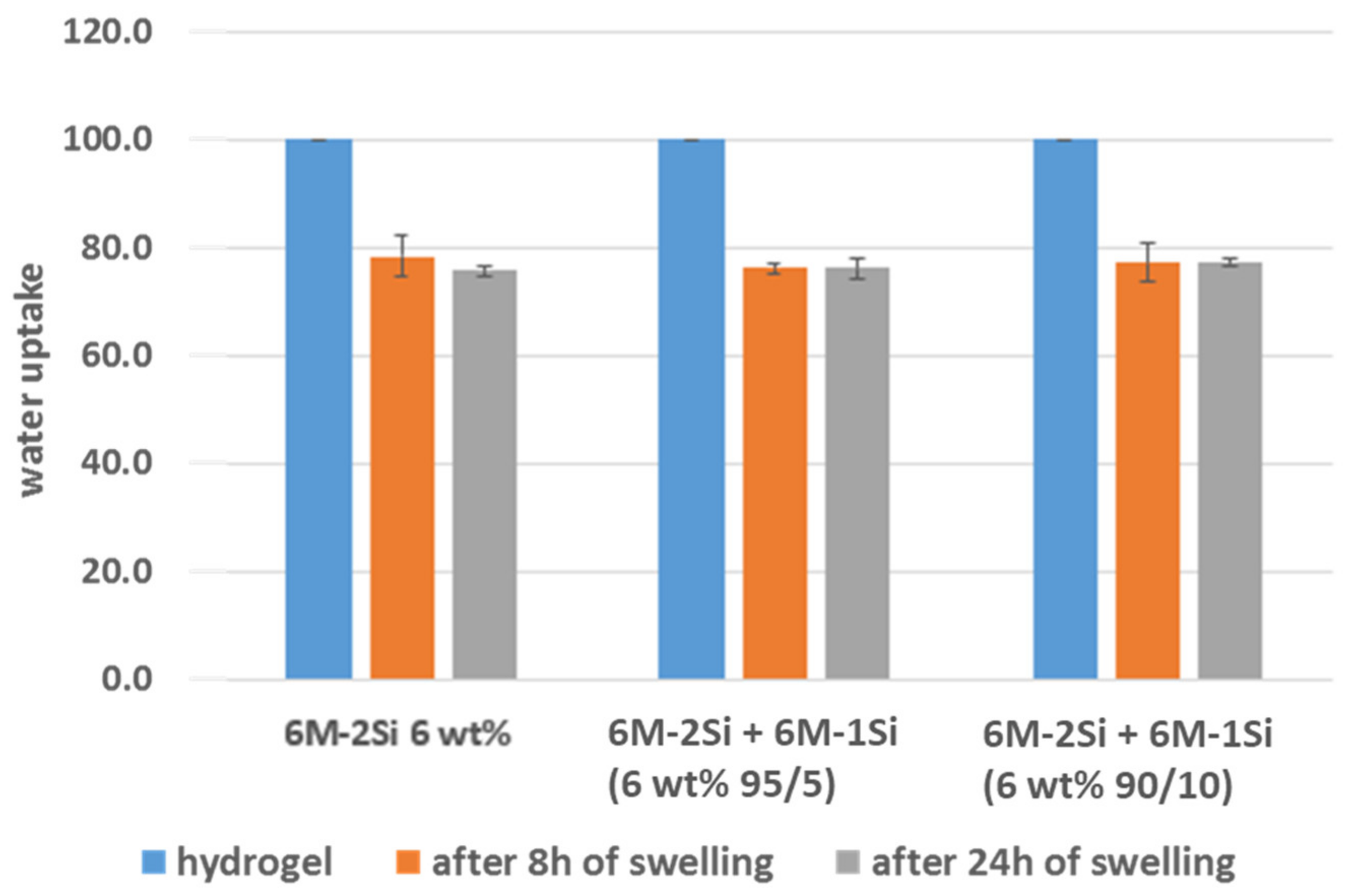
4.8. Swelling Studies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Browne, J.; Anderson, A.; Arciero, R.; Mandelbaum, B.; Moseley, B.; Micheli, L.; Fu, F.; Erggelet, C. Clinical Outcome of Autologous Chondrocyte Implantation at 5 Years in US Subjects. Clin. Orthop. Relat. Res. 2005, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for Articular Cartilage Tissue Engineering: Learning from Biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Watts, A.E.; Ackerman-Yost, J.C.; Nixon, A.J. A Comparison of Three-Dimensional Culture Systems to Evaluate In Vitro Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part. A 2013, 19, 2275–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogrebe, N.J.; Gooch, K.J. Direct Influence of Culture Dimensionality on Human Mesenchymal Stem Cell Differentiation at Various Matrix Stiffnesses Using a Fibrous Self-Assembling Peptide Hydrogel: Effect of Culture Dimensionality on HMSC Differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal Stem Cell Related Therapies for Cartilage Lesions and Osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Rivas, M.; del Valle, L.J.; Alemán, C.; Puiggalí, J. Peptide Self-Assembly into Hydrogels for Biomedical Applications Related to Hydroxyapatite. Gels 2019, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Rackwitz, L.; Nöth, U.; Tuan, R.S. Cartilage Development, Physiology, Pathologies, and Regeneration. In Strategies in Regenerative Medicine; Santin, M., Ed.; Springer: New York, NY, USA, 2009; pp. 1–27. ISBN 978–0-387–74659–3. [Google Scholar]
- Mathieu, S.; Vigier, S.; Labour, M.-N.; Jorgensen, C.; Belamie, E.; Noël, D. Induction of Mesenchymal Stem Cell Differentiation and Cartilage Formation by Cross-Linker-Free Collagen Microspheres. Eur. Cells Mater. 2014, 28, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Zscharnack, M.; Hepp, P.; Richter, R.; Aigner, T.; Schulz, R.; Somerson, J.; Josten, C.; Bader, A.; Marquass, B. Repair of Chronic Osteochondral Defects Using Predifferentiated Mesenchymal Stem Cells in an Ovine Model. Am. J. Sports Med. 2010, 38, 1857–1869. [Google Scholar] [CrossRef]
- Marquass, B.; Schulz, R.; Hepp, P.; Zscharnack, M.; Aigner, T.; Schmidt, S.; Stein, F.; Richter, R.; Osterhoff, G.; Aust, G.; et al. Matrix-Associated Implantation of Predifferentiated Mesenchymal Stem Cells Versus Articular Chondrocytes: In Vivo Results of Cartilage Repair After 1 Year. Am. J. Sports Med. 2011, 39, 1401–1412. [Google Scholar] [CrossRef]
- Ng, H.W.; Zhang, Y.; Naffa, R.; Prabakar, S. Monitoring the Degradation of Collagen Hydrogels by Collagenase Clostridium Histolyticum. Gels 2020, 6, 46. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Wang, M.; Fang, J.; Wang, K.; Ge, X.; Chan, C.W.; Ren, F.; Tan, H.; et al. Mussel-Inspired Dopamine Oligomer Intercalated Tough and Resilient Gelatin Methacryloyl (GelMA) Hydrogels for Cartilage Regeneration. J. Mater. Chem. B 2019, 7, 1716–1725. [Google Scholar] [CrossRef]
- Grigolo, B.; Lisignoli, G.; Desando, G.; Cavallo, C.; Marconi, E.; Tschon, M.; Giavaresi, G.; Fini, M.; Giardino, R.; Facchini, A. Osteoarthritis Treated with Mesenchymal Stem Cells on Hyaluronan-Based Scaffold in Rabbit. Tissue Eng. Part C Methods 2009, 15, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels That Mimic Developmentally Relevant Matrix and N-Cadherin Interactions Enhance MSC Chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [Green Version]
- Erickson, I.E.; Huang, A.H.; Sengupta, S.; Kestle, S.; Burdick, J.A.; Mauck, R.L. Macromer Density Influences Mesenchymal Stem Cell Chondrogenesis and Maturation in Photocrosslinked Hyaluronic Acid Hydrogels. Osteoarthr. Cartil. 2009, 17, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Lee, W.Y.W.; Feng, Q.; Xu, L.; Wang, B.; Man, G.C.W.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic Effects on Mesenchymal Stem Cell-Based Cartilage Regeneration by Chondrogenic Preconditioning and Mechanical Stimulation. Stem Cell Res. Ther. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated Hyaluronic Acid Hydrogels with Retarded Degradation and Enhanced Growth Factor Retention Promote HMSC Chondrogenesis and Articular Cartilage Integrity with Reduced Hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef]
- Toh, W.S.; Lim, T.C.; Kurisawa, M.; Spector, M. Modulation of Mesenchymal Stem Cell Chondrogenesis in a Tunable Hyaluronic Acid Hydrogel Microenvironment. Biomaterials 2012, 33, 3835–3845. [Google Scholar] [CrossRef]
- Diekman, B.O.; Rowland, C.R.; Lennon, D.P.; Caplan, A.I.; Guilak, F. Chondrogenesis of Adult Stem Cells from Adipose Tissue and Bone Marrow: Induction by Growth Factors and Cartilage-Derived Matrix. Tissue Eng. Part A 2010, 16, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.J.; Beck, E.C.; Dennis, S.C.; Converse, G.L.; Hopkins, R.A.; Berkland, C.J.; Detamore, M.S. Decellularized Cartilage May Be a Chondroinductive Material for Osteochondral Tissue Engineering. PLoS ONE 2015, 10, e0121966. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, Y.; Sun, Z.; Sun, X.; Xu, Y.; Li, P.; Meng, H.; Yu, X.; Xiao, B.; Fan, T.; et al. Induction of Mesenchymal Stem Cell Chondrogenic Differentiation and Functional Cartilage Microtissue Formation for in Vivo Cartilage Regeneration by Cartilage Extracellular Matrix-Derived Particles. Acta Biomater. 2016, 33, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Benders, K.E.M.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular Matrix Scaffolds for Cartilage and Bone Regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Pinheiro, A.; Young, C.; Smith, P.; Wright, E. Evaluation of Genipin for Stabilization of Decellularized Porcine Cartilage. J. Orthop. Res. 2017, 35, 1949–1957. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable Chitosan-Based Hydrogels for Cartilage Tissue Engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Li, X.; Jian, Y.; Zhang, K.; Li, G.; Yin, J. Fabrication of Injectable Hydrogels Based on Poly( l -Glutamic Acid) and Chitosan. RSC Adv. 2017, 7, 17005–17019. [Google Scholar] [CrossRef] [Green Version]
- Vinatier, C.; Magne, D.; Weiss, P.; Trojani, C.; Rochet, N.; Carle, G.F.; Vignes-Colombeix, C.; Chadjichristos, C.; Galera, P.; Daculsi, G.; et al. A Silanized Hydroxypropyl Methylcellulose Hydrogel for the Three-Dimensional Culture of Chondrocytes. Biomaterials 2005, 26, 6643–6651. [Google Scholar] [CrossRef]
- Montheil, T.; Maumus, M.; Valot, L.; Lebrun, A.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Inorganic Sol–Gel Polymerization for Hydrogel Bioprinting. ACS Omega 2020, 5, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating Chondrocytes in Degrading PEG Hydrogels with High Modulus: Engineering Gel Structural Changes to Facilitate Cartilaginous Tissue Production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Controlling the Spatial Distribution of ECM Components in Degradable PEG Hydrogels for Tissue Engineering Cartilage. J. Biomed. Mater. Res. 2003, 64A, 70–79. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Hydrogel Properties Influence ECM Production by Chondrocytes Photoencapsulated in Poly(Ethylene Glycol) Hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG Hydrogel Crosslinking Density on Protein Diffusion and Encapsulated Islet Survival and Function. J. Biomed. Mater. Res. Part A 2009, 90A, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Coburn, J.; Gibson, M.; Bandalini, P.A.; Laird, C.; Mao, H.-Q.; Moroni, L.; Seliktar, D.; Elisseeff, J. Biomimetics of the Extracellular Matrix: An Integrated Three-Dimensional Fiber-Hydrogel Composite for Cartilage Tissue Engineering. Smart Struct. Syst. 2011, 7, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kotch, F.W.; Raines, R.T. Self-Assembly of Synthetic Collagen Triple Helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [Green Version]
- Suehiro, T.; Kojima, C.; Tsumura, S.; Harada, A.; Kono, K. Higher Order Structure of Short Collagen Model Peptides Attached to Dendrimers and Linear Polymers. Biopolymers 2010, 93, 640–648. [Google Scholar] [CrossRef]
- Kishimoto, T.; Morihara, Y.; Osanai, M.; Ogata, S.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M. Synthesis of Poly(Pro-Hyp-Gly)n by Direct Polycondensation of (Pro-Hyp-Gly)n, Wheren = 1, 5, and 10, and Stability of the Triple-Helical Structure. Biopolymers 2005, 79, 163–172. [Google Scholar] [CrossRef]
- Cejas, M.A.; Kinney, W.A.; Chen, C.; Leo, G.C.; Tounge, B.A.; Vinter, J.G.; Joshi, P.P.; Maryanoff, B.E. Collagen-Related Peptides: Self-Assembly of Short, Single Strands into a Functional Biomaterial of Micrometer Scale. J. Am. Chem. Soc. 2007, 129, 2202–2203. [Google Scholar] [CrossRef] [PubMed]
- Paramonov, S.E.; Gauba, V.; Hartgerink, J.D. Synthesis of Collagen-like Peptide Polymers by Native Chemical Ligation. Macromolecules 2005, 38, 7555–7561. [Google Scholar] [CrossRef]
- Luo, J.; Tong, Y.W. Self-Assembly of Collagen-Mimetic Peptide Amphiphiles into Biofunctional Nanofiber. ACS Nano 2011, 5, 7739–7747. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Lin, R.-Z.; Melero-Martin, J.M.; Chen, Y.-C. Comparison of Covalently and Physically Cross-Linked Collagen Hydrogels on Mediating Vascular Network Formation for Engineering Adipose Tissue. Artif. Cells Nanomed. Biotechnol. 2018, 1–14. [Google Scholar] [CrossRef]
- Echalier, C.; Jebors, S.; Laconde, G.; Brunel, L.; Verdié, P.; Causse, L.; Bethry, A.; Legrand, B.; Van Den Berghe, H.; Garric, X.; et al. Sol-Gel Synthesis of Collagen-Inspired Peptide Hydrogel. Mater. Today 2017, 20, 59–66. [Google Scholar] [CrossRef]
- Echalier, C.; Pinese, C.; Garric, X.; Van Den Berghe, H.; Jumas Bilak, E.; Martinez, J.; Mehdi, A.; Subra, G. Easy Synthesis of Tunable Hybrid Bioactive Hydrogels. Chem. Mater. 2016, 28, 1261–1265. [Google Scholar] [CrossRef]
- Alauzun, J.; Mehdi, A.; Reyé, C.; Corriu, R.J.P. Direct Synthesis of Bifunctional Mesoporous Organosilicas Containing Chelating Groups in the Framework and Reactive Functional Groups in the Channel Pores. J. Mater. Chem. 2007, 17, 349–356. [Google Scholar] [CrossRef]
- Jebors, S.; Valot, L.; Echalier, C.; Legrand, B.; Mikhaleff, R.; VanDerLee, A.; Arenal, R.; Dumy, P.; Amblard, M.; Martinez, J.; et al. Self-Mineralization and Assembly of a Bis-Silylated Pseudodipeptide to a Structured Bioorganic-Inorganic Material. Mater. Horiz. 2019. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-Assembly of Collagen-Based Biomaterials: Preparation, Characterizations and Biomedical Applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef]
- O’Leary, L.E.R.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-Hierarchical Self-Assembly of a Collagen Mimetic Peptide from Triple Helix to Nanofibre and Hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; O’Leary, L.E.R.; Hartgerink, J.D. Self-Assembly of Fiber-Forming Collagen Mimetic Peptides Controlled by Triple-Helical Nucleation. J. Am. Chem. Soc. 2014, 136, 14417–14424. [Google Scholar] [CrossRef] [PubMed]
- Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical Insights in Bioinks for 3D Printing. Chem. Soc. Rev. 2019, 48, 4049–4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valot, L.; Maumus, M.; Montheil, T.; Martinez, J.; Noël, D.; Mehdi, A.; Subra, G. Biocompatible Glycine-Assisted Catalysis of the Sol-Gel Process: Development of Cell-Embedded Hydrogels. ChemPlusChem 2019, 84, 1720–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, R.Y.; Mockros, L.F. Indentation Tests of Human Articular Cartilage. J. Biomech. 1976, 9, 259–268. [Google Scholar] [CrossRef]
- Pagels, R.F.; Prud’homme, R.K. Polymeric Nanoparticles and Microparticles for the Delivery of Peptides, Biologics, and Soluble Therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Canal, T.; Peppas, N.A. Correlation between Mesh Size and Equilibrium Degree of Swelling of Polymeric Networks. J. Biomed. Mater. Res. Part A 1989, 23, 1183–1193. [Google Scholar] [CrossRef]
- Kim, H.; Woo, Y.; Patel, M.; Jeong, B. Thermogelling Inclusion Complex System for Fine-Tuned Osteochondral Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2020, 21, 3176–3185. [Google Scholar] [CrossRef] [PubMed]




| Hybrid Peptide | Concentration | Gelation Time | Hydrogel Aspect |
|---|---|---|---|
| 6M-2Si | 7 wt% | - a | Above solubility |
| 6M-2Si | 6 wt% | 6 h 30 min–7 h 30 min | White opaque |
| 6M-2Si | 5 wt% | > 8 h | Very weak, white opaque |
| 6M-2Si | 4 wt% | - b | - |
| 6M-2Si + 6M-1Si | 6 wt% (95/5) | 6 h 30 min–7 h | White opaque |
| 6M-2Si + 6M-1Si | 6 wt% (90/10) | 6 h 30 min–7 h | White opaque |
| 6M-2Si + 6M-1Si | 6 wt% (85/15) | - b | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valot, L.; Maumus, M.; Brunel, L.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels 2021, 7, 73. https://doi.org/10.3390/gels7020073
Valot L, Maumus M, Brunel L, Martinez J, Amblard M, Noël D, Mehdi A, Subra G. A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels. 2021; 7(2):73. https://doi.org/10.3390/gels7020073
Chicago/Turabian StyleValot, Laurine, Marie Maumus, Luc Brunel, Jean Martinez, Muriel Amblard, Danièle Noël, Ahmad Mehdi, and Gilles Subra. 2021. "A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering" Gels 7, no. 2: 73. https://doi.org/10.3390/gels7020073
APA StyleValot, L., Maumus, M., Brunel, L., Martinez, J., Amblard, M., Noël, D., Mehdi, A., & Subra, G. (2021). A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels, 7(2), 73. https://doi.org/10.3390/gels7020073








