Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared Spectroscopy
2.2. Morphological Analysis
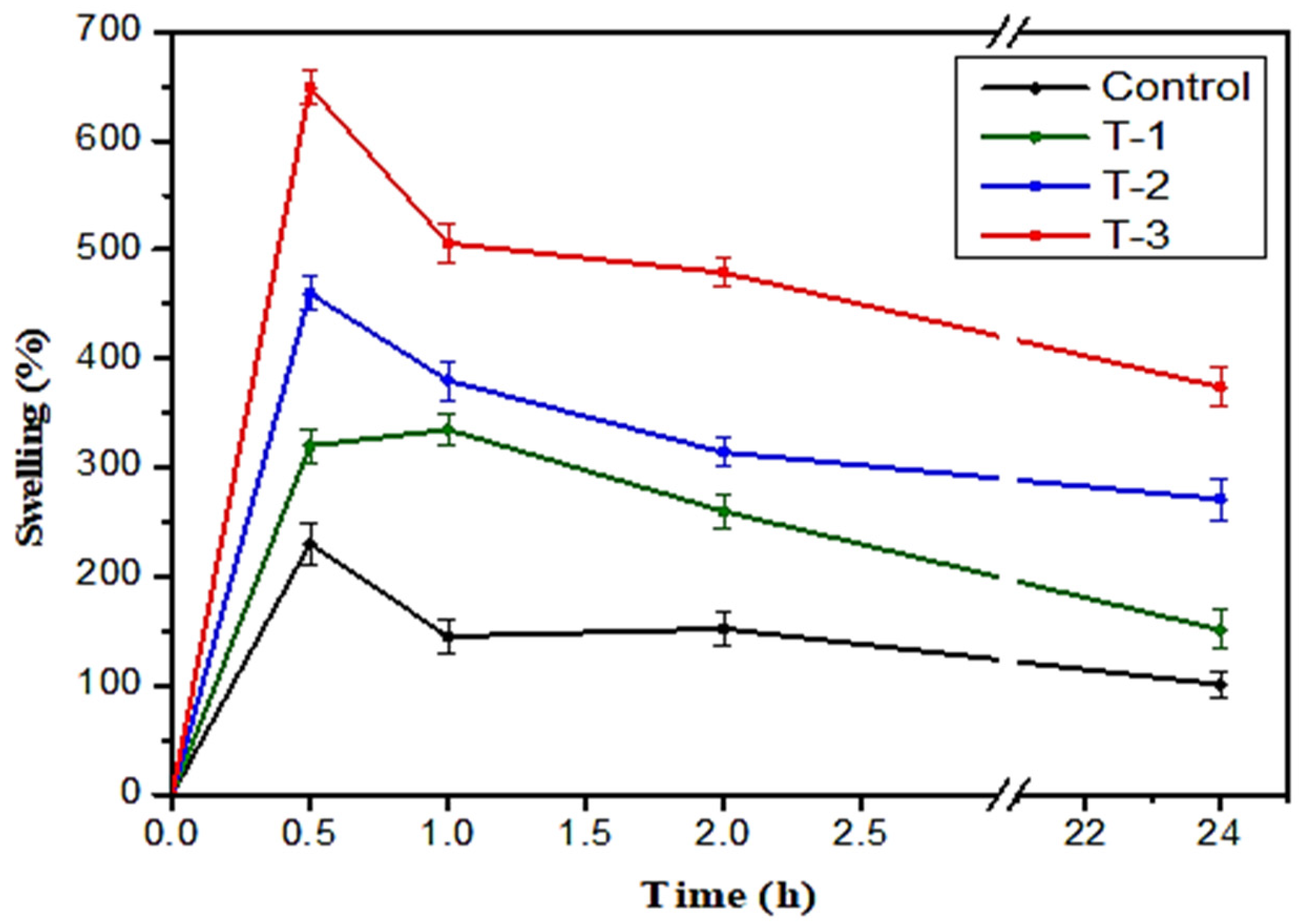
2.3. Swelling Studies
2.4. Thermogravimetric Analysis (TGA)
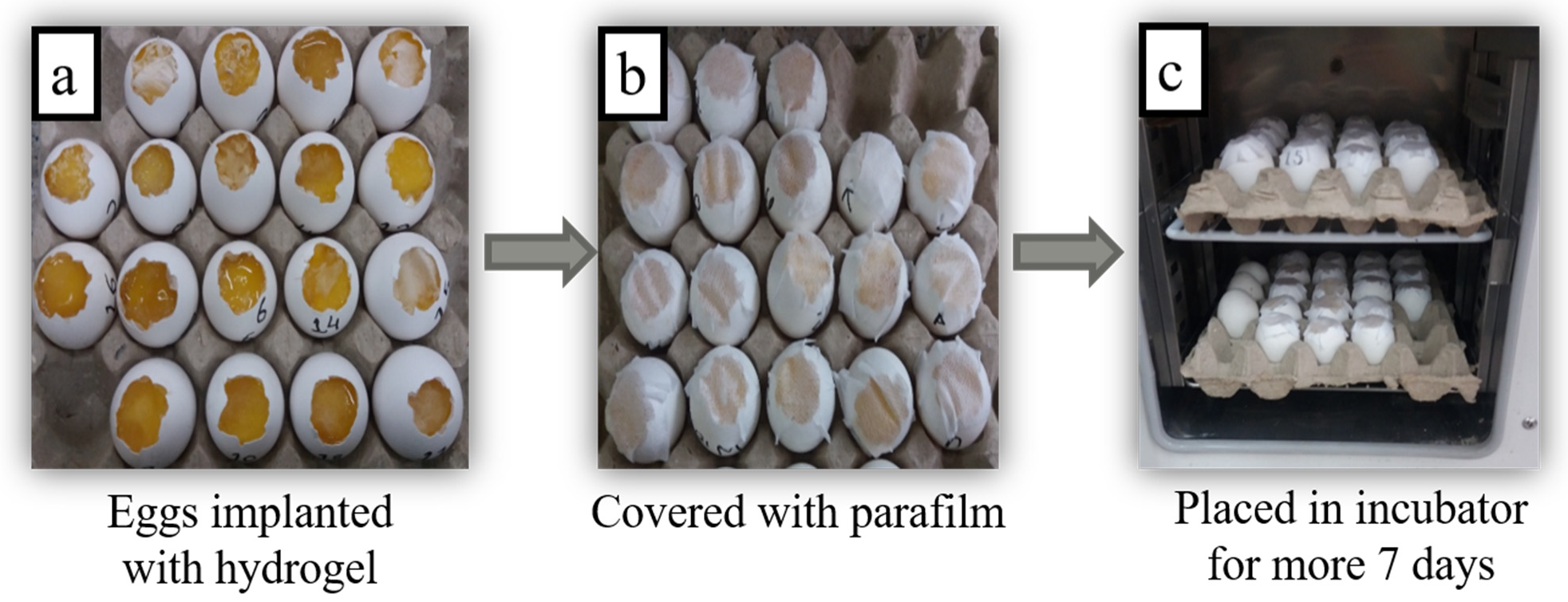
2.5. Chorioallantoic Membrane (CAM) Assay
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation Methods
3.2.1. Raphanus sativus L. Extract Preparation
3.2.2. Sodium Alginate Solution Preparation
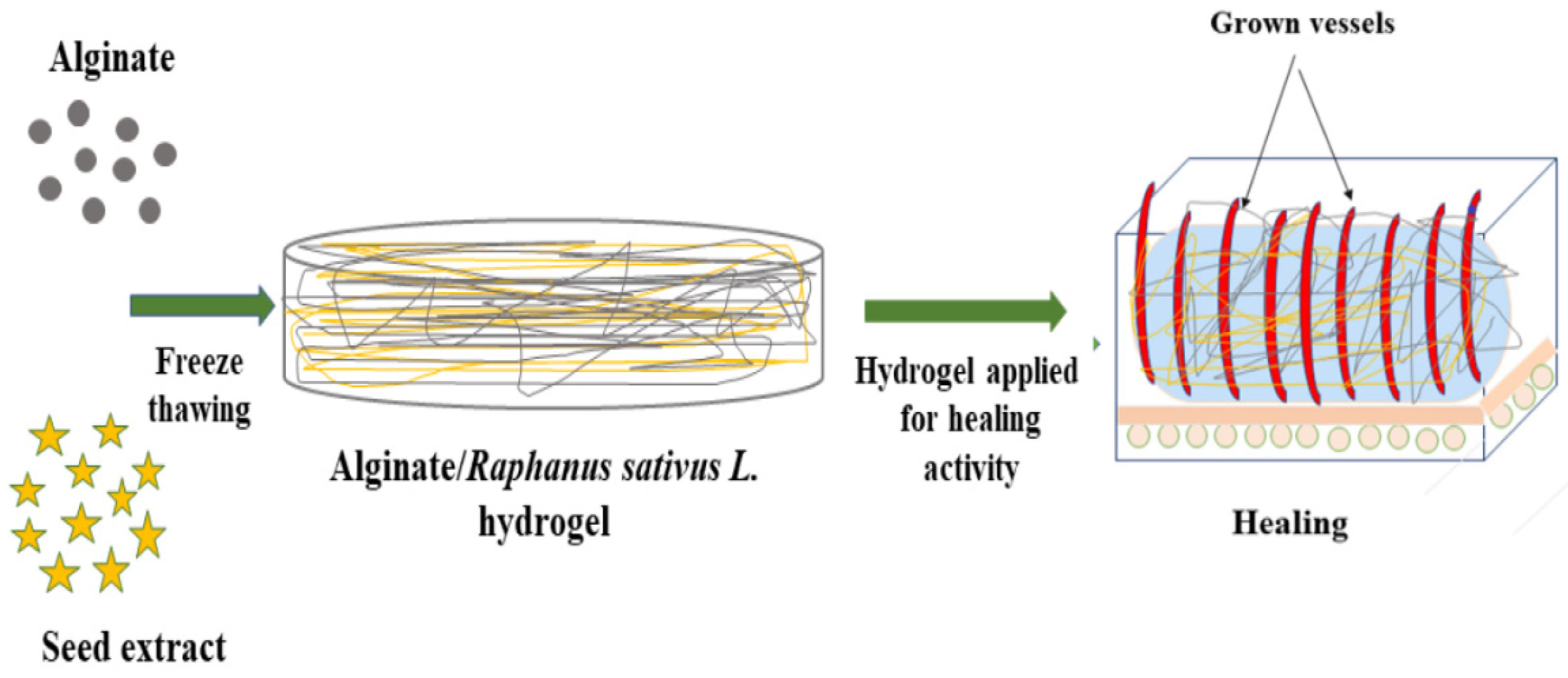
3.3. Synthesis of SA-SE (Sodium Alginate-Seed Extract) Hydrogel by Freeze-Thawing
3.4. Characterization of Synthesized Hydrogels
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SA | Sodium alginate |
References
- Zahid, M.; Lodhi, M.; Rehan, Z.A.; Tayyab, H.; Javed, T.; Shabbir, R.; Mukhtar, A.; El Sabagh, A.; Adamski, R.; Sakran, M.I.; et al. Sustainable development of chitosan/calotropis procera-based hydrogels to stimulate formation of granulation tissue and angiogenesis in wound healing applications. Molecules 2021, 26, 3284. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Islam, J.M.; Kabir, W.; Rahman, A.; Mizan, M.; Rahman, M.F.; Amin, J.; Khan, M.A. Development of hydrocolloid Bi-layer dressing with bio-adhesive and non-adhesive properties. Mater. Sci. Eng. C 2016, 69, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Behera, S.S.; Singh, S.; Rizvi, S.I.; Singh, A.K. Progress in the development and applicability of potential medicinal plant extract-conjugated polymeric constructs for wound healing and tissue regeneration. Phytother. Res. 2016, 30, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly (vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; De Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Niknia, N.; Kadkhodaee, R. Factors affecting microstructure, physicochemical and textural properties of a novel Gum tragacanth-PVA blend cryogel. Carbohydr. Polym. 2017, 155, 475–482. [Google Scholar] [CrossRef]
- Jahani-Javanmardi, A.; Sirousazar, M.; Shaabani, Y.; Kheiri, F. Egg white/poly (vinyl alcohol)/MMT nanocomposite hydrogels for wound dressing. J. Biomater. Sci. Polym Ed. 2016, 27, 262–1276. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Singh, S.K. Evaluation of poly (vinyl alcohol) based cryogel–zinc oxide nanocomposites for possible applications as wound dressing materials. Mater. Sci. Eng. C 2016, 65, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int. J. Nanomed. 2020, 15, 5097. [Google Scholar] [CrossRef] [PubMed]
- Dantas, M.D.M.; Cavalcante, D.R.R.; Araújo, F.E.N.; Barretto, S.R.; Aciole, G.T.S.; Pinheiro, A.L.B.; Ribeiro, M.A.G.; Lima-Verde, I.B.; Melo, C.M.; Cardoso, J.C.; et al. Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J. Photochem. Photobiol. B. Biol. 2011, 105, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y. Alginate fibres: An overview of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Donati, I.; Paoletti, S. Material properties of alginates. In Alginates: Biology and applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–53. [Google Scholar]
- Beevi, S.S.; Mangamoori, L.N.; Dhand, V.; Ramakrishna, D.S. Isothiocyanate profile and selective antibacterial activity of root, stem, and leaf extracts derived from Raphanus sativus L. Foodborne Pathog. Dis. 2009, 6, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Abdou, I.A.; Abou-Zeid, A.A.; El-Sherbeeny, M.R.; Abou-El-Gheat, Z.H. Antimicrobial activities of Allium sativum, Allium cepa, Raphanus sativus, Capsicum frutescens, Eruca sativa, Allium kurrat on bacteria. Qual. Plant. Mater. Veg. 1972, 22, 29–35. [Google Scholar] [CrossRef]
- Ghazanfar, S.A.; Al-Al-Sabahi, A.M. Medicinal plants of northern and central Oman (Arabia). Econ. Bot. 1993, 47, 89–98. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Falahatzadeh, M.; Raoofi, E.; Zarshenas, M.M. An evidence-based review on wound healing herbal remedies from reports of traditional Persian medicine. J. Evid-Based Complementary Altern. Med. 2017, 22, 334–343. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Shin, S.R.; et al. Silver nanoparticles-composing alginate/gelatine hydrogel improves wound healing in vivo. Nanomater. 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B. 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Rani, I.; Akhund, S.; Abro, H. Antimicrobial potential of seed extract of Raphanus sativus. Pak. J. Bot. 2008, 40, 1793–1798. [Google Scholar]
- Aleem, A.R.; Shahzadi, L.; Alvi, F.; Khan, A.F.; Chaudhry, A.A.; ur Rehman, I.; Yar, M. Thyroxin releasing chitosan/collagen based smart hydrogels to stimulate neovascularization. Mater. Des. 2017, 133, 416–425. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I.; Mauro, R.P. Seed coating in direct seeded rice: An innovative and sustainable approach to enhance grain yield and weed management under submerged conditions. Sustainability 2021, 13, 2190. [Google Scholar] [CrossRef]
- Tang, Q.; Pan, D.; Sun, Y.; Cao, J.; Guo, Y. Preparation, characterization and antimicrobial activity of sodium alginate nanobiocomposite films incorporated with ε-Polylysine and cellulose nanocrystals. J. Food Process. Preserv. 2017, 41, e13120. [Google Scholar] [CrossRef]
- Ramya, E.; Jyothi, L.; Rao, D.N. Influence of optical properties of Ag NPs from Raphanus sativus leaf extract on lanthanide complexes. Plasmonics 2017, 12, 1601–1611. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. App. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Malagurski, I.; Levic, S.; Pantic, M.; Matijasevic, D.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Synthesis and antimicrobial properties of Zn-mineralized alginate nanocomposites. Carbohydr. Polym. 2017, 165, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.; Gogoi, A.; Chowdhury, D. Magnetic alginate–Fe3O4 hydrogel fiber capable of ciprofloxacin hydrochloride adsorption/separation in aqueous solution. RSC Adv. 2015, 5, 81573–81582. [Google Scholar] [CrossRef]
- Kusuktham, B.; Prasertgul, J.; Srinun, P. Morphology and property of calcium silicate encapsulated with alginate beads. Silicon 2014, 6, 191–197. [Google Scholar] [CrossRef]
- Khan, N.; Waheed, A.; Hamid, F.S.; Ahmed, N.; Iqbal, Z.; Ali, S.; Gul, H. Phytochemical screening and antibacterial assay of radish seed oil on selected pathogenic bacteria species in vitro. Pak. J. Agric. Res. 2018, 32, 46–51. [Google Scholar]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]


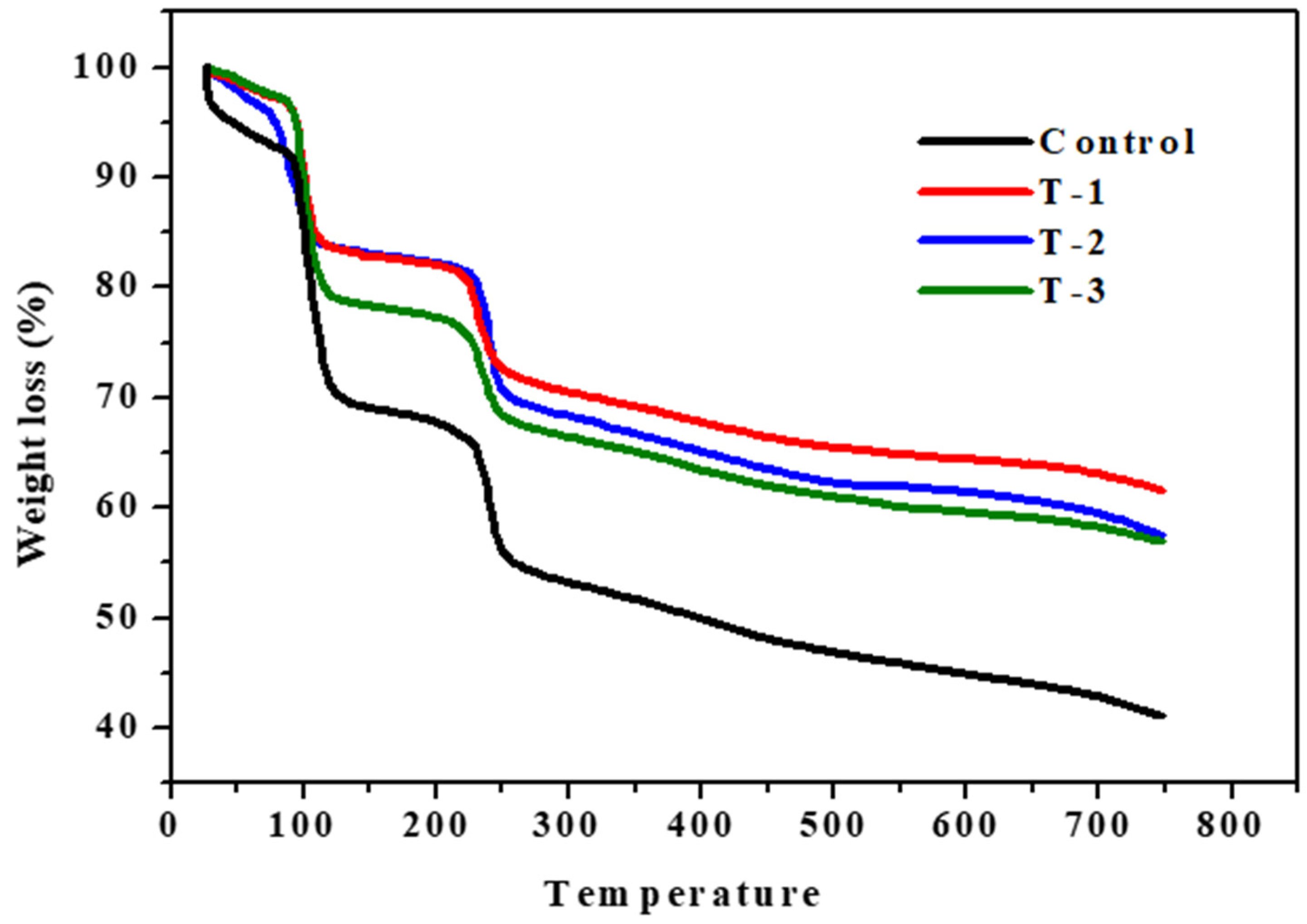

| Sample | Stages | T-onset (°C) | T-endset (°C) | W (%) |
|---|---|---|---|---|
| Control | I | 92.2 | 119.2 | 20 |
| II | 137.3 | 217.3 | 3 | |
| III | 229.8 | 249.8 | 9 | |
| T-1 | I | 93.7 | 112.3 | 9 |
| II | 122.3 | 214.8 | 2 | |
| III | 221.8 | 242.3 | 7 | |
| T-2 | I | 72.2 | 107.4 | 11 |
| II | 117.3 | 222.3 | 2 | |
| III | 229.8 | 249.8 | 10 | |
| T-3 | I | 92.2 | 117.3 | 16 |
| II | 124.3 | 212.3 | 3 | |
| III | 219.8 | 247.3 | 8 |
| Samples | SA (wt %) | SE (wt %) |
|---|---|---|
| Control | 100 | nil |
| T-1 | 70 | 30 |
| T-2 | 80 | 20 |
| T-3 | 90 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. https://doi.org/10.3390/gels7030107
Zahid M, Lodhi M, Afzal A, Rehan ZA, Mehmood M, Javed T, Shabbir R, Siuta D, Althobaiti F, Dessok ES. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels. 2021; 7(3):107. https://doi.org/10.3390/gels7030107
Chicago/Turabian StyleZahid, Muhammad, Maria Lodhi, Ayesha Afzal, Zulfiqar Ahmad Rehan, Muzzamil Mehmood, Talha Javed, Rubab Shabbir, Dorota Siuta, Fayez Althobaiti, and Eldessoky S. Dessok. 2021. "Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application" Gels 7, no. 3: 107. https://doi.org/10.3390/gels7030107






