Poly (Amidehydrazide) Hydrogel Particles for Removal of Cu2+ and Cd2+ Ions from Water
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
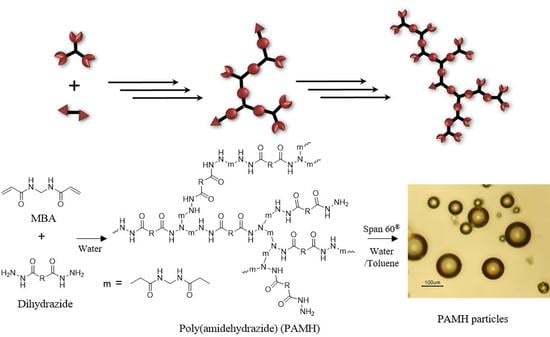
4.2.1. Synthesis of Poly(Amidehydrazide) Particles
4.2.2. Measurement of Metal Ion Sorption Test
4.2.3. Measurement of Trace Amount Metal Ion Sorption Test (Packed Column Method)
4.2.4. ATR-IR of Water-Swollen and Metal-Absorbed ADH-PAMH1.75
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, S.A.; Van Sickle, J.; Herlihy, A.T.; Hughes, R.M. Mercury concentration in fish from streams and rivers throughout the western United States. Environ. Sci. Technol. 2007, 41, 58–65. [Google Scholar] [CrossRef]
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Widespread copper and lead contamination of household drinking water, New South Wales, Australia. Environ. Res. 2016, 151, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, K.M.; Ogawa, Y.; Zakir, H.M.; Otomo, K.; Shikazono, N. Heavy metals contamination in water and sediments of an urban river in a developing country. Int. J. Environ. Sci. Technol. 2011, 8, 723. [Google Scholar] [CrossRef] [Green Version]
- Rzętała, M.A. Cadmium contamination of sediments in the water reservoirs in Silesian Upland (southern Poland). J. Soils Sediments 2016, 16, 2458–2470. [Google Scholar] [CrossRef] [Green Version]
- Idrees, N.; Tabassum, B.; Abd Allah, E.F.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West, U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Q.; Huang, H.; Xue, F.; Lin, W.; Zhou, H.; Wei, W. Biomass bagasse-based hyperbranched adsorbent for the complete removal of low-level Cr(VI). Cellulose 2020, 27, 8121–8134. [Google Scholar] [CrossRef]
- Falahian, Z.; Torki, F.; Faghihian, H. Synthesis and Application of Polypyrrole/Fe 3 O 4 Nanosize Magnetic Adsorbent for Efficient Separation of Hg 2+ from Aqueous Solution. Glob. Challenges 2018, 2, 1700078. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Wu, Q.; He, H.; Zhou, H.; Xue, F.; Zhu, H.; Zhou, S.; Wang, L.; Wang, S. Multiple active sites cellulose-based adsorbent for the removal of low-level Cu(II), Pb(II) and Cr(VI) via multiple cooperative mechanisms. Carbohydr. Polym. 2020, 233, 115860. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Shao, T.; Zhao, X.; Peng, H.; Gong, Y.; Wan, H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018, 339, 322–333. [Google Scholar] [CrossRef]
- Rahman, M.L.; Fui, C.J.; Ting, T.X.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Polymer ligands derived from jute fiber for heavy metal removal from electroplating wastewater. Polymers 2020, 12, 2521. [Google Scholar] [CrossRef]
- Azizkhani, S.; Mahmoudi, E.; Abdullah, N.; Ismail, M.H.S.; Mohammad, A.W.; Hussain, S.A. Synthesis and characterisation of graphene oxide-silica-chitosan for eliminating the Pb(II) from aqueous solution. Polymers 2020, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, R.; Martín-Lara, M.A.; Moreno, J.A.; Blázquez, G.; Calero, M. Effective removal of zinc from industrial plating wastewater using hydrolyzed olive cake: Scale-up and preparation of zinc-Based biochar. J. Clean. Prod. 2019, 227, 634–644. [Google Scholar] [CrossRef]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Johnson, J.H.; Goddard, W.A. Dendrimer enhanced ultrafiltration. 1. Recovery of Cu(II) from aqueous solutions using PAMAM dendrimers with ethylene diamine core and terminal NH 2 groups. Environ. Sci. Technol. 2005, 39, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Cahill, B.P.; Papastavrou, G.; Koper, G.J.M.; Borkovec, M. Adsorption of poly(amido amine) (PAMAM) dendrimers on silica: Importance of electrostatic three-body attraction. Langmuir 2008, 24, 465–473. [Google Scholar] [CrossRef]
- Gosika, M.; Maiti, P.K. PH and generation dependent morphologies of PAMAM dendrimers on a graphene substrate. Soft Matter 2018, 14, 1925–1938. [Google Scholar] [CrossRef]
- Ren, B.; Wang, K.; Zhang, B.; Li, H.; Niu, Y.; Chen, H.; Yang, Z.; Li, X.; Zhang, H. Adsorption behavior of PAMAM dendrimers functionalized silica for Cd(II) from aqueous solution: Experimental and theoretical calculation. J. Taiwan Inst. Chem. Eng. 2019, 101, 80–91. [Google Scholar] [CrossRef]
- Sohail, I.; Bhatti, I.A.; Ashar, A.; Sarim, F.M.; Mohsin, M.; Naveed, R.; Yasir, M.; Iqbal, M.; Nazir, A. Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ions from aqueous solution. J. Mater. Res. Technol. 2020, 9, 498–506. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Zhang, G.; Zhao, Y.; Lv, C.; Liu, X.; Chen, L. Preparation of PVDF/hyperbranched-nano- palygorskite composite membrane for efficient removal of heavy metal ions. Polymers 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelwahab, H.E.; Hassan, S.Y.; Mostafa, M.A.; El Sadek, M.M. Synthesis and characterization of glutamic-chitosan hydrogel for copper and nickel removal from wastewater. Molecules 2016, 21, 684. [Google Scholar] [CrossRef] [Green Version]
- Paraskevopoulou, P.; Raptopoulos, G.; Leontaridou, F.; Papastergiou, M.; Sakellari, A.; Karavoltsos, S. Evaluation of polyurea-crosslinked alginate aerogels for seawater decontamination. Gels 2021, 7, 27. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.F.; Lebrun, L. Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J. Memb. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Corti, C.; Rampazzi, L.; Monticelli, D.; Scalarone, D.; Sassella, A.; Canevali, C. Optimization of Copper Stain Removal from Marble through the Formation of Cu (II) Complexes in Agar Gels. Gels 2021, 7, 111. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Thakur, S.; Mamba, G.; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Lee, S.; Eom, Y.; Park, J.; Lee, J.; Kim, S.Y. Micro-hydrogel Particles Consisting of Hyperbranched Polyamidoamine for the Removal of Heavy Metal Ions from Water. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Okesola, B.O.; Suravaram, S.K.; Parkin, A.; Smith, D.K. Selective Extraction and In Situ Reduction of Precious Metal Salts from Model Waste To Generate Hybrid Gels with Embedded Electrocatalytic Nanoparticles. Angew. Chem. 2016, 128, 191–195. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Versatile supramolecular pH-tolerant hydrogels which demonstrate pH-dependent selective adsorption of dyes from aqueous solution. Chem. Commun. 2013, 49, 11164–11166. [Google Scholar] [CrossRef]
- bt Johari, I.S.; Yusof, N.A.; Haron, M.J.; Mohd Nor, S.M. Preparation and characterization of poly(ethyl hydrazide) grafted oil palm empty fruit bunch for removal of ni(ii) ion in aqueous environment. Polymers 2013, 5, 1056–1067. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)-hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Shin, S.S.; Park, C.H.; Jeon, S.; Gwon, J.; Lee, S.Y.; Kim, S.J.; Kim, H.J.; Lee, J.H. Poly(acryloyl hydrazide)-grafted cellulose nanocrystal adsorbents with an excellent Cr(VI) adsorption capacity. J. Hazard. Mater. 2020, 394, 122512. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Eom, Y.; Lee, S.; Kim, S.Y. Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles. Molecules 2019, 24, 3866. [Google Scholar] [CrossRef] [Green Version]
- Dirtu, D.; Odochian, L.; Pui, A.; Humelnicu, I. Thermal decomposition of ammonia. N2H4-An intermediate reaction product. Cent. Eur. J. Chem. 2006, 4, 666–673. [Google Scholar] [CrossRef]
- Tzabar, N.; ter Brake, H.J.M. Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 2016, 22, 901–914. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Diao, K.; Tan, X.; Lei, F.; Jiang, J.; Goodman, B.A.; Ma, Y.; Liu, S. Mechanisms of adsorption of heavy metal cations from waters by an amino bio-based resin derived from Rosin. Polymers 2019, 11, 969. [Google Scholar] [CrossRef] [Green Version]
- Raddatz, S.; Mueller-Ibeler, J.; Kluge, J.; Wäß, L.; Burdinski, G.; Havens, J.R.; Onofrey, T.J.; Wang, D.; Schweitzer, M. Hydrazide oligonucleotides: New chemical modification for chip array attachment and conjugation. Nucleic Acids Res. 2002, 30, 4793–4802. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Poly(amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions. Polym. Chem. 2013, 4, 2164–2167. [Google Scholar] [CrossRef]
- Li, Q.; Bai, Q.; Xu, F.; Zhang, Q.; Chu, S. Absorptive Removal of Copper from Aqueous Solution by Biosorbents. In Proceedings of the 2011 International Conference on Chemistry and Chemical Process, Bangkok, Thailand, 7–9 May 2011; Volume 10, pp. 156–160. [Google Scholar]
- Benaïssa, H.; Elouchdi, M.A. Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem. Eng. Process. Process. Intensif. 2007, 46, 614–622. [Google Scholar] [CrossRef]
- Vakili, M.R.; Zahmatkesh, S.; Panahiyan, M.J.; Jafarizadeh, T. Poly(amide-hydrazide-imide)s containing L-aspartic acid: Synthesis, characterization, and their applications in removal of heavy metal ions. Des. Monomers Polym. 2015, 18, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Bahareh, V.D.W. Cu(II) Ion Adsorption by Aniline Grafted Chitosan and Its Responsive Fluorescence Properties. Molecules 2020, 25, 1052. [Google Scholar]
- Sun, H.; Zhang, X.; He, Y.; Zhang, D.; Feng, X.; Zhao, Y.; Chen, L. Preparation of PVDF-g-PAA-PAMAM membrane for efficient removal of copper ions. Chem. Eng. Sci. 2019, 209, 115186. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Hamdi, H.; Mlayah, A. Use of lignite as a low-cost material for cadmium and copper removal from aqueous solutions: Assessment of adsorption characteristics and exploration of involved mechanisms. Water 2021, 13, 164. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-rahman, A.A.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Trakulsujaritchok, T.; Noiphom, N.; Tangtreamjitmun, N.; Saeeng, R. Adsorptive features of poly(glycidyl methacrylate-co-hydroxyethyl methacrylate): Effect of porogen formulation on heavy metal ion adsorption. J. Mater. Sci. 2011, 46, 5350–5362. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.M.; Ye, H.; Zhao, B.X. Effective removal of heavy metal ions Cd 2+, Zn 2+, Pb 2+, Cu 2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef]




| Heavy Metal Ion | Adsorbents | Sorption Capacity (mg/g) | References |
|---|---|---|---|
| Cu2+ | Activated carbon | 23.0 | [39] |
| Bacteria isolated from soil | 16.3 | [40] | |
| Dried sunflower leaves | 89.4 | [41] | |
| Poly (amide-hydrazide-imide)s containing L-aspartic acid | 34.3 | [42] | |
| Aniline grafted chitosan | 106.6 | [43] | |
| PVDF-g-PAA-PAMAM membranes | 101.0 | [44] | |
| Cellulose nanofibril-polyethyleneimine | 52.3 | [45] | |
| Lignite | 21.4 | [46] | |
| ADH-PAMH1.25 | 85.3 | This work | |
| Cd2+ | Activated carbon | 33.6 | [47] |
| Alginate beads | 31.4 | [48] | |
| Alginate-chitosan composite | 90.8 | [49] | |
| HEMA-PGMA functionalized DETA | 35.9 | [50] | |
| Fe3O4@APS@AA-co-CA MNPs | 29.6 | [51] | |
| Poly (amide-hydrazide-imide)s containing L-aspartic acid | 79.6 | [42] | |
| Lignite | 38.0 | [46] | |
| SDH-PAMH1.25 | 47.6 | This work |
| Amine (g/g) | Amide (g/g) | Cu2+ Sorption at 1000 ppm Solution (mg/g) | |
|---|---|---|---|
| ADH-PAMH1.25 | 0.087 | 0.528 | 85.27 |
| ADH-PAMH1.5 | 0.079 | 0.531 | 84.34 |
| ADH-PAMH1.75 | 0.072 | 0.533 | 74.65 |
| ADH-PAMH2 | 0.066 | 0.535 | 71.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kim, T.; Kim, S.Y. Poly (Amidehydrazide) Hydrogel Particles for Removal of Cu2+ and Cd2+ Ions from Water. Gels 2021, 7, 121. https://doi.org/10.3390/gels7030121
Choi H, Kim T, Kim SY. Poly (Amidehydrazide) Hydrogel Particles for Removal of Cu2+ and Cd2+ Ions from Water. Gels. 2021; 7(3):121. https://doi.org/10.3390/gels7030121
Chicago/Turabian StyleChoi, Hojung, Taehyoung Kim, and Sang Youl Kim. 2021. "Poly (Amidehydrazide) Hydrogel Particles for Removal of Cu2+ and Cd2+ Ions from Water" Gels 7, no. 3: 121. https://doi.org/10.3390/gels7030121