Chondroitin Sulfate-Based Cryogels for Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
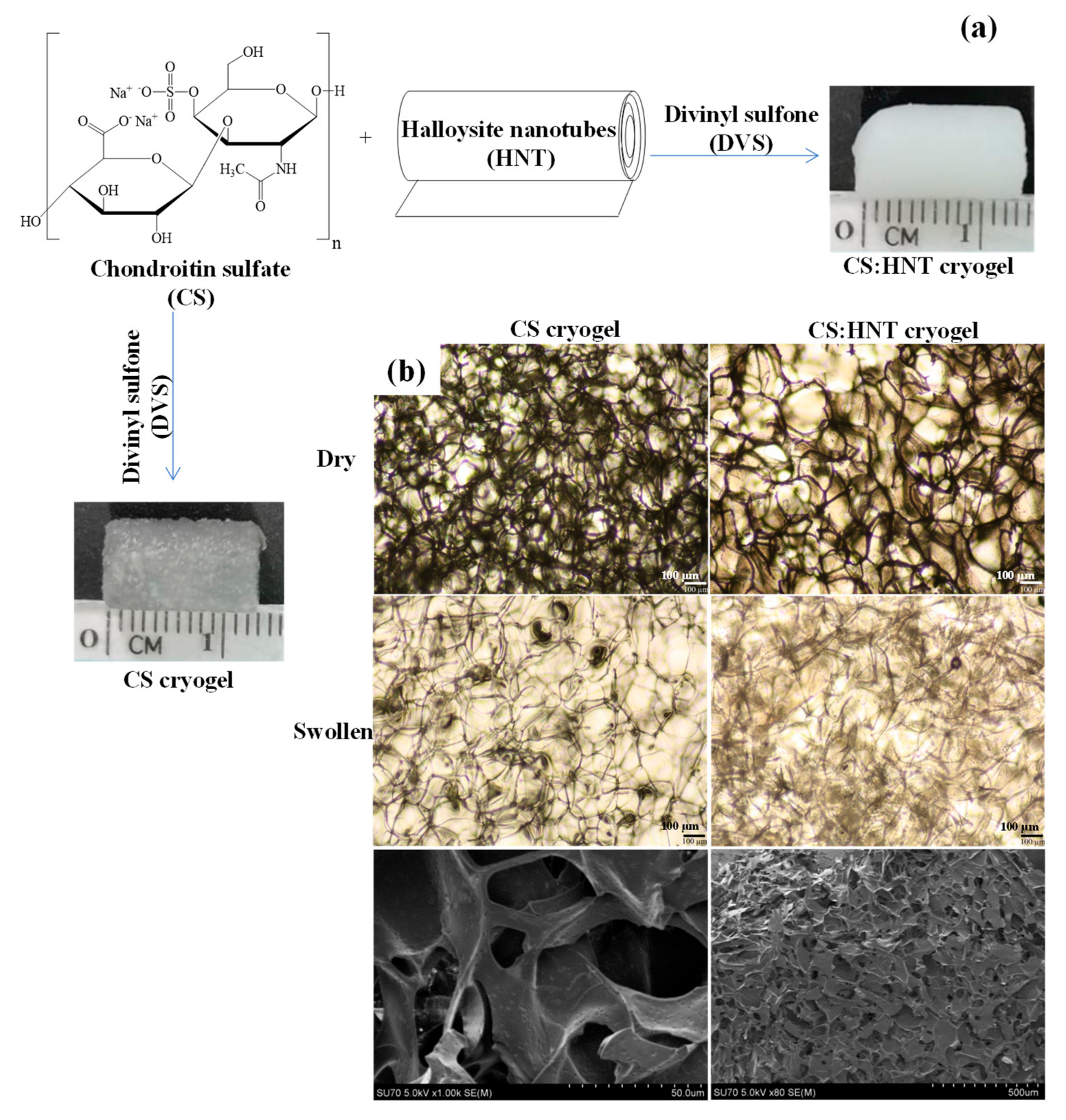
2.1. Synthesis and Characterization of CS-Based Cryogels
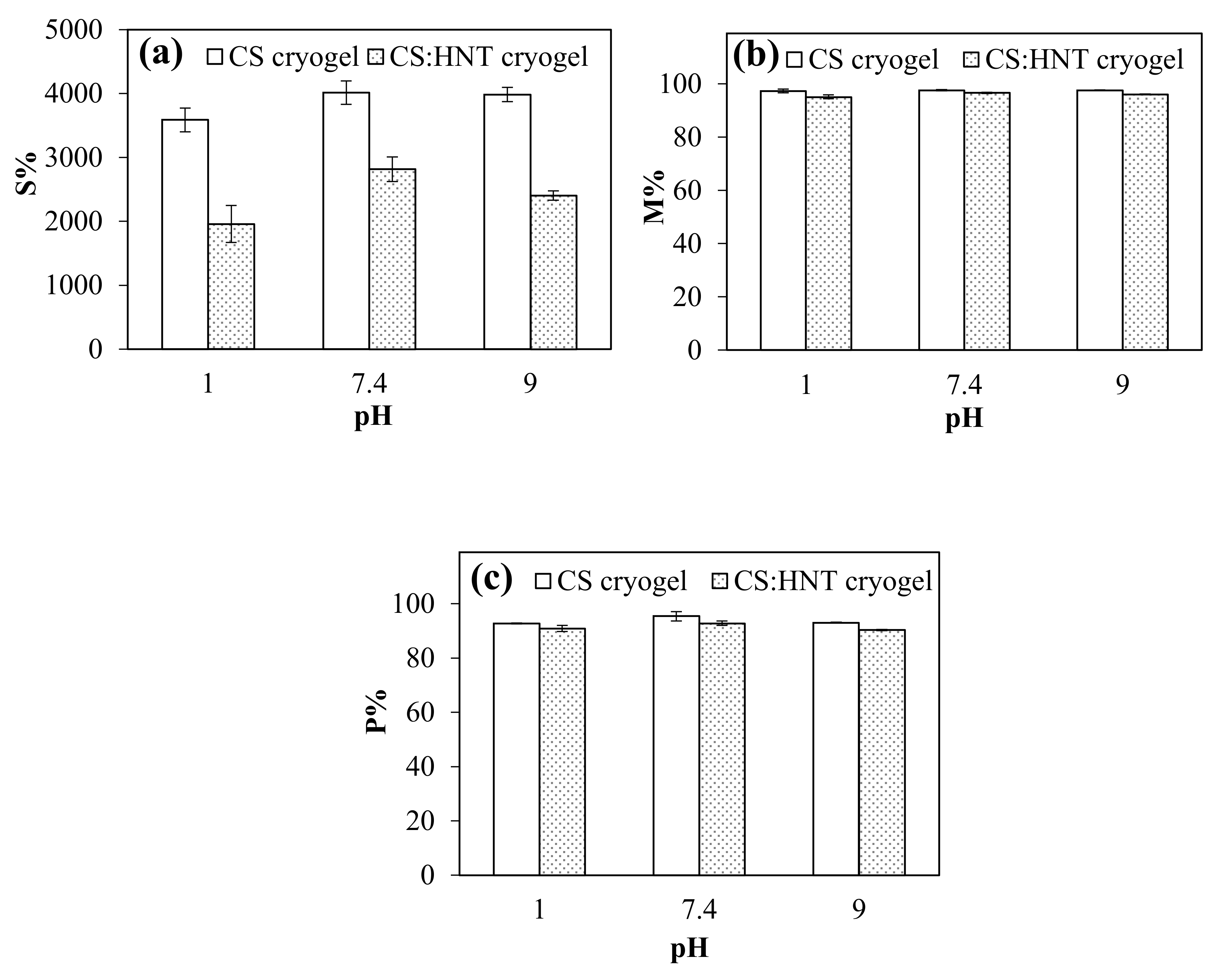
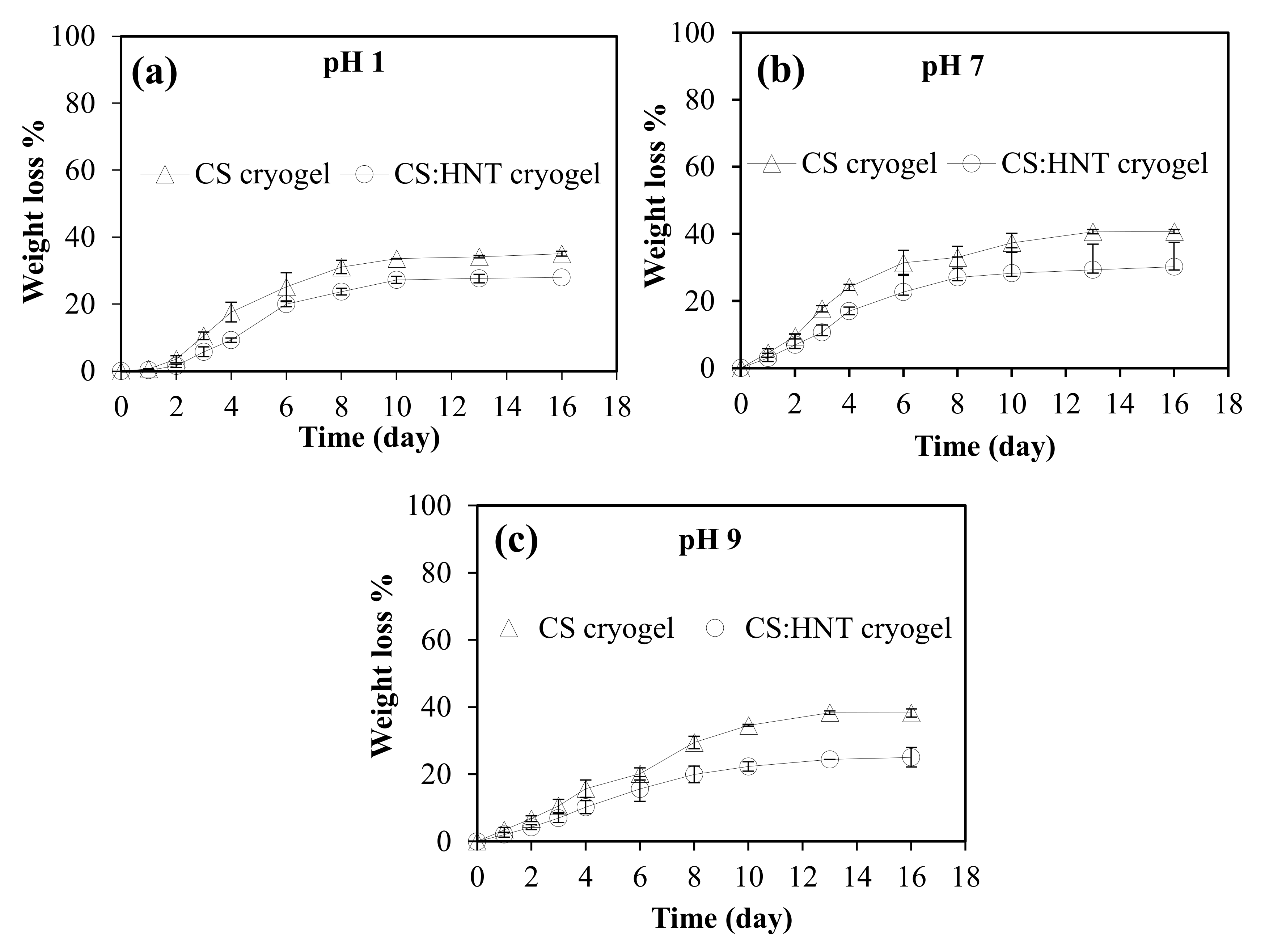
2.2. Hydrolytic Degradationof CS-Based Cryogels
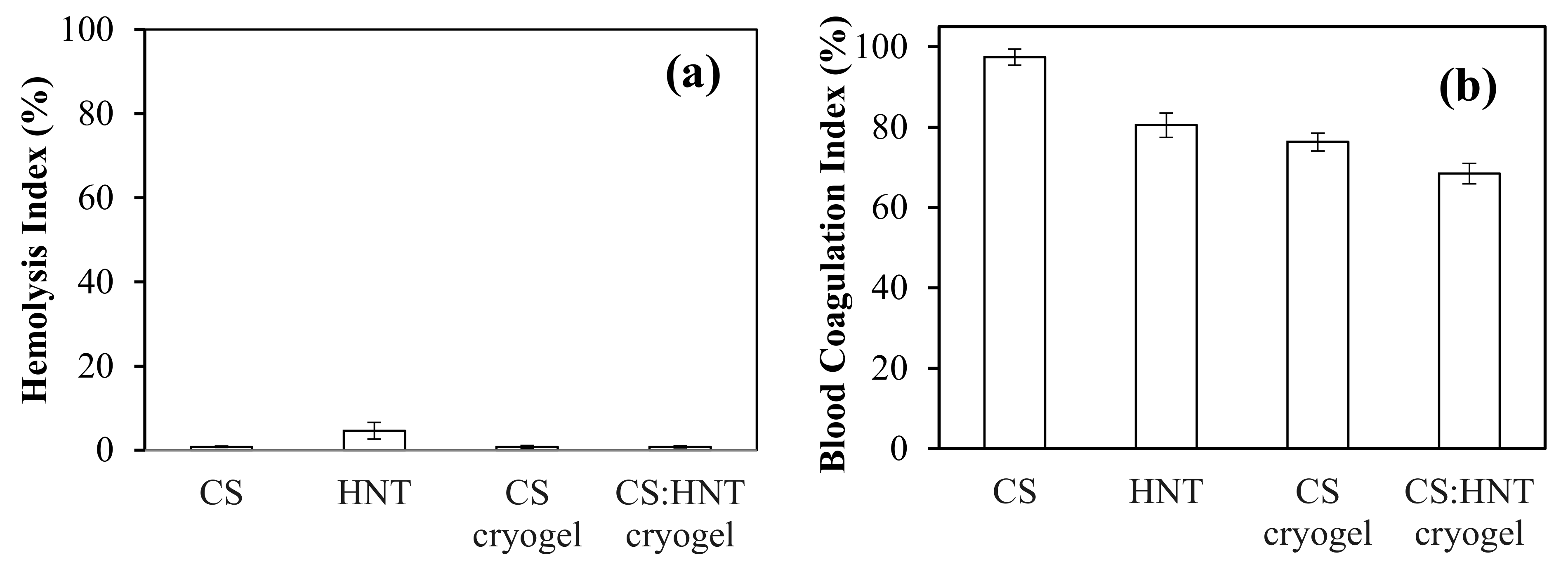
2.3. Hemoompatibility Assay of Cryogels
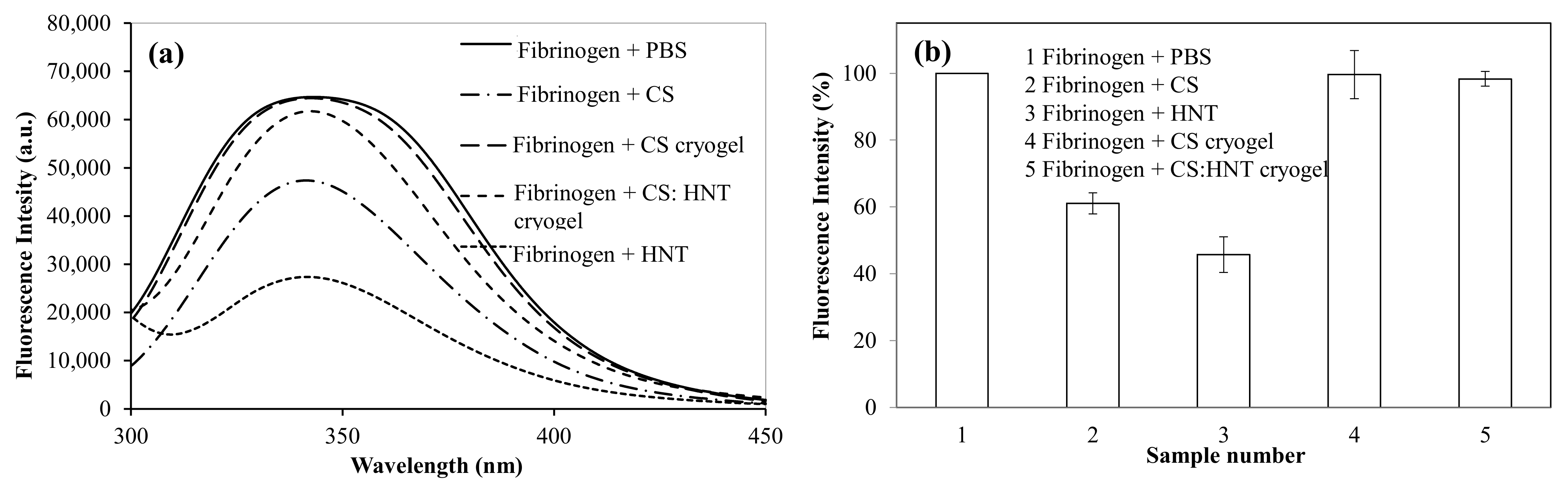
2.4. The Interaction of CS-Based Cryogel with Fibrinogen
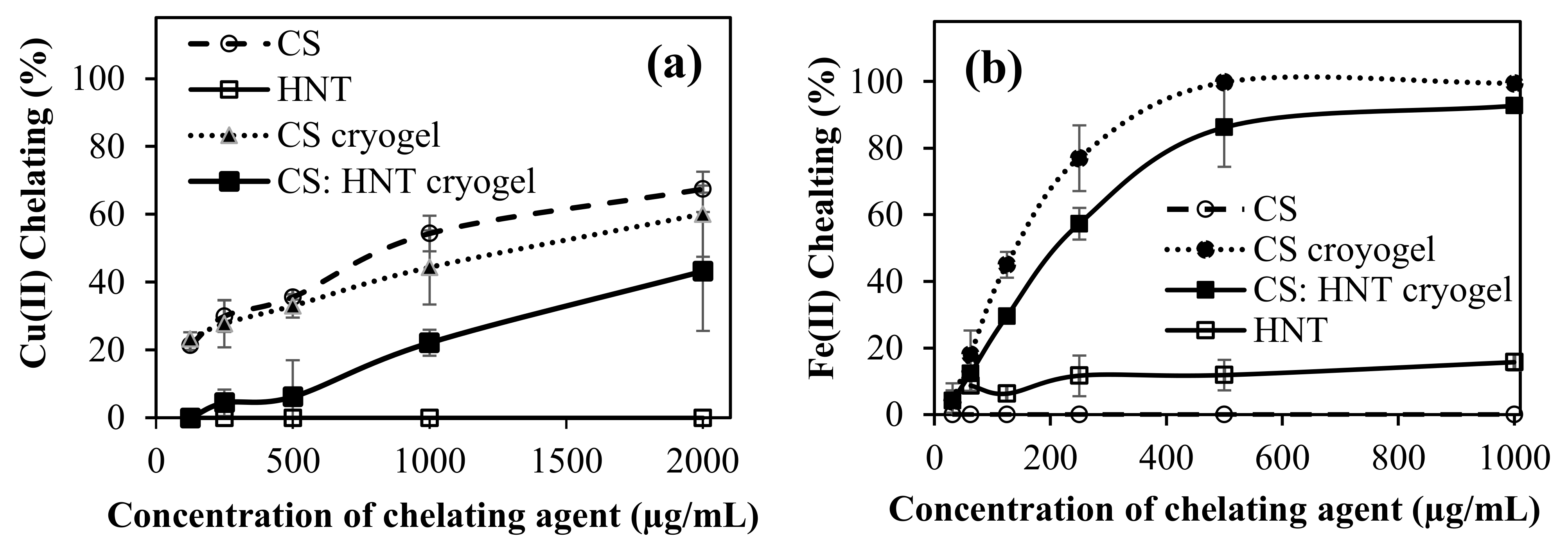
2.5. Metal Chelating Activities of CS-Based Cryogels
2.6. α-Glucosidase Interaction with CS-Based Cryogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterisation of Cryogels
4.3. Swelling Behavior and Hydrolytic Degradation of Cryogels
4.4. Hemocompatibilitiy Assay of Cryogels
4.5. Fibrinogen Interactions of Cryogels
4.6. Metal Ion Chelating Capabilitiesof CS Cryogels
4.7. The Effect of Cryogel on α-Glucosidase Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pang, H.; Li, J.; Miao, Z.; Li, S.J. Inhibitory effects of chondroitin sulfate on alpha-amylase activity: A potential hypoglycemic agent. Int. J. Biol. Macromol. 2021, 184, 289–296. [Google Scholar] [CrossRef]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; García, A.G.; Verges, J.; Montell, E.; López, M.G. Antioxidant, antiinflammatory and neuroprotective actions of chondroitin sulfate and proteoglycans. Osteoarthr. Cartil. 2010, 18, S24–S27. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.; Avenoso, A.; Campo, S.; Ferlazzo, A.; Calatroni, A. Chondroitin Sulphate: Antioxidant Properties and Beneficial Effects. Mini-Rev. Med. Chem. 2006, 6, 1311–1320. [Google Scholar] [CrossRef]
- Sahiner, M.; Sagbas Suner, S. Poli(Rutin) micro/nanogels for biomedical applications. Hittite J. Sci. Eng. 2021, 8, 179–187. [Google Scholar] [CrossRef]
- Tan, T.; Yang, Q.; Chen, D.; Zhao, J.; Xiang, L.; Feng, J.; Song, X.; Fu, Y.; Gong, T. Chondroitin sulfate-mediated albumin corona nanoparticles for the treatment of breast cancer. Asian J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Demirci, S.; Suner, S.S.; Sahiner, M.; Sahiner, N. Superporous hyaluronic acid cryogel composites embedding synthetic polyethyleneimine microgels and Halloysite Nanotubes as natural clay. Eur. Polym. J. 2017, 93, 775–784. [Google Scholar] [CrossRef]
- Sengel, S.B.; Sahiner, M.; Aktas, N.; Sahiner, N. Halloysite-carboxymethyl cellulose cryogel composite from natural sources. Appl. Clay Sci. 2017, 140, 66–74. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020, 309, 113077. [Google Scholar] [CrossRef]
- Goda, E.S.; Gab-Allah, M.A.; Singu, B.S.; Yoon, K.R. Halloysite nanotubes based electrochemical sensors: A review. Microchem. J. 2019, 147, 1083–1096. [Google Scholar] [CrossRef]
- Pereira, I.; Saleh, M.; Nunes, C.; Reis, S.; Veiga, F.; Paiva-Santos, A.C. Preclinical developments of natural-occurring halloysite clay nanotubes in cancer therapeutics. Adv. Colloid Interface Sci. 2021, 291, 102406. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Fu, Y.; Gong, C.; Wang, W.; Zhang, L.; Ivanov, E.; Lvov, Y. Antifouling Thermoplastic Composites with Maleimide Encapsulated in Clay Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 30083–30091. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Andrabi, S.M.; Singh, A.; Majumder, S.; Kumar, A. Designing cryogels through cryostructuring of polymeric matrices for biomedical applications. Eur. Polym. J. 2021, 144, 110234. [Google Scholar] [CrossRef]
- Lozinsky, V. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Su, E.; Okay, O. Cryogenic formation-structure-property relationships of poly (2-acrylamido-2-methyl-1-propanesulfonic acid) cryogels. Polymer 2019, 178, 121603. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Development of chitosan-poly(ethyleneimine) based double network cryogels and their application as superadsorbents for phosphate. Carbohydr. Polym. 2019, 210, 17–25. [Google Scholar] [CrossRef]
- Tavsanli, B.; Okay, O. Macroporous methacrylated hyaluronic acid cryogels of high mechanical strength and flow-dependent viscoelasticity. Carbohydr. Polym. 2020, 229, 115458. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2021, 166, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Dolak, İ.; Canpolat, G.; Onat, R.; Keçili, R.; Baysal, Z.; Ziyadanoğulları, B.; Ersöz, A.; Say, R. A novel lanthanide-chelate based molecularly imprinted cryogel for purification of hemoglobin from blood serum: An alternative method for thalassemia diagnosis. Process Biochem. 2020, 91, 189–196. [Google Scholar] [CrossRef]
- Zoughaib, M.; Luong, D.; Garifullin, R.; Gatina, D.Z.; Fedosimova, S.V.; Abdullin, T.I. Enhanced angiogenic effects of RGD, GHK peptides and copper (II) compositions in synthetic cryogel ECM model. Mater. Sci. Eng. C 2021, 120, 111660. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Lai, J.Y.; Chou, S.F.; Hsueh, Y.J.; Ma, D.H.K. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, E.; Damania, A.; Shakya, A.K.; Kumar, A.; Sarin, S.K.; Kumar, A. Fabrication of macroporous cryogels as potential hepatocyte carriers for bioartificial liver support. Colloids Surf. B Biointerfaces 2015, 136, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Eigel, D.; Schuster, R.; Männel, M.J.; Thiele, J.; Panasiuk, M.J.; Andreae, L.C.; Varricchio, C.; Brancale, A.; Welzel, P.B.; Huttner, W.B.; et al. Sulfonated cryogel scaffolds for focal delivery in ex-vivo brain tissue cultures. Biomaterials 2021, 271, 120712. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Han, M.E.; Kang, B.J.; Kim, S.H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Kim, H.D.; Lee, E.A.; An, Y.-H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef]
- Li, Q.; Williams, C.G.; Sun, D.D.N.; Wang, J.; Leong, K.; Elisseeff, J.H. Photocrosslinkable polysaccharides based on chondroitin sulfate. J. Biomed. Mater. Res. 2004, 68, 28–33. [Google Scholar] [CrossRef]
- Ramdhani, D. Formulation and Characterization of Chondroitin Sulfate Nanoparticle with Chitosan as Polymer and Kappa Carrageenan as Crosslinker Using the Ionic Gelation Method. J. Nanomed. Nanotechnol. 2017, S8, 2. [Google Scholar] [CrossRef]
- Deng, L.; Qi, Y.; Liu, Z.; Xi, Y.; Xue, W. Effect of tannic acid on blood components and functions. Colloids Surf. B Biointerfaces 2019, 184, 110505. [Google Scholar] [CrossRef]
- Martinez, M.; Weisel, J.W.; Ischiropoulos, H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic. Biol. Med. 2013, 65, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N. Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications. Molecules 2021, 26, 2429. [Google Scholar] [CrossRef]
- Kaviani, S.; Shahab, S.; Sheikhi, M.; Khaleghian, M.; Al Saud, S. Characterization of the binding affinity between some anti-Parkinson agents and Mn2+, Fe3+ and Zn2+ metal ions: A DFT insight. Inorg. Chem. Commun. 2021, 128, 108582. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, X.; Ge, K.; Yin, Y.; Machuki, J.O.; Yang, Y.; Shi, H.; Geng, D.; Gao, F. Carbon nitride-based nanocaptor: An intelligent nanosystem with metal ions chelating effect for enhanced magnetic targeting phototherapy of Alzheimer’s disease. Biomaterials 2021, 267, 120483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiao, J.; Nagy, T.; Xiong, M.P. ROS-triggered degradable iron-chelating nanogels: Safely improving iron elimination in vivo. J. Control. Release 2018, 283, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surf. B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, J.; Yu, J.; Chen, X.; Zhang, S.; Cai, Y.; Li, L. A new functionality study of vanillin as the inhibitor for α-glucosidase and its inhibition kinetic mechanism. Food Chem. 2021, 353, 129448. [Google Scholar] [CrossRef]
- Moghimi, S.; Salarinejad, S.; Toolabi, M.; Firoozpour, L.; Esmaeil Sadat Ebrahimi, S.; Safari, F.; Madani-Qamsari, F.; Mojtabavi, S.; Faramarzi, M.A.; Karima, S.; et al. Synthesis, in-vitro evaluation, molecular docking, and kinetic studies of pyridazine-triazole hybrid system as novel α-glucosidase inhibitors. Bioorg. Chem. 2021, 109, 104670. [Google Scholar] [CrossRef]
- Sahiner, M.; Sahiner, N.; Sagbas, S.; Fullerton, M.L.; Blake, D.A. Fabrication of Biodegradable Poly(naringin) Particles with Antioxidant Activity and Low Toxicity. ACS Omega 2018, 3, 17359–17367. [Google Scholar] [CrossRef]
- Sahiner, M.; Blake, D.A.; Fullerton, M.L.; Suner, S.S.; Sunol, A.K.; Sahiner, N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019, 137, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.Q.; Wechter, E.; Siegmund, L.A. Glycogen Storage Disease Type l: Don’t Miss the Signs. J. Nurse Pract. 2020, 16, 442–446. [Google Scholar] [CrossRef]
- Marusic, T.; Zerjav Tansek, M.; Sirca Campa, A.; Mezek, A.; Berden, P.; Battelino, T.; Groselj, U. Normalization of obstructive cardiomyopathy and improvement of hepatopathy on ketogenic diet in patient with glycogen storage disease (GSD) type IIIa. Mol. Genet. Metab. Rep. 2020, 24, 100628. [Google Scholar] [CrossRef] [PubMed]
- Tager, J.M.; Oude Elferink, R.P.J.; Reuser, A.; Kroos, M.; Ginsel, L.A.; Fransen, J.A.M.; Klumperman, J. α-Glucosidase Deficiency (Pompe’s Disease). Enzyme 1987, 38, 0280–0285. [Google Scholar] [CrossRef] [PubMed]
- Newland, B.; Ehret, F.; Hoppe, F.; Eigel, D.; Pette, D.; Newland, H.; Welzel, P.B.; Kempermann, G.; Werner, C. Macroporous heparin-based microcarriers allow long-term 3D culture and differentiation of neural precursor cells. Biomaterials 2020, 230, 119540. [Google Scholar] [CrossRef] [PubMed]
- Eigel, D.; Werner, C.; Newland, B. Cryogel biomaterials for neuroscience applications. Neurochem. Int. 2021, 147, 105012. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Nitrogen-Doped Arginine Carbon Dots and Its Metal Nanoparticle Composites as Antibacterial Agent. C J. Carbon Res. 2020, 6, 58. [Google Scholar] [CrossRef]


| Cryogel Type | Vp (mL.g-1) | Density (g·mL−1) | Gel Content% | Cryogel Type |
|---|---|---|---|---|
| CS cryogel | 31.55 ± 6.37 | 0.11 ± 0.03 | 60 ± 3.31 | CS cryogel |
| CS:HNT cryogel | 17.50 ± 3.15 | 0.17 ± 0.09 | 76 ± 0.84 | CS:HNT cryogel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S.; Sahiner, M.; Ari, B.; Sunol, A.K.; Sahiner, N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels 2021, 7, 127. https://doi.org/10.3390/gels7030127
Demirci S, Sahiner M, Ari B, Sunol AK, Sahiner N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels. 2021; 7(3):127. https://doi.org/10.3390/gels7030127
Chicago/Turabian StyleDemirci, Sahin, Mehtap Sahiner, Betul Ari, Aydin K. Sunol, and Nurettin Sahiner. 2021. "Chondroitin Sulfate-Based Cryogels for Biomedical Applications" Gels 7, no. 3: 127. https://doi.org/10.3390/gels7030127
APA StyleDemirci, S., Sahiner, M., Ari, B., Sunol, A. K., & Sahiner, N. (2021). Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels, 7(3), 127. https://doi.org/10.3390/gels7030127









