Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption
Abstract
:1. Introduction
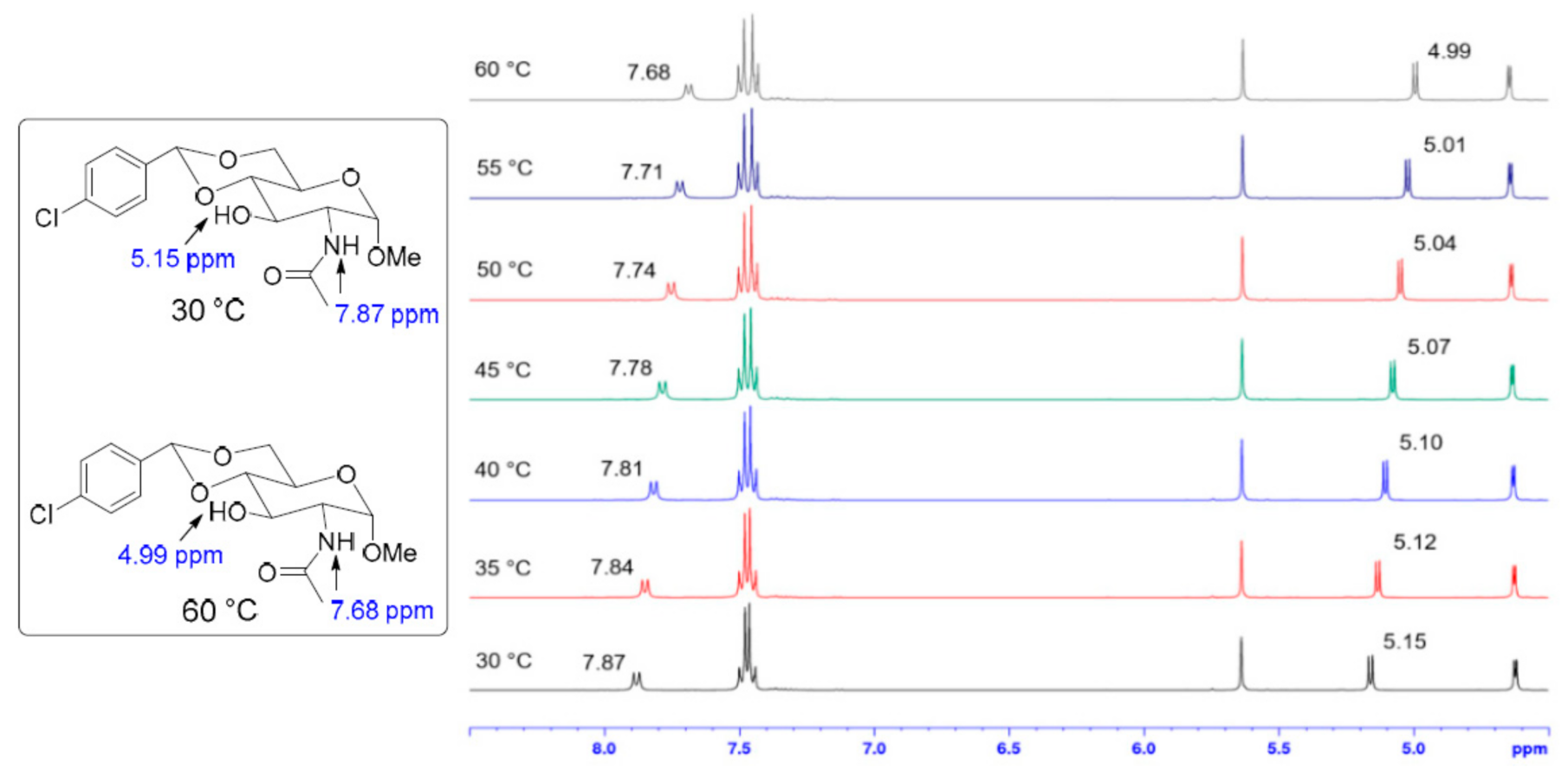
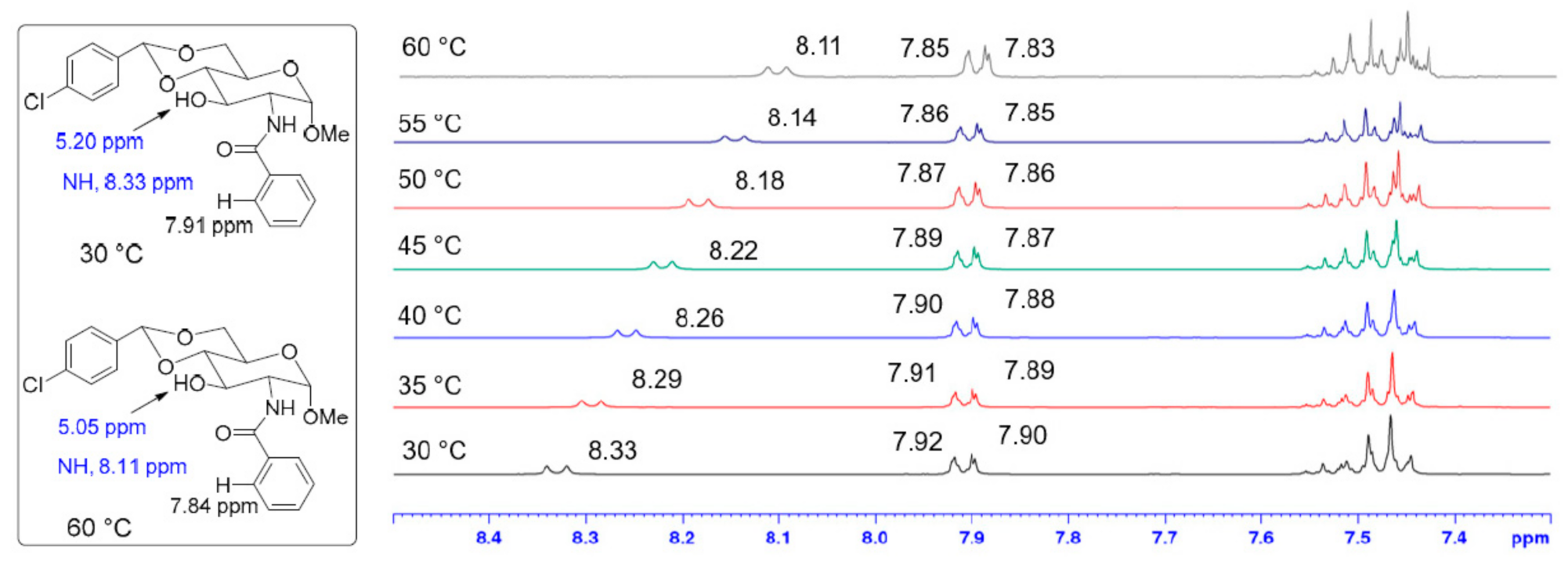
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Methods and Materials
4.2. Optical Microscopy
4.3. Atomic Force Microscopy
4.4. Gelation Tests
4.5. Naproxen Trapping and Release Studies
4.6. Chloramphenicol Trapping and Release Studies
4.7. Dye Absorption Studies
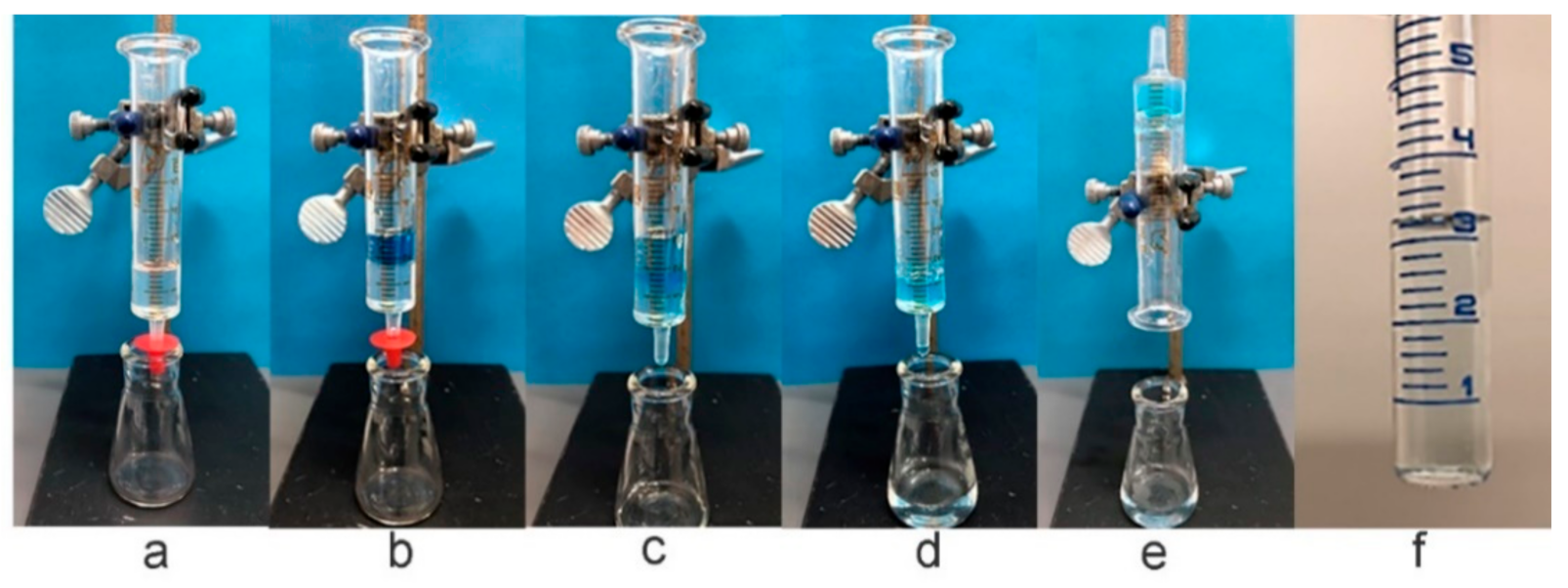
4.8. Extrusion Studies
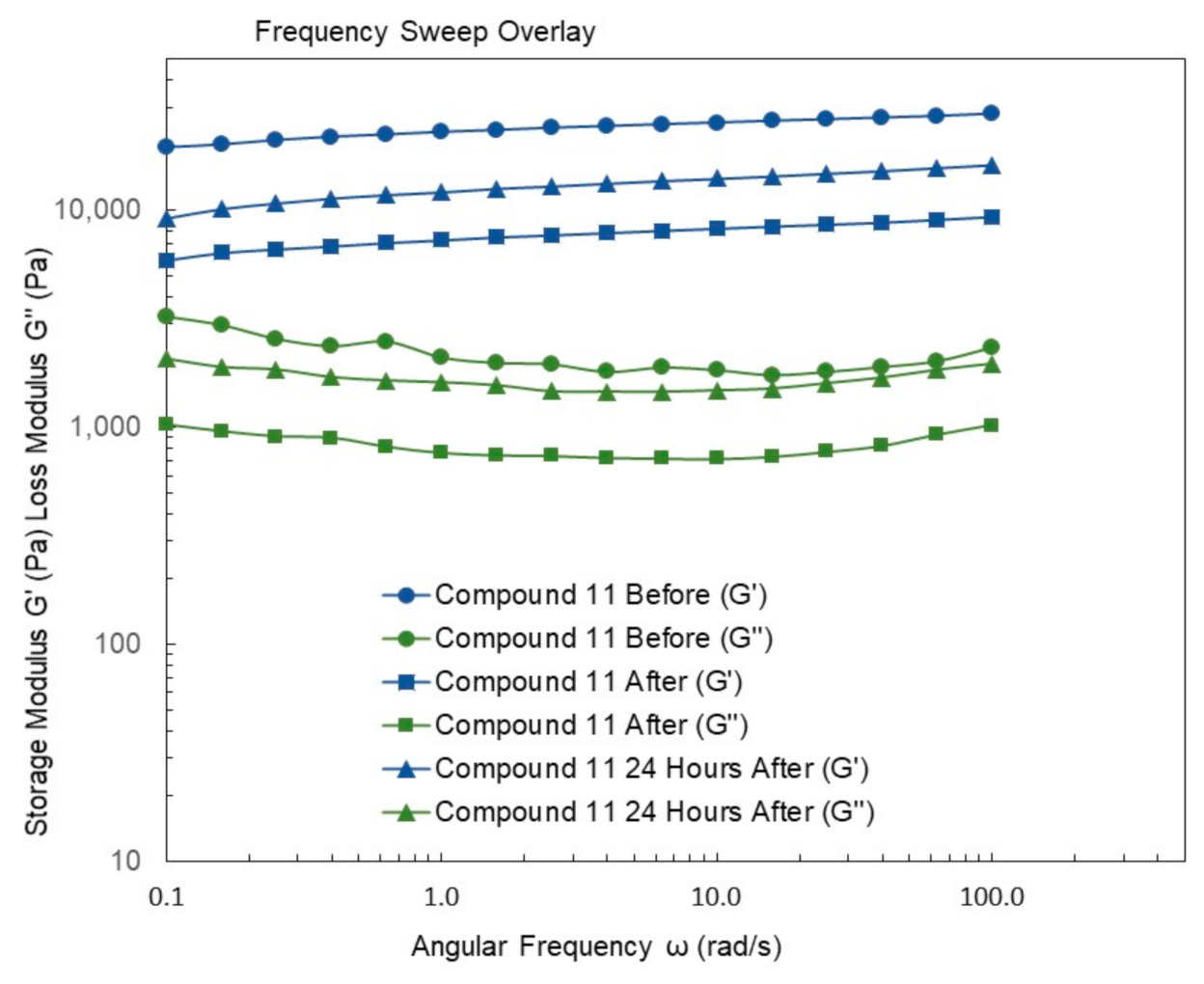
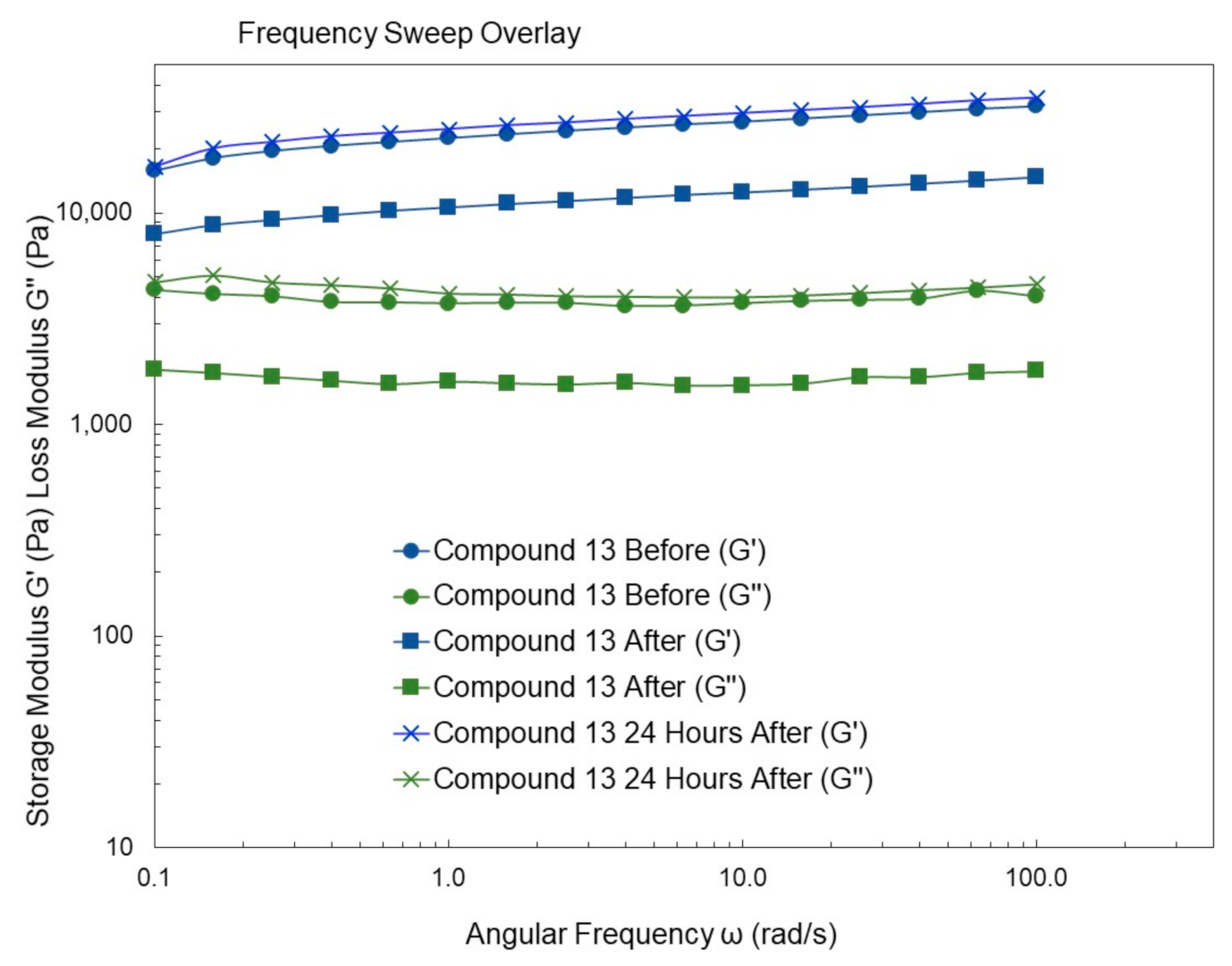
4.9. Rheological Studies
4.10. Synthesis and Characterization Data for Compounds 3–18
4.10.1. Synthesis of Compound 3
4.10.2. Synthesis of Compound 4
4.10.3. General Procedure for the Synthesis of Amides 5–18
4.10.4. General Procedure for the Synthesis of Acid Chlorides
4.10.5. Synthesis of Compound 5
4.10.6. Synthesis of Compound 6
4.10.7. Synthesis of Compound 7
4.10.8. Synthesis of Compound 8
4.10.9. Synthesis of Compound 9
4.10.10. Synthesis of Compound 10
4.10.11. Synthesis of Compound 11
4.10.12. Synthesis of Compound 12
4.10.13. Synthesis of Compound 13
4.10.14. Synthesis of Compound 14
4.10.15. Synthesis of Compound 15
4.10.16. Synthesis of Compound 16
4.10.17. Synthesis of Compound 17
4.10.18. Synthesis of Compound 18
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, G.; Niu, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Muthuvijayan, V.; Prasad, E. In vitro study of a glucose attached poly(aryl ether) dendron based gel as a drug carrier for a local anaesthetic. New J. Chem. 2017, 41, 7453–7462. [Google Scholar] [CrossRef]
- Sharma, K.; Joseph, J.P.; Sahu, A.; Yadav, N.; Tyagi, M.; Singh, A.; Pal, A.; Kartha, K.P.R. Supramolecular gels from sugar-linked triazole amphiphiles for drug entrapment and release for topical application. RSC Adv. 2019, 9, 19819–19827. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Wang, D.; Bietsch, J.; Wang, G. Synthesis and characterization of pentaerythritol derived glycoconjugates as supramolecular gelators. Org. Biomol. Chem. 2019, 17, 6043–6056. [Google Scholar] [CrossRef]
- Zhou, J.; O’Keeffe, M.; Liao, G.; Zhao, F.; Terhorst, C.; Xu, B. Design and synthesis of nanofibers of self-assembled de novo glycoconjugates towards mucosal lining restoration and anti-inflammatory drug delivery. Tetrahedron 2016, 72, 6078–6083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vibhute, A.M.; Muvvala, V.; Sureshan, K.M. A Sugar-Based Gelator for Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2016, 55, 7782–7785. [Google Scholar] [CrossRef]
- Pathak, N.P.; Chatterjee, D.; Paul, A.; Yadav, S. Arabinose based gelators: Rheological characterization of the gels and phase selective organogelation of crude-oil. RSC Adv. 2016, 6, 92225–92234. [Google Scholar] [CrossRef]
- Mukherjee, S.; Shang, C.; Chen, X.; Chang, X.; Liu, K.; Yu, C.; Fang, Y. N-Acetylglucosamine-based efficient, phase-selective organogelators for oil spill remediation. Chem. Commun. 2014, 50, 13940–13943. [Google Scholar] [CrossRef]
- Guan, X.; Fan, K.; Gao, T.; Ma, A.; Zhang, B.; Song, J. A novel multi-stimuli responsive gelator based on D-gluconic acetal and its potential applications. Chem. Commun. 2016, 52, 962–965. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, A.; Morris, J.; Wang, G. Stimuli-responsive gelators from carbamoyl sugar derivatives and their responses to metal ions and tetrabutylammonium salts. RSC Adv. 2020, 10, 40068–40083. [Google Scholar] [CrossRef]
- Piepenbrock, M.O.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, J.; Chen, M.; Yang, Z. Self-assembling small molecules for the detection of important analytes. Chem. Soc. Rev. 2014, 43, 7257–7266. [Google Scholar] [CrossRef] [PubMed]
- Thamizhanban, A.; Lalitha, K.; Sarvepalli, G.P.; Maheswari, C.U.; Sridharan, V.; Rayappan, J.B.B.; Nagarajan, S. Smart supramolecular gels of enolizable amphiphilic glycosylfuran. J. Mater. Chem. B 2019, 7, 6238–6246. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Bietsch, J.; Chen, A.; Sharma, P. Synthesis of Dendritic Glycoclusters and Their Applications for Supramolecular Gelation and Catalysis. J. Org. Chem. 2020, 85, 16136–16156. [Google Scholar] [CrossRef]
- Diaz Diaz, D.; Kuhbeck, D.; Koopmans, R.J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar] [CrossRef] [PubMed]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef]
- John, G.; Vijai Shankar, B.; Jadhav, S.R.; Vemula, P.K. Biorefinery: A Design Tool for Molecular Gelators. Langmuir 2010, 26, 17843–17851. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Kozlowski, P.; Wang, G. Synthesis and Characterization of Hybrid Glycolipids as Functional Organogelators and Hydrogelators. Langmuir 2019, 35, 14639–14650. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Adhikari, S.B.; Mays, K.; Wang, G. Synthesis and Study of Molecular Assemblies Formed by 4,6-O-(2-Phenylethylidene)-Functionalized D-Glucosamine Derivatives. Langmuir 2017, 33, 8076–8089. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Cheuk, S.; Wang, G. Synthesis and characterization of D-glucosamine-derived low molecular weight gelators. Tetrahedron 2010, 66, 5962–5971. [Google Scholar] [CrossRef]
- Wang, G.; Chen, A.; Mangunuru, H.P.R.; Yerabolu, J.R. Synthesis and characterization of amide linked triazolyl glycolipids as molecular hydrogelators and organogelators. RSC Adv. 2017, 7, 40887–40895. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Friggeri, A.; Koumoto, K.; Amaike, M.; Shinkai, S.; Reinhoudt, D.N. Molecular Design of “Super” Hydro-Gelators: Understanding the Gelation Process of Azobenzene-Based Sugar Derivatives in Water. Org. Lett. 2002, 4, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, C.; Tan, M.; Wang, L.; Kong, D.; Yang, Z. A structure-gelation ability study in a short peptide-based ‘Super Hydrogelator’ system. Soft Matter 2011, 7, 3897–3905. [Google Scholar] [CrossRef]
- Yan, N.; He, G.; Zhang, H.; Ding, L.; Fang, Y. Glucose-based fluorescent low-molecular mass compounds: Creation of simple and versatile supramolecular gelators. Langmuir 2010, 26, 5909–5917. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Luo, H.; Zhang, B.; Wang, F.; Song, J. Porous amorphous powder form phase-selective organogelator for rapid recovery of leaked aromatics and spilled oils. J. Hazard. Mater. 2020, 384, 121460. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, R.; Lan, H.; Lu, Y.; Li, Y.; Xiao, S.; Yi, T. Halogen Effect on Non-Conventional Organogel Assisted by Balanced π-π Interaction. ChemistrySelect 2017, 2, 5421–5426. [Google Scholar] [CrossRef]
- Chen, L.; Revel, S.; Morris, K.; Serpell, L.C.; Adams, D.J. Effect of Molecular Structure on the Properties of Naphthalene-Dipeptide Hydrogelators. Langmuir 2010, 26, 13466–13471. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.K.; Manheri, M.K. Towards a fragment-based approach in gelator design: Halogen effects leading to thixotropic, mouldable and self-healing systems in aryl-triazolyl amino acid-based gelators! Chem. Commun. 2017, 53, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef]
- Pizzi, A.; Lascialfari, L.; Demitri, N.; Bertolani, A.; Maiolo, D.; Carretti, E.; Metrangolo, P. Halogen bonding modulates hydrogel formation from Fmoc amino acids. CrystEngComm 2017, 19, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Corradi, E.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G. Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly Processes. Angew. Chem. Int. Ed. 2000, 39, 1782–1786. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Li, Z.; Zhang, Y.; Cao, W.; Wang, L.; Liu, S. Unusual C-I···O Halogen Bonding in Triazole Derivatives: Gelation Solvents at Two Extremes of Polarity and Formation of Superorganogels. Langmuir 2017, 33, 311–321. [Google Scholar] [CrossRef]
- Mac Cormack, A.S.; Busch, V.M.; Japas, M.L.; Giovanetti, L.; Di Salvo, F.; Di Chenna, P.H. The effect of vicinal di-halo substituents on the organogelling properties of aromatic supramolecular gelators and their application as soft templates. New J. Chem. 2020, 44, 8198–8208. [Google Scholar] [CrossRef]
- Chen, A.; Samankumara, L.P.; Garcia, C.; Bashaw, K.; Wang, G. Synthesis and characterization of 3-O-esters of N-acetyl-D-glucosamine derivatives as organogelators. New J. Chem. 2019, 43, 7950–7961. [Google Scholar] [CrossRef]
- Chen, A.; Okafor, I.S.; Garcia, C.; Wang, G. Synthesis and self-assembling properties of 4,6-O-benzylidene acetal protected D-glucose and D-glucosamine β-1,2,3-triazole derivatives. Carbohydr. Res. 2018, 461, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Goyal, N.; Mangunuru, H.P.R.; Yang, H.; Cheuk, S.; Reddy, P.V.N. Preparation and self-assembly study of amphiphilic and bispolar diacetylene-containing glycolipids. J. Org. Chem. 2015, 80, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Mangunuru, H.P.R.; Parikh, B.; Shrestha, S.; Wang, G. Synthesis and characterization of pH responsive D-glucosamine based molecular gelators. Beilstein J. Org. Chem. 2014, 10, 3111–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]




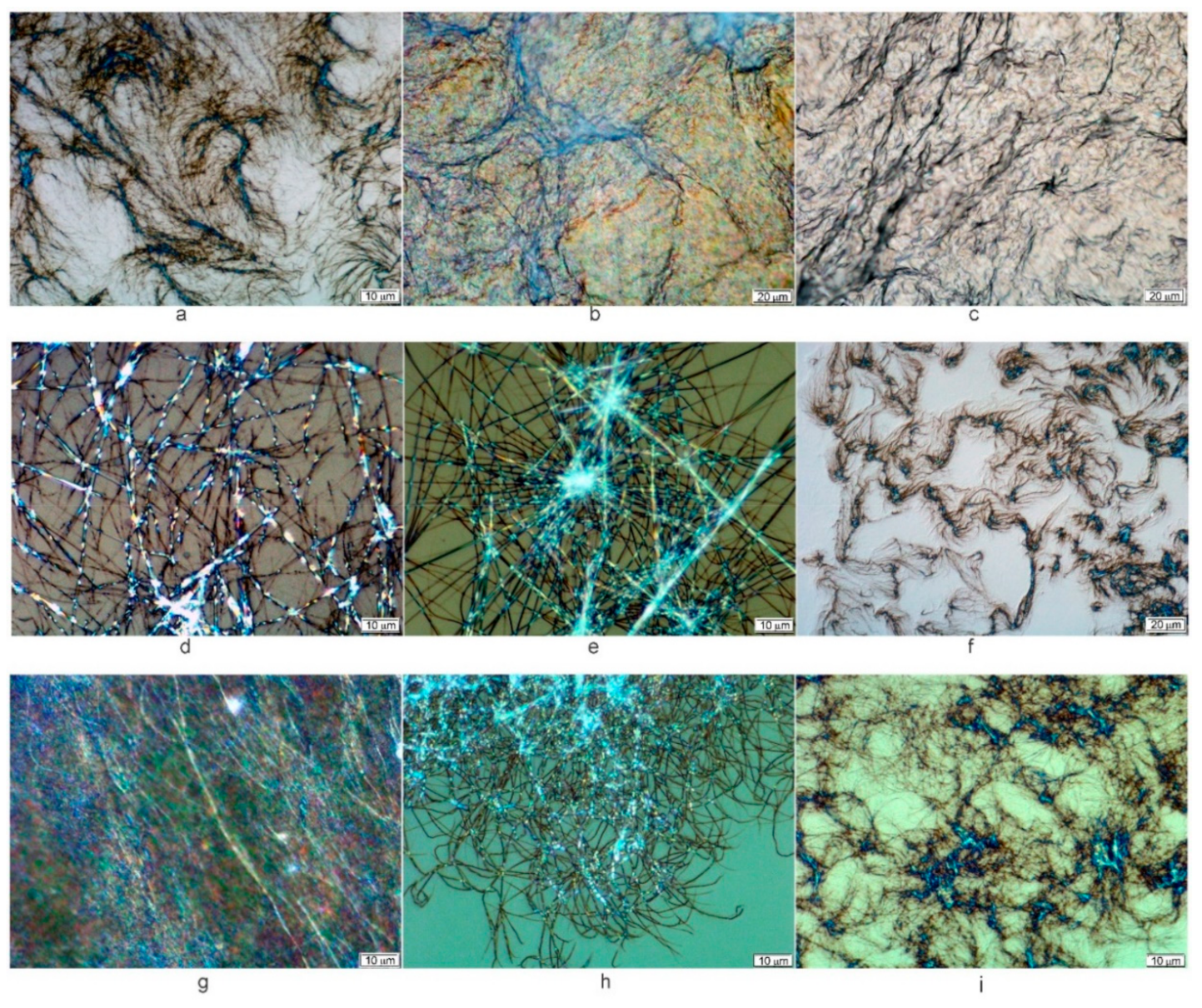
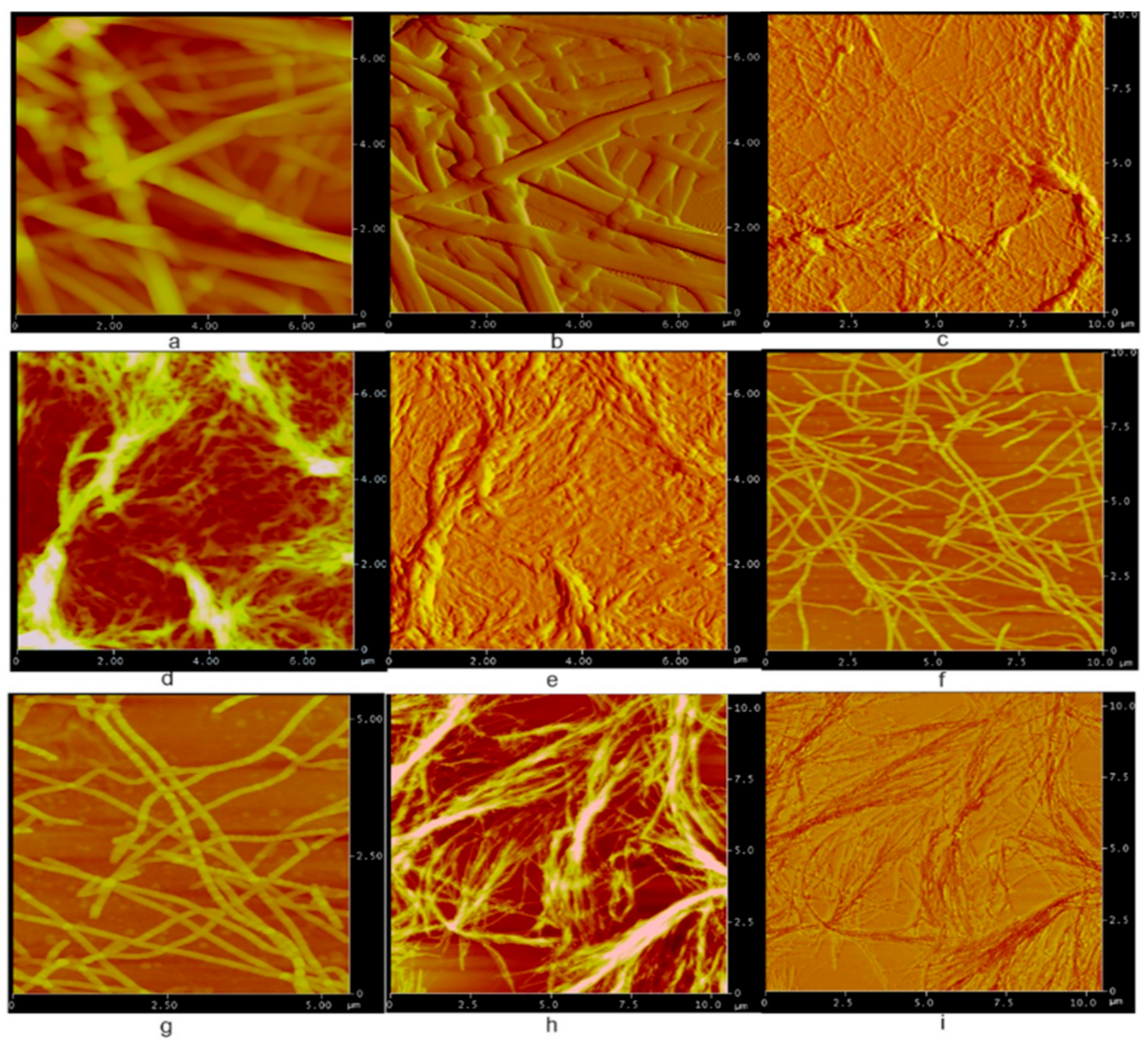




| Comp # | R = | Hex. | Tol. | n-BuOH | n-PrOH | i-PrOH | EtOH | TEG | EG | Gly | H2O | EtOH/H2O1:1 | EtOH/H2O1:2 | DMSO/H2O1:1 | DMSO/H2O1:2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 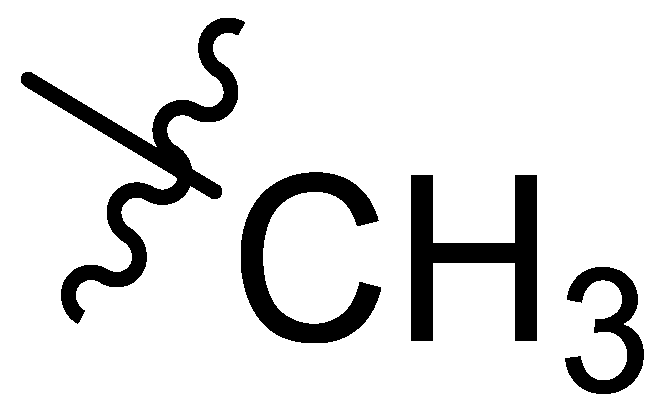 | I | G2.9O | G10.0T | G3.3O | G20.0O | G20.0O | G10.0T | G3.3C | G4.0C | I | G6.7O | P | G20.0O | G5.0O |
| 5 | 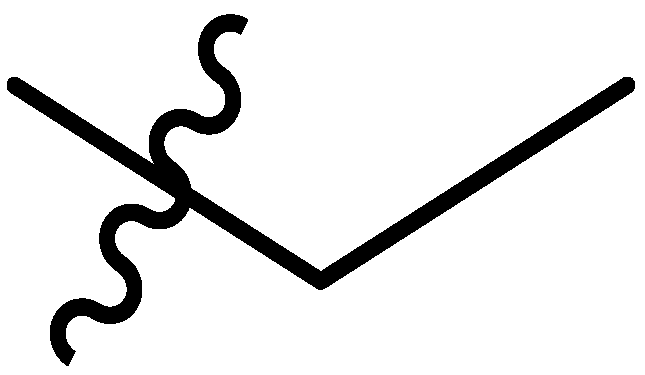 | I | P | P | G20.0O | G20.0O | G10.0O | S | G6.7c | I | I | P | P | P | P |
| 6 | 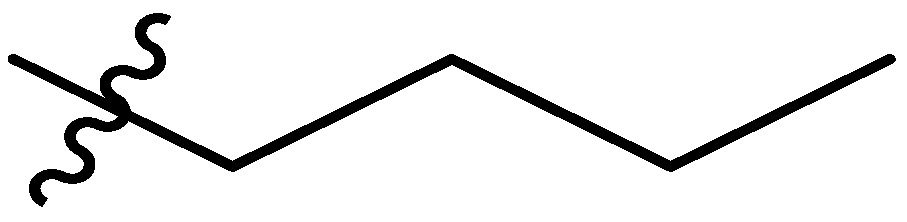 | I | P | G1.0O | G20.0O | G6.7T | G20.0T | S | G6.7T | I | I | G0.74T | G10.0O | 0.74T | G1.0T |
| 7 | 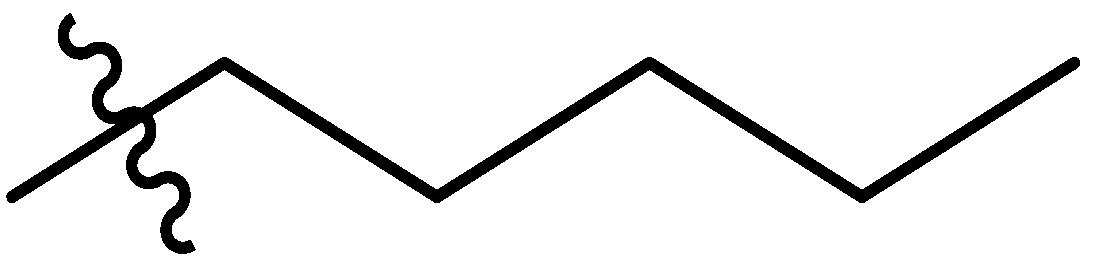 | I | G10.0O | G20.0T | P | G20.0T | G20.0T | G20.0O | G20.0T | I | I | P | P | G0.91T | G6.7T |
| 8 | 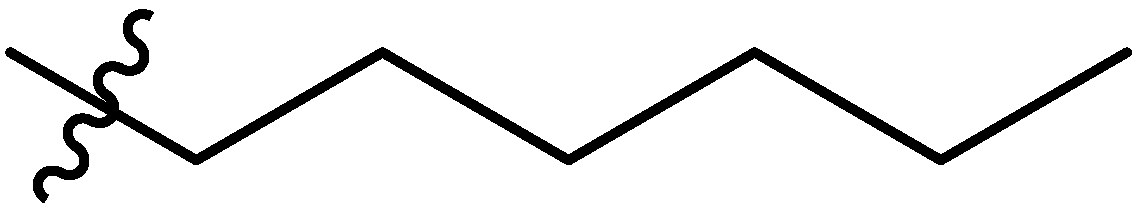 | I | G10.0T | G10.0O | G20.0O | G10.0O | P | S | I | I | I | P | P | G1.3T | G0.80T |
| 9 |  | I | G20.0C | G6.7O | G6.7O | G3.3C | G6.7T | S | G6.7T | G3.3C | I | G1.3T | G2.5T | G1.5T | G2.2T |
| 10 | 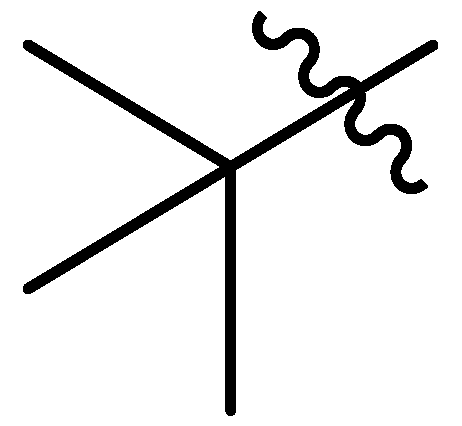 | G5.0O | S | S | S | S | S | S | G20.0O | G20.0O | I | G20.0O | P | G20.0O | P |
| 11 | 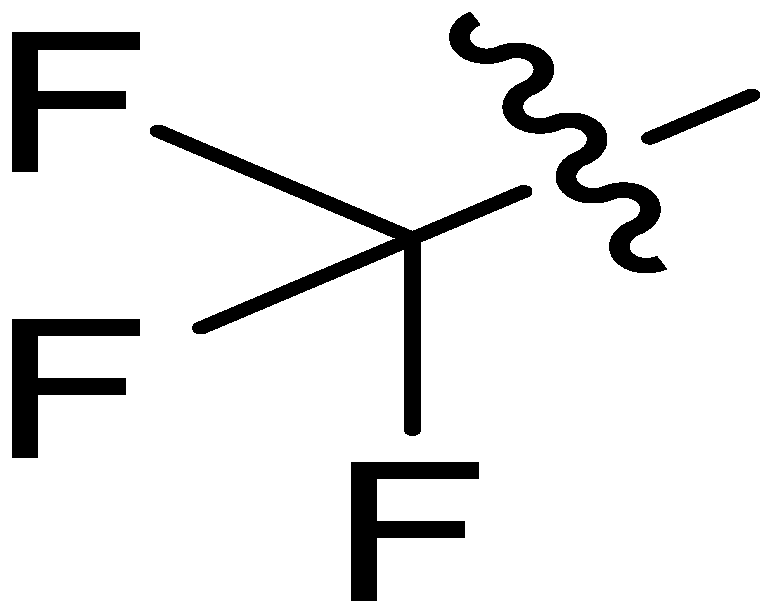 | I | P | G20.0O | G10.0O | G20.0O | G10.0C | S | G10.0C | I | I | G2.0C | G1.4T | G2.2C | G1.5C |
| 12 | 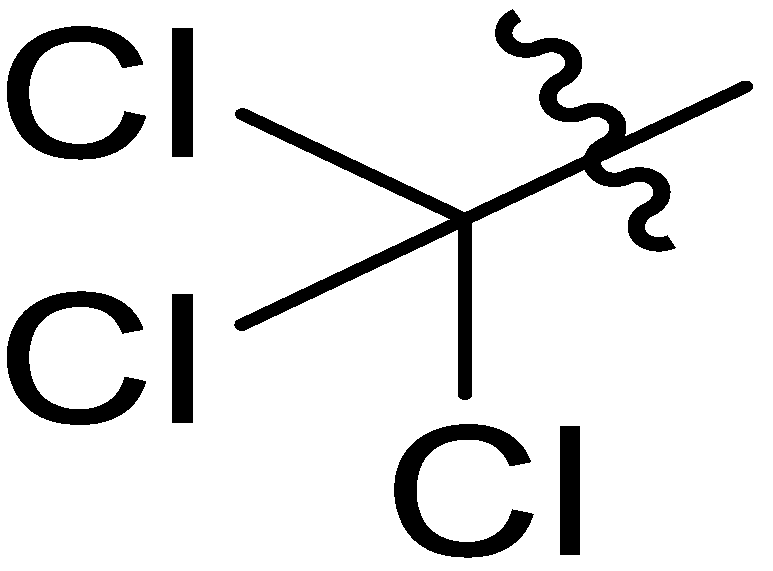 | I | S | S | S | S | S | S | S | G10.0O | I | G0.67C | G0.91T | G2.0T | G3.3T |
| 13 | 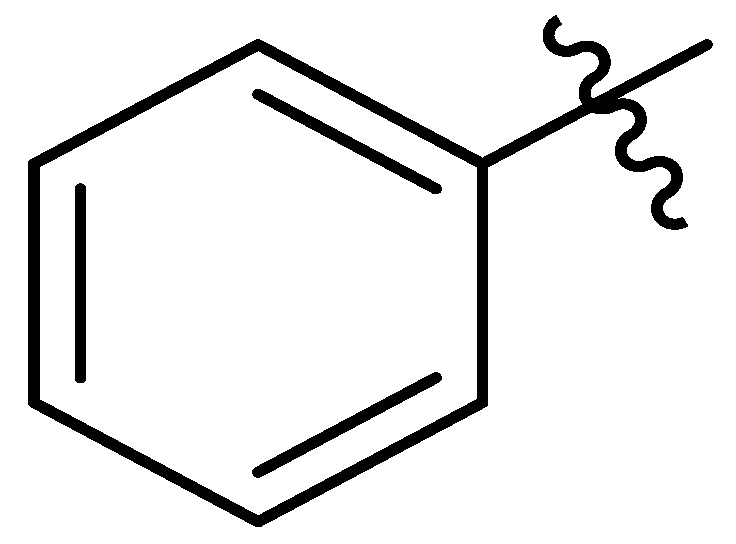 | I | G10.0T | P | P | P | G5.0O | S | G10.0O | G5.0O | G0.36T | G0.54C | G0.36T | G0.54C | G0.46C |
| 14 | 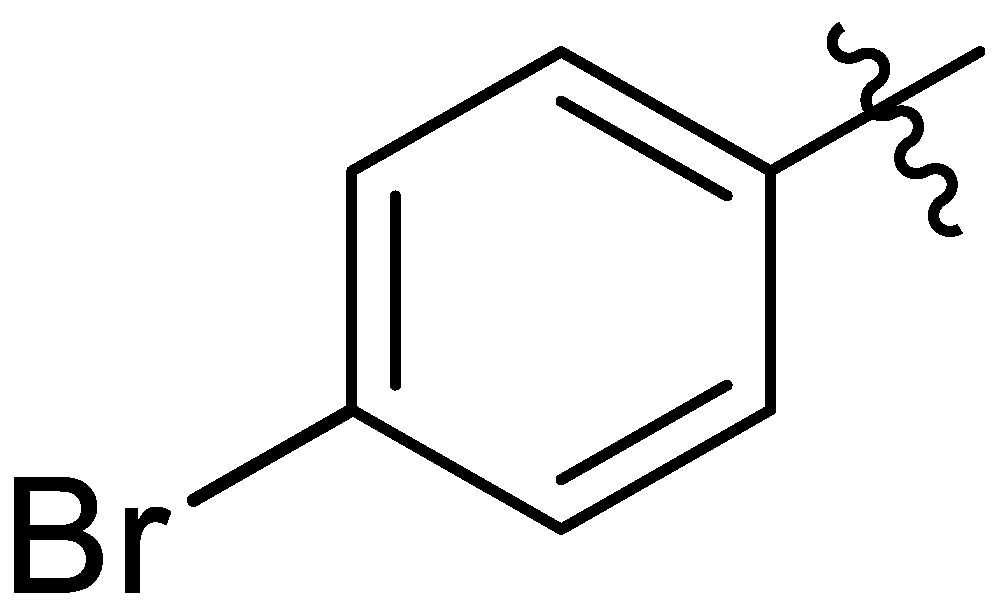 | I | I | G20.0O | P | I | P | S | G10.0O | I | I | I | I | G20.0O | G20.0O |
| 15 | 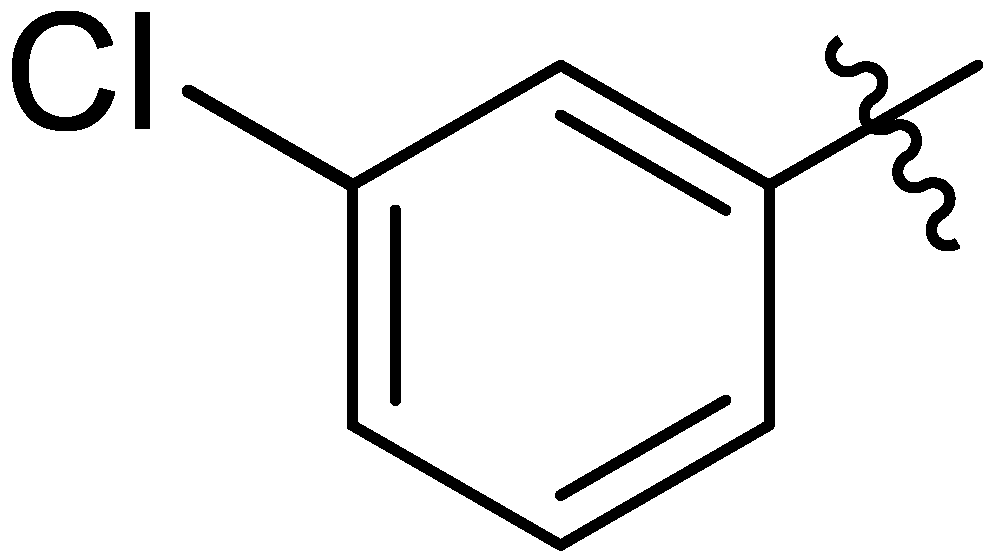 | I | G10.0O | G10.0O | G10.0O | G6.7O | P | S | G10.0O | G6.7O | I | G1.3O | G10.0O | G0.71O | G1.3O |
| 16 | 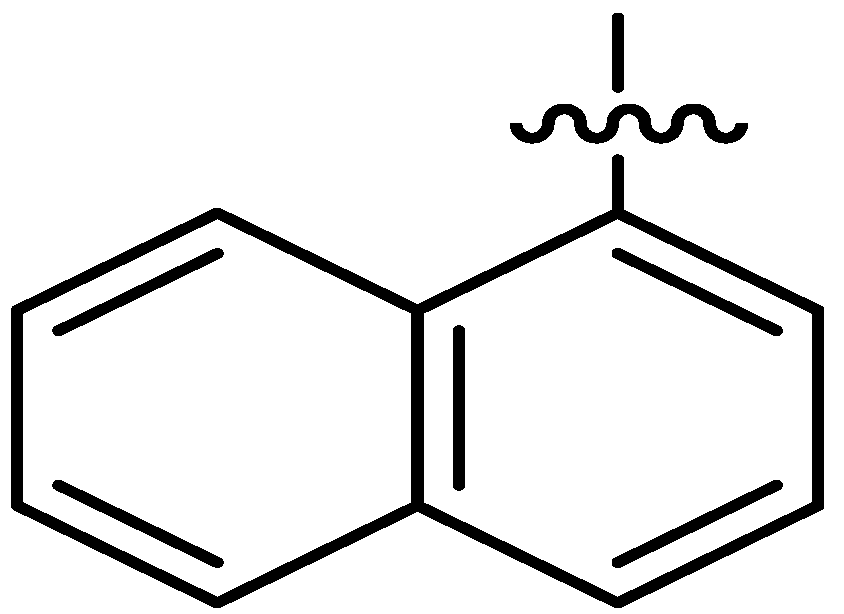 | I | P | G10.0O | G10.0C | G10.0O | G20.0C | S | G10.0O | G6.7T | I | I | I | G5.0O | G2.9O |
| 17 | 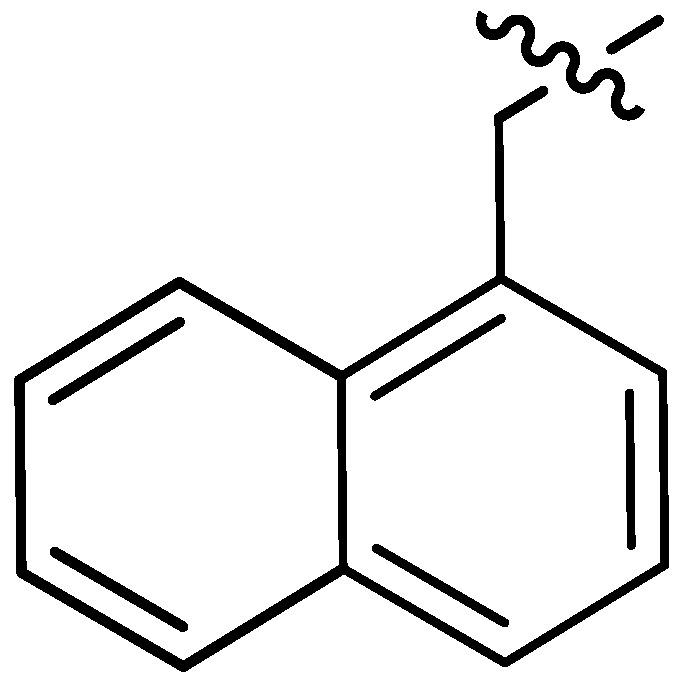 | I | P | P | G10.0T | I | I | S | G5.0T | G6.7T | I | P | I | P | I |
| 18 | 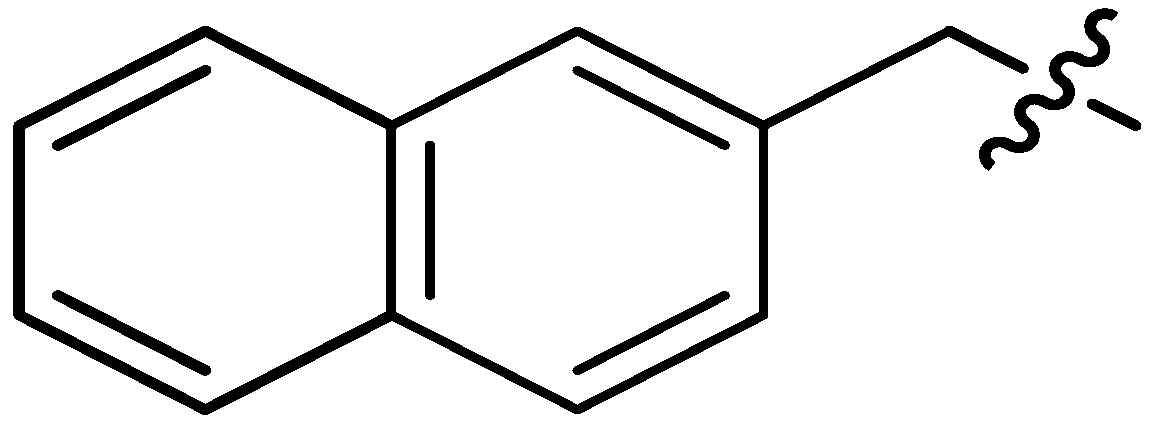 | I | P | I | I | I | I | S | G10.0T | I | I | I | I | P | I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bietsch, J.; Olson, M.; Wang, G. Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption. Gels 2021, 7, 134. https://doi.org/10.3390/gels7030134
Bietsch J, Olson M, Wang G. Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption. Gels. 2021; 7(3):134. https://doi.org/10.3390/gels7030134
Chicago/Turabian StyleBietsch, Jonathan, Mary Olson, and Guijun Wang. 2021. "Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption" Gels 7, no. 3: 134. https://doi.org/10.3390/gels7030134
APA StyleBietsch, J., Olson, M., & Wang, G. (2021). Fine-Tuning of Molecular Structures to Generate Carbohydrate Based Super Gelators and Their Applications for Drug Delivery and Dye Absorption. Gels, 7(3), 134. https://doi.org/10.3390/gels7030134







