Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption
Abstract
:1. Introduction
2. Results and Discussion
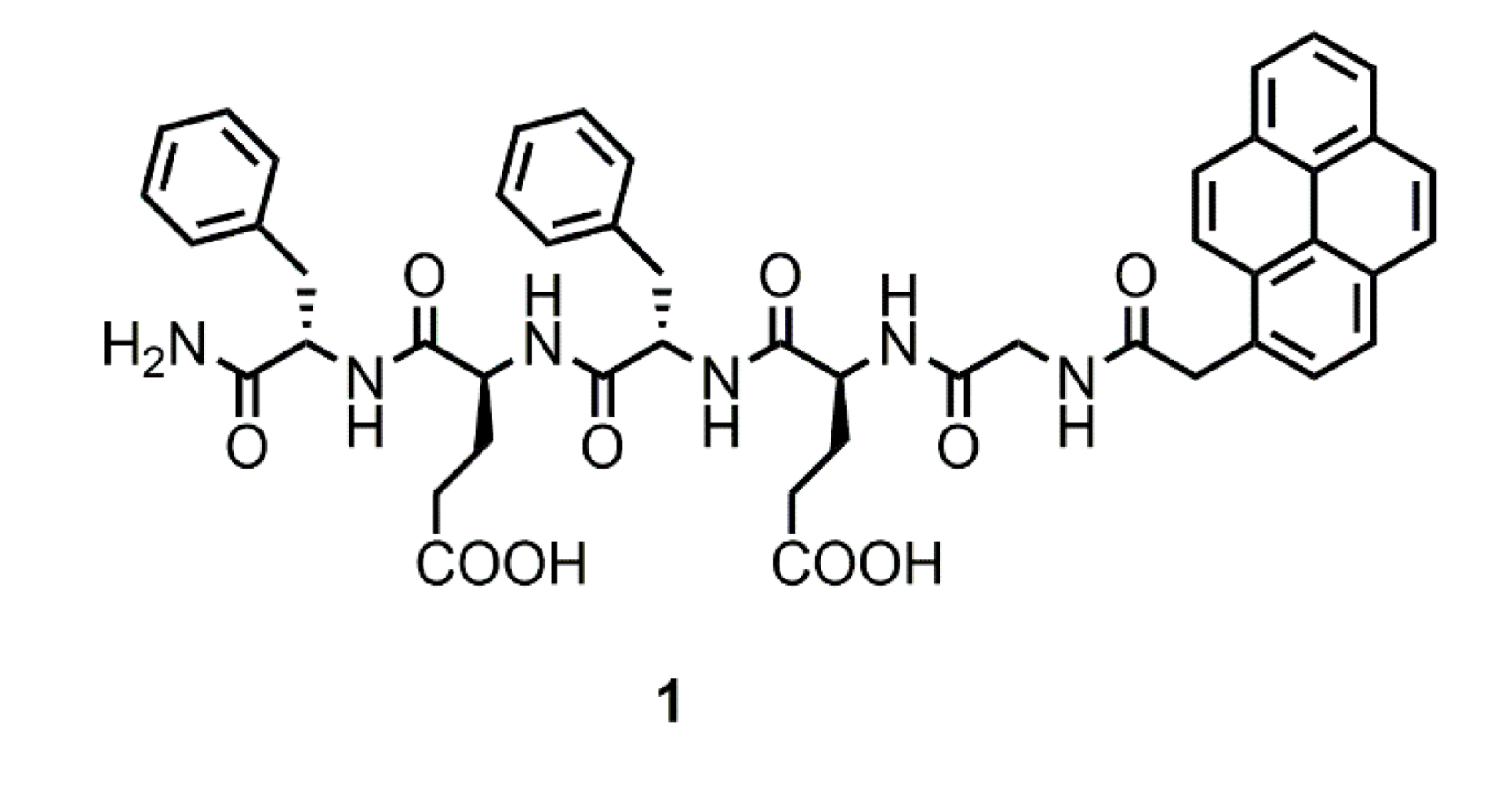
2.1. Design and Synthesis of 1
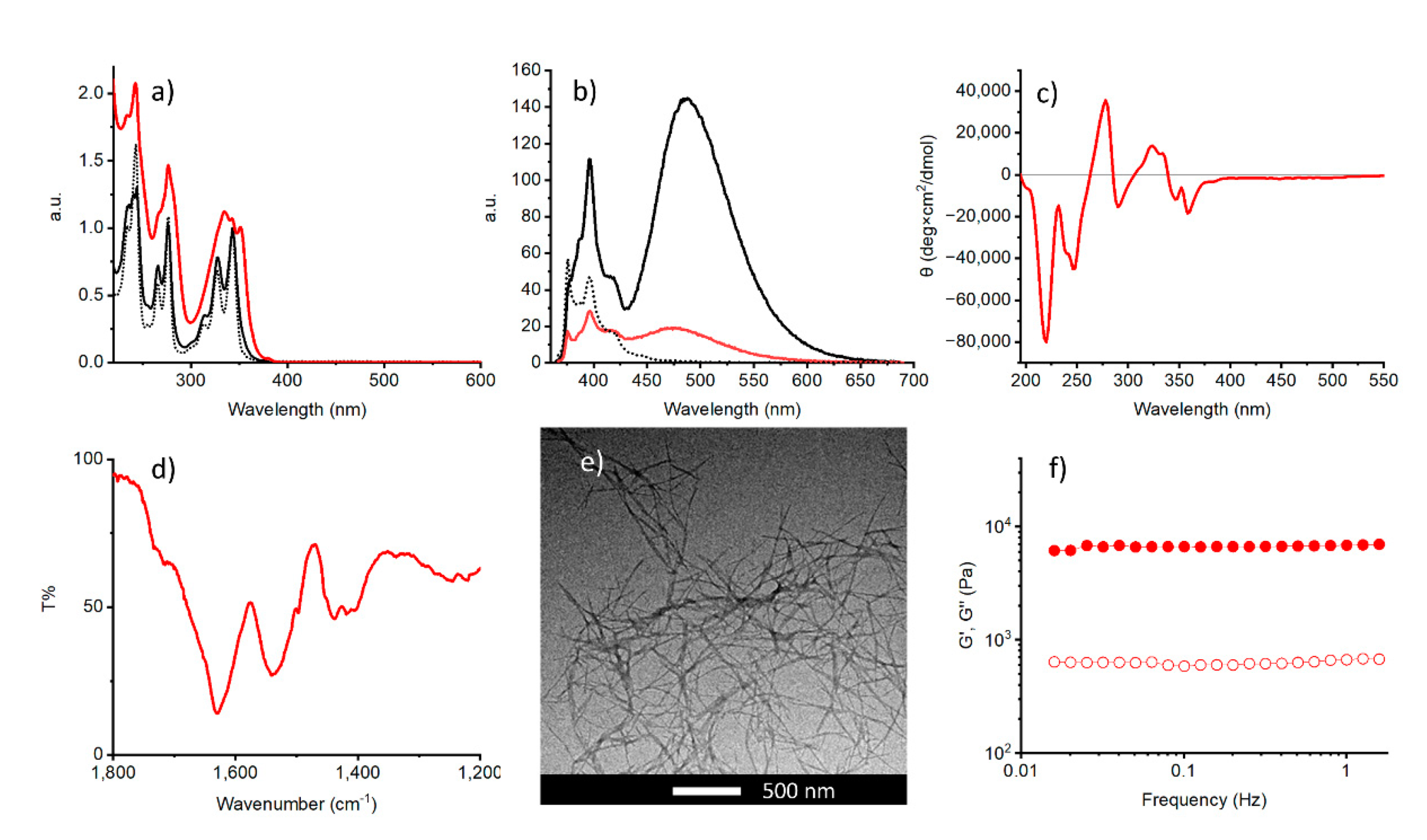
2.2. pH-Triggered Gelation
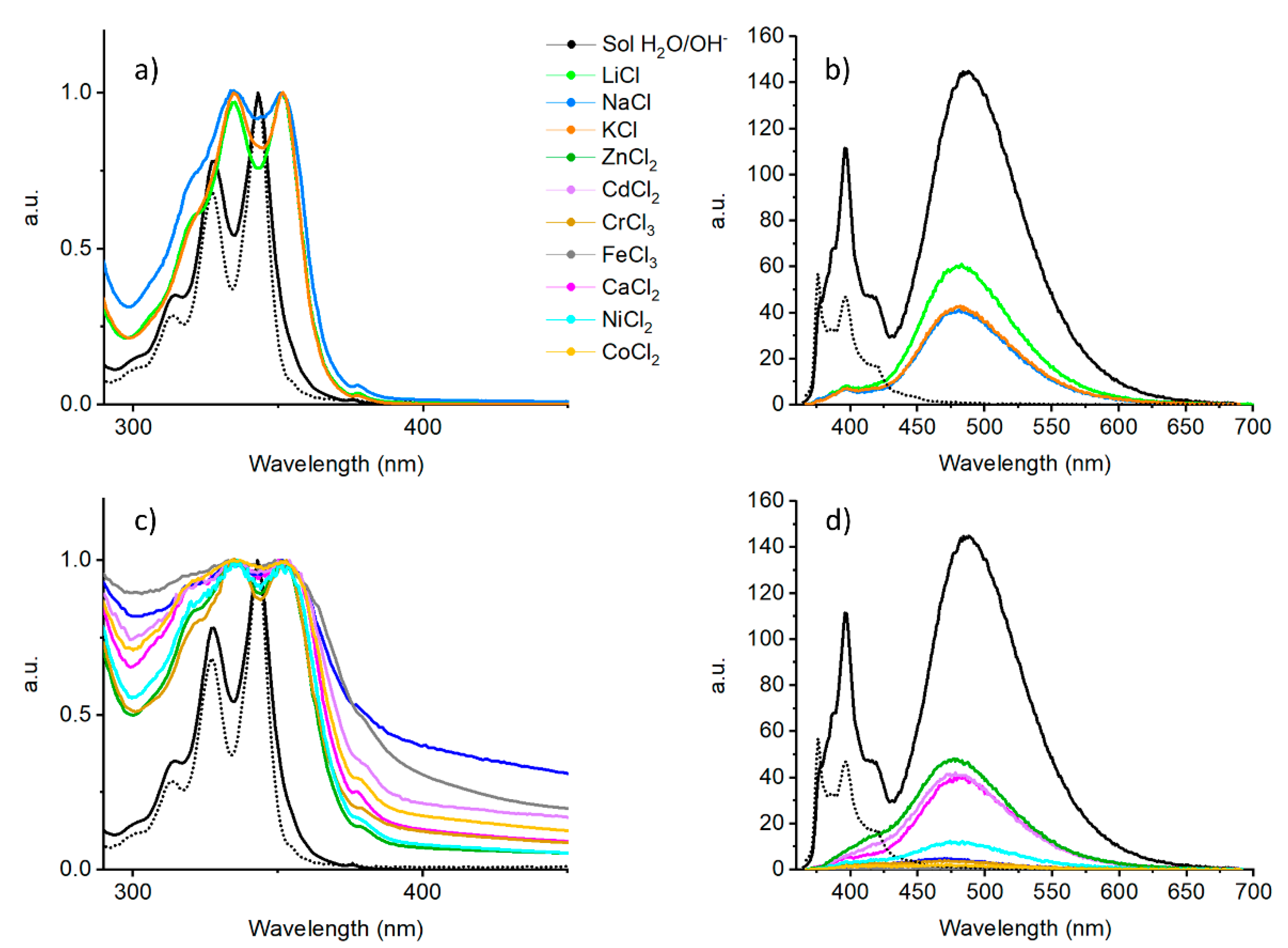
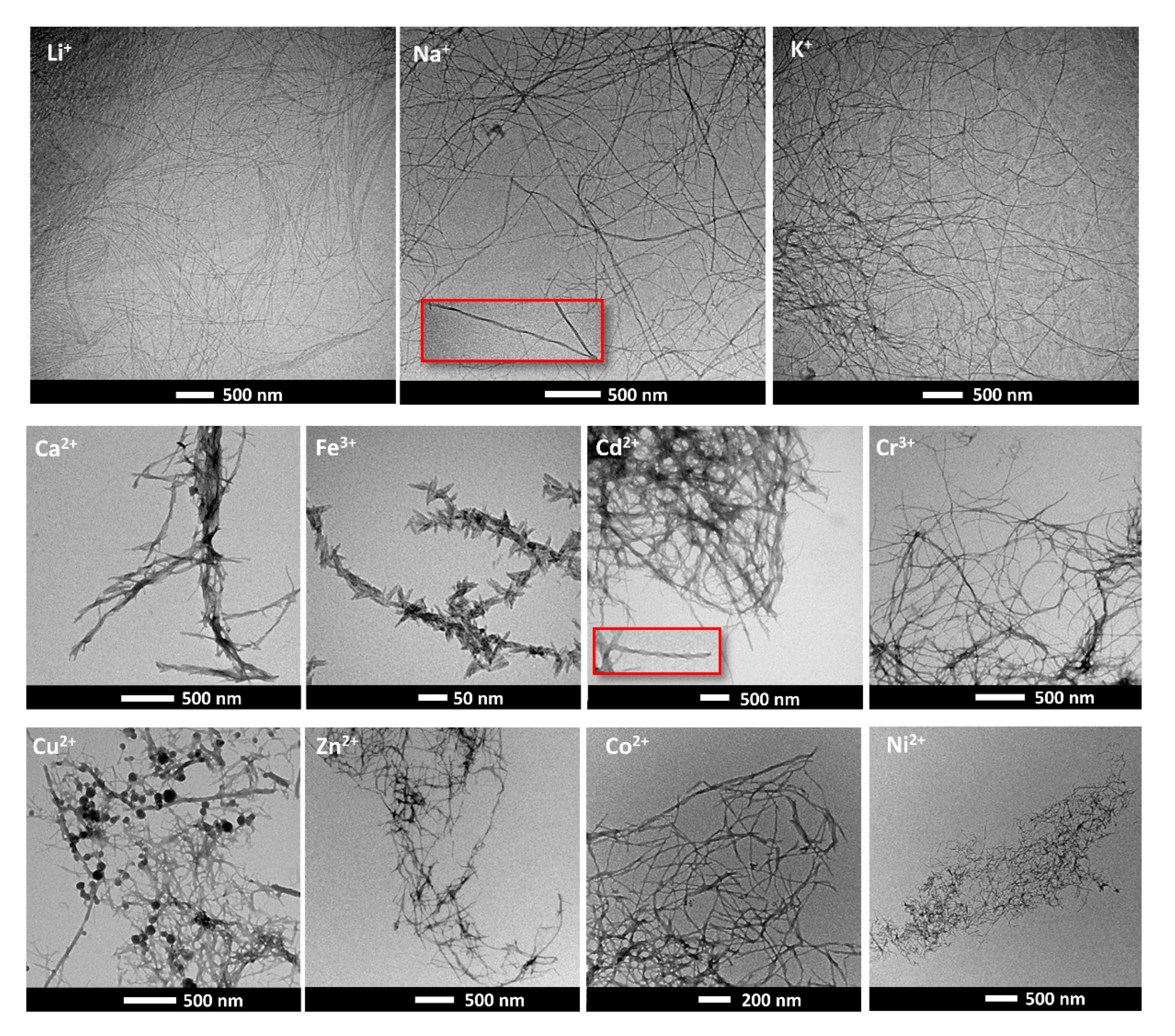
2.3. Metal Ion Triggered Gelation
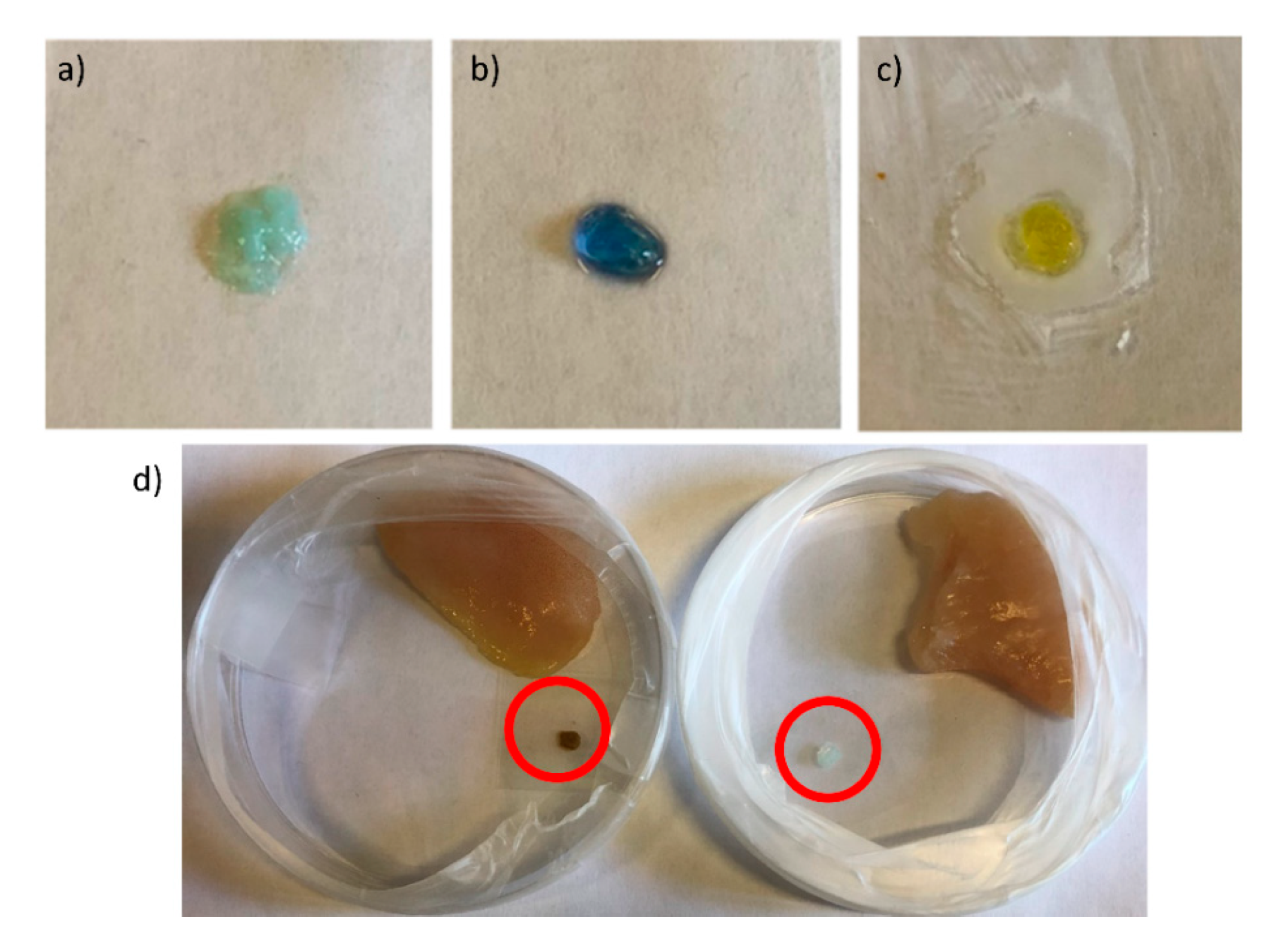
2.4. Detection of Amines and Meat Freshness Monitoring
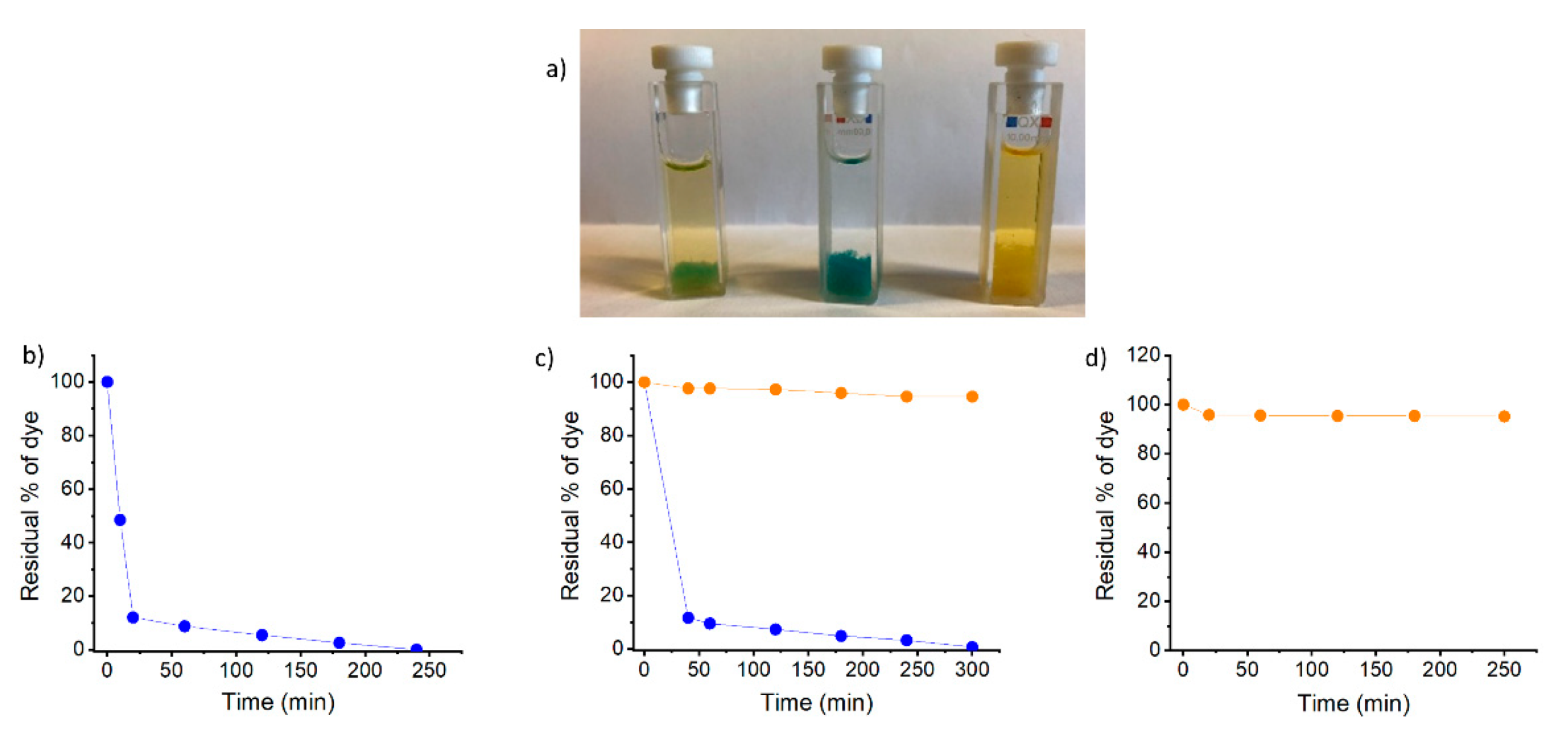
2.5. Selective Cationic Dye Adsorption
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Synthesis of 1
4.3. Gel Preparation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Du, X.W.; Zhou, J.; Shi, J.F.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Escuder, B.; Miravet, J.F. Functional Molecular Gels; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.W.; Ou, B.; Jiang, S.T.; Yin, G.Q.; Chen, L.J.; Xu, L.; Li, X.P.; Yang, H.B. Cross-linked AIE supramolecular polymer gels with multiple stimuli-responsive behaviours constructed by hierarchical self-assembly. Polym. Chem. 2018, 9, 2021–2030. [Google Scholar] [CrossRef]
- Sun, Z.F.; Huang, Q.Y.; He, T.; Li, Z.Y.; Zhang, Y.; Yi, L.Z. Multistimuli-Responsive Supramolecular Gels: Design Rationale, Recent Advances, and Perspectives. ChemPhysChem 2014, 15, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mat. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.-L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, Applicability, and Advanceability. Adv. Mater. 2019, 31, 1806204. [Google Scholar] [CrossRef]
- Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Recent advances in coordination-driven polymeric gel materials: Design and applications. Dalton Trans. 2020, 49, 7658–7672. [Google Scholar] [CrossRef]
- Huang, J.; He, L.; Zhang, J.; Chen, L.; Su, C.-Y. Dynamic functionalised metallogel: An approach to immobilised catalysis with improved activity. J. Mol. Catal. A: Chem. 2010, 317, 97–103. [Google Scholar] [CrossRef]
- Naseer, F.; Ajmal, M.; Bibi, F.; Farooqi, Z.H.; Siddiq, M. Copper and cobalt nanoparticles containing poly(acrylic acid-co-acrylamide) hydrogel composites for rapid reduction of 4-nitrophenol and fast removal of malachite green from aqueous medium. Polym. Compos. 2018, 39, 3187–3198. [Google Scholar]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Wei, T.-B.; Li, H.; Zhang, Y.-M. Rationally introduce multi-competitive binding interactions in supramolecular gels: A simple and efficient approach to develop multi-analyte sensor array. Chem. Sci. 2016, 7, 5341–5346. [Google Scholar] [PubMed] [Green Version]
- Liu, J.; He, P.; Yan, J.; Fang, X.; Peng, J.; Liu, K.; Fang, Y. An organometallic super-gelator with multiple-stimulus responsive properties. Adv. Mater. 2008, 20, 2508–2511. [Google Scholar]
- Sun, Z.; Li, Z.; He, Y.; Shen, R.; Deng, L.; Yang, M.; Liang, Y.; Zhang, Y. Ferrocenoyl phenylalanine: A new strategy toward supramolecular hydrogels with multistimuli responsive properties. J. Am. Chem. Soc. 2013, 135, 13379–13386. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Fujita, N.; Tani, T.; Kaneko, K.; Shinkai, S. Organogel of an 8-quinolinol platinum (II) chelate derivative and its efficient phosphorescence emission effected by inhibition of dioxygen quenching. Chem. Commun. 2005, 4149–4151. [Google Scholar] [CrossRef]
- Dixit, M.K.; Mahendar, C.; Dubey, M. Cd2+-induced Fluorescent Metallogel: A case of CHEF and ACQ phenomenon. Chem. Asian, J. 2019. [Google Scholar] [CrossRef]
- Basak, S.; Nanda, J.; Banerjee, A. Multi-stimuli responsive self-healing metallo-hydrogels: Tuning of the gel recovery property. Chem. Commun. 2014, 50, 2356–2359. [Google Scholar]
- Häring, M.; Díaz, D.D. Supramolecular metallogels with bulk self-healing properties prepared by in situ metal complexation. Chem. Commun. 2016, 52, 13068–13081. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Singh, A.; Sarma, T.K.; Sardana, N.; Pal, A. Chirality control of multi-stimuli responsive and self-healing supramolecular metallo-hydrogels. New, J. Chem. 2018, 42, 6427–6432. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. Part. A 2016, 104, 1002–1016. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X. Biomimetic Self-Assembling Peptide Hydrogels for Tissue Engineering Applications. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 297–312. [Google Scholar]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-assembled peptide hydrogel as a smart biointerface for enzyme-based electrochemical biosensing and cell monitoring. ACS Appl. Mater. Interfaces 2016, 8, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.F.; Silva, E.R.; Alves, W.A. Nanostructured antigen-responsive hydrogels based on peptides for leishmaniasis detection. J. Braz. Chem. Soc. 2017, 28, 1619–1629. [Google Scholar] [CrossRef]
- King, P.J.; Saiani, A.; Bichenkova, E.V.; Miller, A.F. A de novo self-assembling peptide hydrogel biosensor with covalently immobilised DNA-recognising motifs. Chem. Commun. 2016, 52, 6697–6700. [Google Scholar]
- Naskar, J.; Palui, G.; Banerjee, A. Tetrapeptide-Based Hydrogels: For Encapsulation and Slow Release of an Anticancer Drug at Physiological pH. J. Phys. Chem. B 2009, 113, 11787–11792. [Google Scholar] [CrossRef] [PubMed]
- Thota, C.K.; Yadav, N.; Chauhan, V.S. A novel highly stable and injectable hydrogel based on a conformationally restricted ultrashort peptide. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Xu, Q.; Dong, C.; Lee, S.S.; Gao, L.; Li, Y.; D’Ortenzio, M.; Wu, J. Self-assembling peptide nanofibrous hydrogel as a versatile drug delivery platform. Curr. Pharm. Des. 2015, 21, 4342–4354. [Google Scholar] [CrossRef]
- Stupp, S.I.; Palmer, L.C. Supramolecular chemistry and self-assembly in organic materials design. Chem. Mater. 2014, 26, 507–518. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mat. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Controlling the Assembly and Properties of Low-Molecular-Weight Hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Kaur, H.; Sharma, P.; Patel, N.; Pal, V.K.; Roy, S. Accessing Highly Tunable Nanostructured Hydrogels in a Short Ionic Complementary Peptide Sequence via pH Trigger. Langmuir 2020, 36, 12107–12120. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Gao, Y.; Zhang, Y.; Pan, Y.; Xu, B. Calcium Ions to Cross-Link Supramolecular Nanofibers to Tune the Elasticity of Hydrogels over Orders of Magnitude. Langmuir 2011, 27, 14425–14431. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Pont, G.; Morris, K.; Lotze, G.; Squires, A.; Serpell, L.C.; Adams, D.J. Salt-induced hydrogelation of functionalised-dipeptides at high pH. Chem. Commun. 2011, 47, 12071–12073. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; McDonald, T.O.; Adams, D.J. Salt-induced hydrogels from functionalised-dipeptides. RSC Adv. 2013, 3, 8714–8720. [Google Scholar] [CrossRef]
- Shao, T.; Falcone, N.; Kraatz, H.B. Supramolecular Peptide Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, I.; Kraatz, H.-B. Ion-Dependent Modulation of Self-Healing Hydrogels. ChemistrySelect 2017, 2, 451–457. [Google Scholar] [CrossRef]
- Ji, W.; Yuan, C.; Zilberzwige-Tal, S.; Xing, R.; Chakraborty, P.; Tao, K.; Gilead, S.; Yan, X.; Gazit, E. Metal-Ion Modulated Structural Transformation of Amyloid-Like Dipeptide Supramolecular Self-Assembly. ACS Nano 2019, 13, 7300–7309. [Google Scholar] [CrossRef]
- Fu, W.; Farhadi Sabet, Z.; Liu, J.; You, M.; Zhou, H.; Wang, Y.; Gao, Y.; Li, J.; Ma, X.; Chen, C. Metal ions modulation of the self-assembly of short peptide conjugated nonsteroidal anti-inflammatory drugs (NSAIDs). Nanoscale 2020, 12, 7960–7968. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, H. Cation-based approach to morphological diversity of diphenylalanine dipeptide structures. Soft Matter 2021. [Google Scholar] [CrossRef]
- McEwen, H.; Du, E.Y.; Mata, J.P.; Thordarson, P.; Martin, A.D. Tuning hydrogels through metal-based gelation triggers. J. Mat. Chem. B 2017, 5, 9412–9417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, H.; Roy, S. Inducing Differential Self-Assembling Behavior in Ultrashort Peptide Hydrogelators Using Simple Metal Salts. Biomacromol. 2019, 20, 2610–2624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nandi, S.K.; Suman, S.; Haldar, D. A new dipeptide as a selective gelator of Cu(ii), Zn(ii), and Pb(ii). CrystEngComm 2020, 22, 7975–7982. [Google Scholar] [CrossRef]
- Micklitsch, C.M.; Knerr, P.J.; Branco, M.C.; Nagarkar, R.; Pochan, D.J.; Schneider, J.P. Zinc-Triggered Hydrogelation of a Self-Assembling beta-Hairpin Peptide. Angew. Chem. Int. Ed. 2011, 50, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Misra, R.; Saseendran, A.; Pahan, S.; Gopi, H.N. Metal-Coordinated Supramolecular Polymers from the Minimalistic Hybrid Peptide Foldamers. Angew. Chem. Int. Ed. 2021, 60, 9863–9868. [Google Scholar] [CrossRef] [PubMed]
- Gayen, K.; Basu, K.; Bairagi, D.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Amino-Acid-Based Metallo-Hydrogel That Acts Like an Esterase. ACS Appl. Bio Mater. 2018, 1, 1717–1724. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Kuang, Y.; Gao, Y.; Shi, J.; Zhang, X.X.; Xu, B. A Redox Responsive, Fluorescent Supramolecular Metallohydrogel Consists of Nanofibers with Single-Molecule Width. J. Am. Chem. Soc. 2013, 135, 5008–5011. [Google Scholar] [CrossRef] [Green Version]
- Aneja, V.P.; Chauhan, J.; Walker, J. Characterization of atmospheric ammonia emissions from swine waste storage and treatment lagoons. J. Geophys. Res. Atmos. 2000, 105, 11535–11545. [Google Scholar] [CrossRef] [Green Version]
- Aneja, V.P.; Roelle, P.A.; Murray, G.C.; Southerland, J.; Erisman, J.W.; Fowler, D.; Asman, W.A.; Patni, N. Atmospheric nitrogen compounds II: Emissions, transport, transformation, deposition and assessment. Atmos. Environ. 2001, 35, 1903–1911. [Google Scholar] [CrossRef]
- Gao, T.; Tillman, E.S.; Lewis, N.S. Detection and classification of volatile organic amines and carboxylic acids using arrays of carbon black-dendrimer composite vapor detectors. Chem. Mater. 2005, 17, 2904–2911. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M.; de Las Rivas, B.; Marcobal, A.; Muñoz, R. Molecular methods for the detection of biogenic amine-producing bacteria on foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic amines in foods. Food Sci. Biotech. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzzi, G.; Gardini, F. Biogenic amines in dry fermented sausages: A review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Biogenic amines in raw and processed seafood. Front. Microbiol. 2012, 3, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.H.; Bechtel, P.J. Ammonia, dimethylamine, trimethylamine, and trimethylamine oxide from raw and processed fish by-products. J. Aquat. Food Prod.Technol. 2008, 17, 27–38. [Google Scholar] [CrossRef]
- Devarayan, K.; Motcham, V.V.; Kathavarayan, M.; Anjappan, H. Real-Time Detection of Packaged Seer Fish Spoilage Using Halochromic Optical Nose. J. Aquat. Food Prod.Technol. 2021, 30, 484–495. [Google Scholar] [CrossRef]
- Steiner, M.-S.; Meier, R.J.; Duerkop, A.; Wolfbeis, O.S. Chromogenic sensing of biogenic amines using a chameleon probe and the red− green− blue readout of digital camera images. Anal. Chem. 2010, 82, 8402–8405. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, J.; Yu, J.; Cui, B. A colorimetric sensor for qualitative discrimination and quantitative detection of volatile amines. Sensors 2010, 10, 6463–6476. [Google Scholar] [CrossRef]
- Fan, J.; Chang, X.; He, M.; Shang, C.; Wang, G.; Yin, S.; Peng, H.; Fang, Y. Functionality-Oriented Derivatization of Naphthalene Diimide: A Molecular Gel Strategy-Based Fluorescent Film for Aniline Vapor Detection. ACS Appl. Mater. Interfaces 2016, 8, 18584–18592. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, Q.; Li, Y.; Gao, A.; Chang, X. Continuous multi-channel sensing of volatile acid and organic amine gases using a fluorescent self-assembly system. J. Mater. Chem C. 2019, 7, 133–142. [Google Scholar] [CrossRef]
- Pang, X.; Yu, X.; Lan, H.; Ge, X.; Li, Y.; Zhen, X.; Yi, T. Visual Recognition of Aliphatic and Aromatic Amines Using a Fluorescent Gel: Application of a Sonication-Triggered Organogel. ACS Appl. Mater. Interfaces 2015, 7, 13569–13577. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, A.; Hou, J.-T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Xue, P.; Sun, J.; Yao, B.; Gong, P.; Zhang, Z.; Qian, C.; Zhang, Y.; Lu, R. Strong Emissive Nanofibers of Organogels for the Detection of Volatile Acid Vapors. Chem. Eur. J. 2015, 21, 4712–4720. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yao, B.; Wang, P.; Gong, P.; Zhang, Z.; Lu, R. Strong Fluorescent Smart Organogel as a Dual Sensing Material for Volatile Acid and Organic Amine Vapors. Chem. Eur. J. 2015, 21, 17508–17515. [Google Scholar] [CrossRef]
- Wang, S.S.; Xue, P.C.; Wang, P.P.; Yao, B.Q. Emission enhanced two-component gels for the detection of organic amine vapors. New J. Chem. 2015, 39, 6874–6881. [Google Scholar] [CrossRef]
- Sengupta, S.; Mondal, R. A novel low molecular weight supergelator showing an excellent gas adsorption, dye adsorption, self-sustaining and chemosensing properties in the gel state. RSC Adv. 2016, 6, 14009–14015. [Google Scholar] [CrossRef]
- Tang, L.Y.; Liao, S.S.; Qu, J.Q. Self-Healing and Multistimuli-Responsive Hydrogels Formed via a Cooperation Strategy and Their Application in Detecting Biogenic Amines. ACS Appl. Mater. Interfaces 2018, 10, 27365–27373. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, W.; Lin, D.; Qin, X.; Yang, Y.; Wang, F.; Pan, Q.; Su, Z. Water-stable lanthanide-based metal–organic gel for the detection of organic amines and white-light emission. J. Mater. Chem C. 2020, 8, 13648–13654. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2011, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting–self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; Palui, G.; Banerjee, A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter 2009, 5, 3452–3460. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L. Removing Organic Dyes by Using a Small Peptide Hydrogel. Chem. Lett. 2016, 45, 1253–1255. [Google Scholar] [CrossRef]
- Basak, S.; Nandi, N.; Paul, S.; Hamley, I.W.; Banerjee, A. A tripeptide-based self-shrinking hydrogel for waste-water treatment: Removal of toxic organic dyes and lead (Pb2+) ions. Chem. Commun. 2017, 53, 5910–5913. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Chetia, M.; Sarkar, A.K.; Chatterjee, S. Co-assembly of charge complementary peptides and their applications as organic dye/heavy metal ion (Pb2+, Hg2+) absorbents and arsenic(iii/v) detectors. RSC Adv. 2020, 10, 42062–42075. [Google Scholar] [CrossRef]
- Chetia, M.; Debnath, S.; Chowdhury, S.; Chatterjee, S. Self-assembly and multifunctionality of peptide organogels: Oil spill recovery, dye absorption and synthesis of conducting biomaterials. RSC Adv. 2020, 10, 5220–5233. [Google Scholar] [CrossRef] [Green Version]
- Mondal, B.; Bairagi, D.; Nandi, N.; Hansda, B.; Das, K.S.; Edwards-Gayle, C.J.C.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Peptide-Based Gel in Environmental Remediation: Removal of Toxic Organic Dyes and Hazardous Pb2+ and Cd2+ Ions from Wastewater and Oil Spill Recovery. Langmuir 2020, 36, 12942–12953. [Google Scholar] [CrossRef]
- Qin, L.; Xie, F.; Duan, P.F.; Liu, M.H. A Peptide Dendron-Based Shrinkable Metallo-Hydrogel for Charged Species Separation and Stepwise Release of Drugs. Chem. Eur. J. 2014, 20, 15419–15425. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Das, A.K.; Banerjee, A. pH-Responsive, Bolaamphiphile-Based Smart Metallo-Hydrogels as Potential Dye-Adsorbing Agents, Water Purifier, and Vitamin B12 Carrier. Chem. Mater. 2007, 19, 1633–1639. [Google Scholar] [CrossRef]
- Davies, R.; Aggeli, A.; Beevers, A.; Boden, N.; Carrick, L.; Fishwick, C.; McLeish, T.; Nyrkova, I.; Semenov, A. Self-assembling β-sheet tape forming peptides. Supramol. Chem. 2006, 18, 435–443. [Google Scholar] [CrossRef]
- Lee, N.R.; Bowerman, C.J.; Nilsson, B.L. Sequence length determinants for self-assembly of amphipathic β-sheet peptides. Pept. Sci. 2013, 100, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Bowerman, C.J.; Nilsson, B.L. Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules 2013, 14, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Winnik, F.M. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Yang, H.-K.; Su, M.-M.; Ren, L.-J.; Zheng, P.; Wang, W. Enhanced thermal stability of organogels through self-reinforcing supramolecular assembly of a cholesterol–polyoxomatalate–cholesterol hybrid gelator. RSC Adv. 2014, 4, 1138–1145. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, K.; Chen, X.; Chen, Y.; Zhang, S.; Peng, J.; Fang, Y. A novel calix[4]arene-based dimeric-cholesteryl derivative: Synthesis, gelation and unusual properties. New J. Chem. 2015, 39, 639–649. [Google Scholar] [CrossRef]
- Sawada, H.; Yamanaka, M. Synthesis of a Bis-Urea Dimer and Its Effects on the Physical Properties of an Amphiphilic Tris-Urea Supramolecular Hydrogel. Chem. Asian J. 2018, 13, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Requena, J.V.; Grijalvo, S.; Sampedro, D.; Mayr, J.; Saldías, C.; Marrero-Tellado, J.J.; Eritja, R.; Herrera, R.P.; Díaz, D.D. Sulfonamide as amide isostere for fine-tuning the gelation properties of physical gels. RSC Adv. 2020, 10, 11481–11492. [Google Scholar] [CrossRef] [Green Version]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerget. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. Comprehensive Chiroptical Spectroscopy: Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 2. [Google Scholar]
- Garifullin, R.; Guler, M.O. Supramolecular chirality in self-assembled peptide amphiphile nanostructures. Chem. Commun. 2015, 51, 12470–12473. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, A.; Mba, M. Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption. Gels 2021, 7, 85. https://doi.org/10.3390/gels7030085
Fortunato A, Mba M. Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption. Gels. 2021; 7(3):85. https://doi.org/10.3390/gels7030085
Chicago/Turabian StyleFortunato, Anna, and Miriam Mba. 2021. "Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption" Gels 7, no. 3: 85. https://doi.org/10.3390/gels7030085
APA StyleFortunato, A., & Mba, M. (2021). Metal Cation Triggered Peptide Hydrogels and Their Application in Food Freshness Monitoring and Dye Adsorption. Gels, 7(3), 85. https://doi.org/10.3390/gels7030085







