Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases
Abstract
:1. Introduction
2. The Preparation and Main Properties of the Micro-/Nanoparticle Hybrid Hydrogel Platform
3. Micro-/Nanoparticle Hybrid Hydrogel Platform Applied in Articular Cartilage-Related Diseases
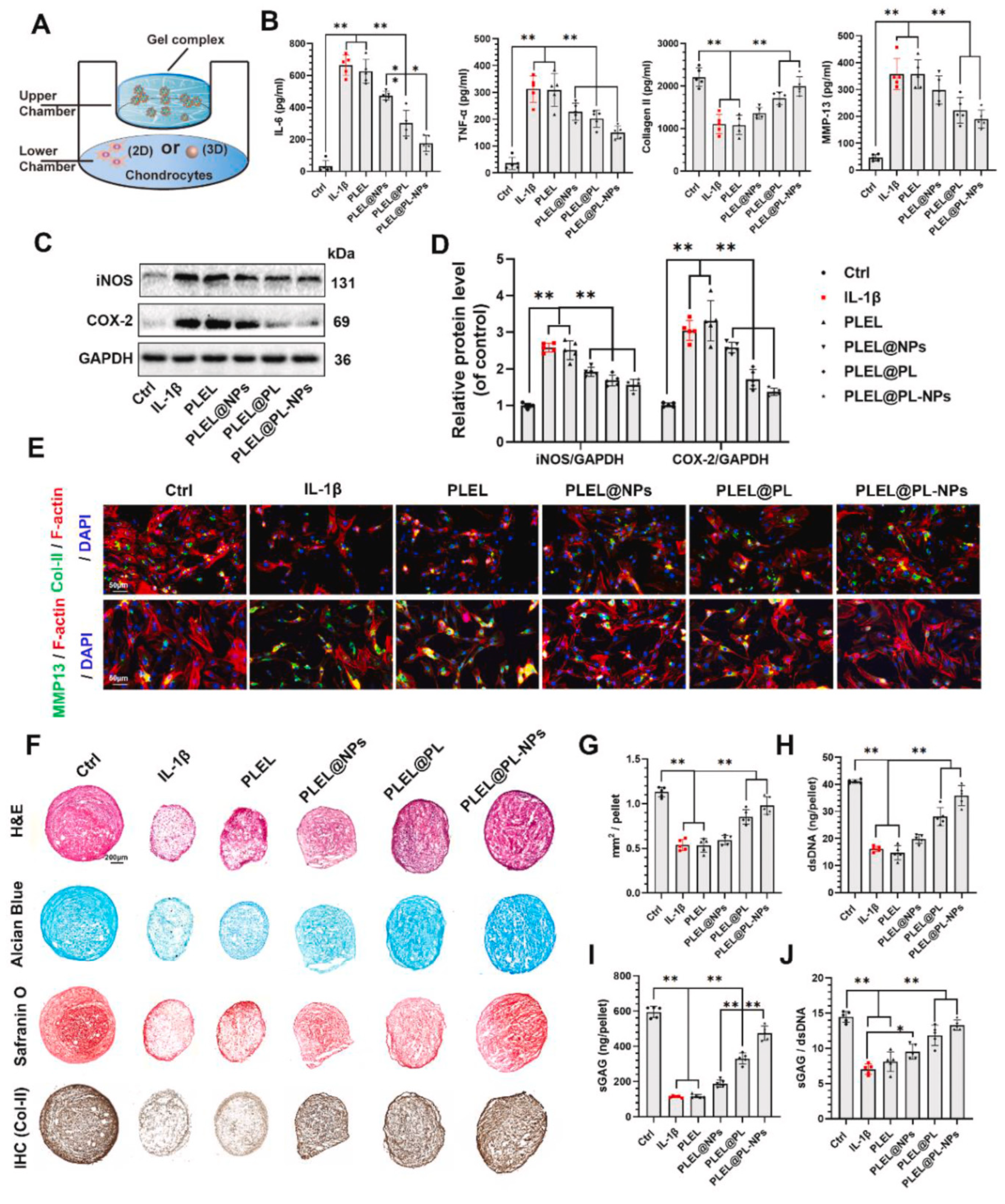
3.1. Micro-/Nanoparticle Hybrid Hydrogel Platform in Symptomatic Treatment
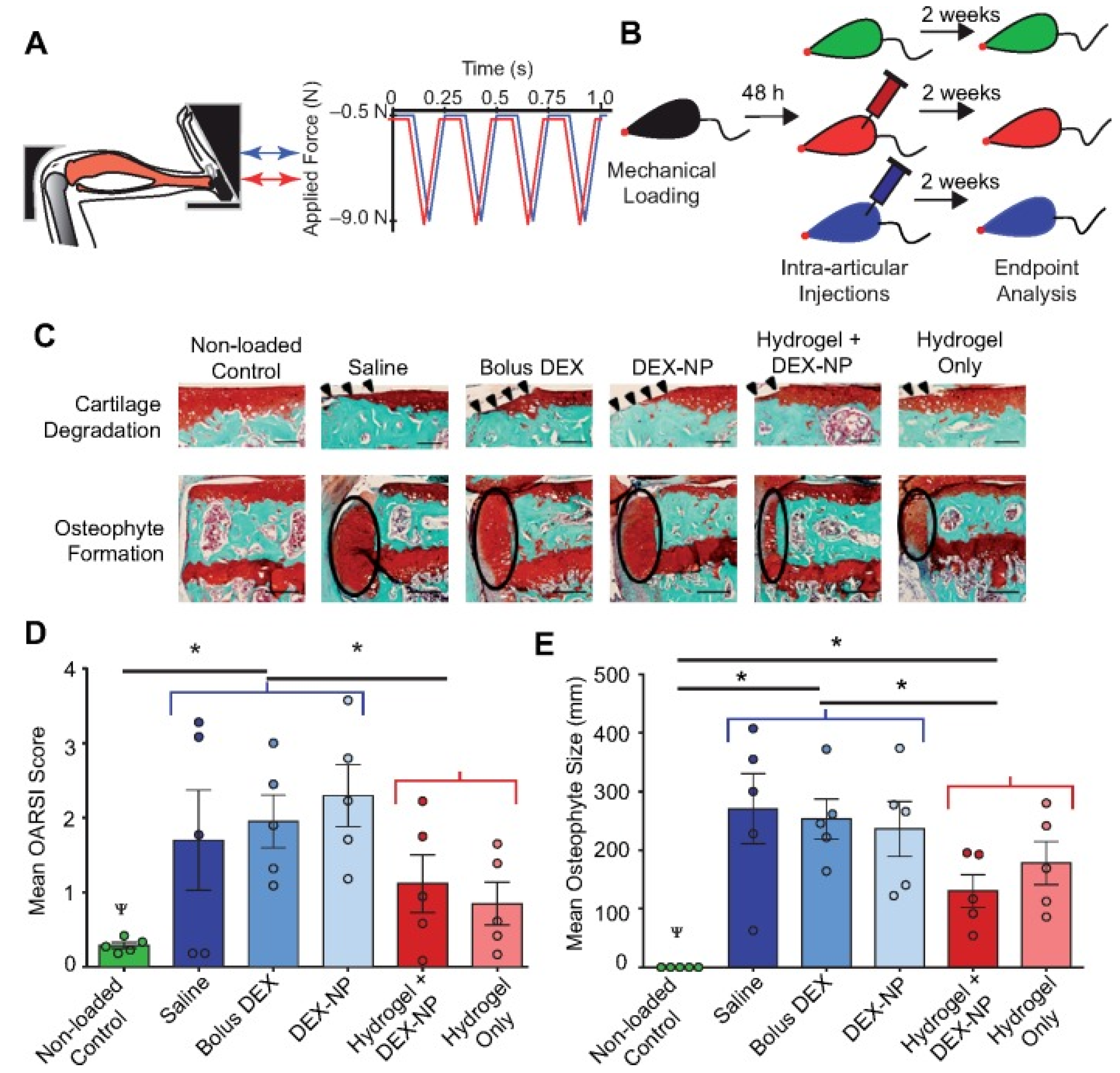
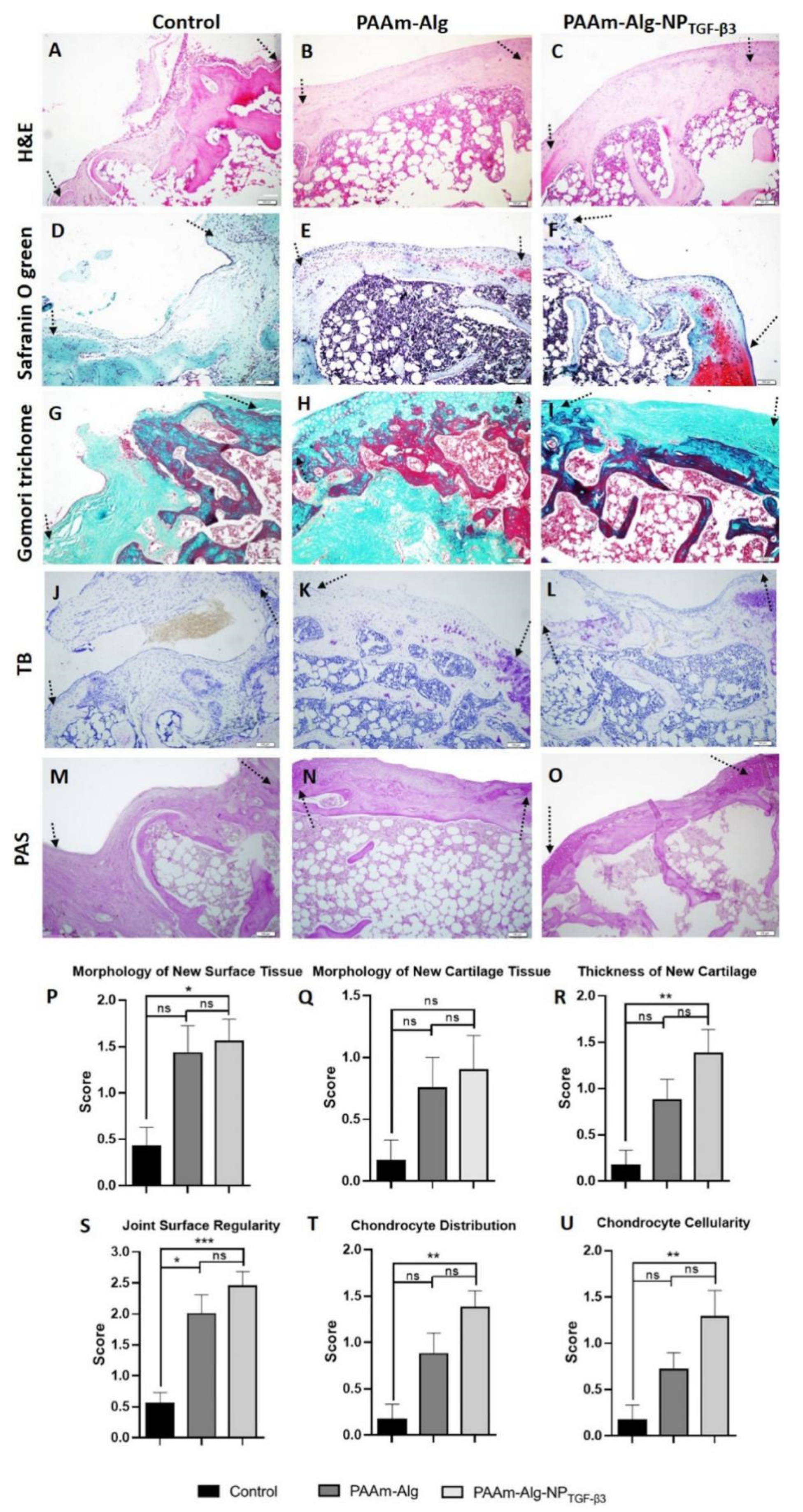
3.2. Micro-/Nanoparticle Hybrid Hydrogel Platform in Cartilage Defect
4. Prospect and Challenges
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, R. Osteoarthritis. Lancet 2018, 391, 1985. [Google Scholar] [CrossRef]
- Schett, G.; Tanaka, Y.; Isaacs, J.D. Why remission is not enough: Underlying disease mechanisms in RA that prevent cure. Nat. Rev. Rheumatol. 2021, 17, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, X. Innate Lymphocytes in Inflammatory Arthritis. Front. Immunol. 2020, 11, 565275. [Google Scholar] [CrossRef]
- Zhu, G.G.; Nafa, K.; Agaram, N.; Zehir, A.; Benayed, R.; Sadowska, J.; Borsu, L.; Kelly, C.; Tap, W.D.; Fabbri, N.; et al. Genomic Profiling Identifies Association of IDH1/IDH2 Mutation with Longer Relapse-Free and Metastasis-Free Survival in High-Grade Chondrosarcoma. Clin. Cancer Res. 2020, 26, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Latourte, A.; Rat, A.C.; Ngueyon Sime, W.; Ea, H.K.; Bardin, T.; Mazières, B.; Roux, C.; Guillemin, F.; Richette, P. Chondrocalcinosis of the Knee and the Risk of Osteoarthritis Progression: Data From the Knee and Hip Osteoarthritis Long-term Assessment Cohort. Arthritis Rheumatol. 2020, 72, 726–732. [Google Scholar] [CrossRef]
- Dai, J.; Yu, D.; Wang, Y.; Chen, Y.; Sun, H.; Zhang, X.; Zhu, S.; Pan, Z.; Heng, B.C.; Zhang, S.; et al. Kdm6b regulates cartilage development and homeostasis through anabolic metabolism. Ann. Rheum. Dis. 2017, 76, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Haslag-Minoff, J.; Regunath, H. Relapsing Polychondritis. N. Engl. J. Med. 2018, 378, 1715. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.C.; Liu, F.; Blankenbaker, D.G.; Woo, K.M.; Kijowski, R. Juvenile Osteochondritis Dissecans: Cartilage T2 Mapping of Stable Medial Femoral Condyle Lesions. Radiology 2018, 288, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Zeng, C.; Wei, J.; Persson, M.S.M.; Sarmanova, A.; Doherty, M.; Xie, D.; Wang, Y.; Li, X.; Li, J.; Long, H.; et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: A systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med. 2018, 52, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef]
- Palmer, J.S.; Monk, A.P.; Hopewell, S.; Bayliss, L.E.; Jackson, W.; Beard, D.J.; Price, A.J. Surgical interventions for symptomatic mild to moderate knee osteoarthritis. Cochrane Database Syst. Rev. 2019, 7, Cd012128. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef]
- Mehta, S.; He, T.; Bajpayee, A.G. Recent advances in targeted drug delivery for treatment of osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 94–109. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Grodzinsky, A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017, 13, 183–193. [Google Scholar] [CrossRef]
- Chahal, J.; Lansdown, D.A.; Davey, A.; Davis, A.M.; Cole, B.J. The Clinically Important Difference and Patient Acceptable Symptomatic State for Commonly Used Patient-Reported Outcomes After Knee Cartilage Repair. Am. J. Sports Med. 2021, 49, 193–199. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Deng, C.; Xu, C.; Zhou, Q.; Cheng, Y. Advances of nanotechnology in osteochondral regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- He, T.; Zhang, C.; Vedadghavami, A.; Mehta, S.; Clark, H.A.; Porter, R.M.; Bajpayee, A.G. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J. Control. Release 2020, 318, 109–123. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Z.; Liu, B.; Xiang, J.; Qiu, D.; Tian, Y.; Qu, X.; Yang, Z. An Injectable Strong Hydrogel for Bone Reconstruction. Adv. Healthc. Mater. 2019, 8, e1900709. [Google Scholar] [CrossRef]
- Adak, A.; Ghosh, S.; Gupta, V.; Ghosh, S. Biocompatible Lipopeptide-Based Antibacterial Hydrogel. Biomacromolecules 2019, 20, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Umair Hassan, M.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, Y.; Wu, H.; Zhou, C.; Tao, L. An antioxidant self-healing hydrogel for 3D cell cultures. J. Mater. Chem. B 2020, 8, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, T.; Yan, Y.; Ji, X.; Sun, Y.; Zhao, X.; Qi, J.; Cui, W.; Deng, L.; Zhang, H. Cartilage matrix-inspired biomimetic superlubricated nanospheres for treatment of osteoarthritis. Biomaterials 2020, 242, 119931. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Cai, Q.; Zhang, H.; Yao, X.; Zhu, W. Full Poly(ethylene glycol) Hydrogels with High Ductility and Self-Recoverability. ACS Appl. Mater. Interfaces 2020, 12, 37549–37560. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Li, X.; Huang, K.; Shao, W.; Yao, D.; Huang, C. Redox-responsive blend hydrogel films based on carboxymethyl cellulose/chitosan microspheres as dual delivery carrier. Int. J. Biol. Macromol. 2019, 134, 413–421. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.; Cao, Z.; Ye, F.; Lin, Z.; Li, Y. Development of CaCO(3) microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration. Biomater. Sci. 2019, 7, 3614–3626. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Fu, Y.N.; Li, Y.; Li, G.; Yang, L.; Yuan, Q.; Tao, L.; Wang, X. Adaptive Chitosan Hollow Microspheres as Efficient Drug Carrier. Biomacromolecules 2017, 18, 2195–2204. [Google Scholar] [CrossRef]
- Kudva, A.K.; Dikina, A.D.; Luyten, F.P.; Alsberg, E.; Patterson, J. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019, 90, 287–299. [Google Scholar] [CrossRef]
- Mao, W.; Son, Y.J.; Yoo, H.S. Gold nanospheres and nanorods for anti-cancer therapy: Comparative studies of fabrication, surface-decoration, and anti-cancer treatments. Nanoscale 2020, 12, 14996–15020. [Google Scholar] [CrossRef]
- Wu, M.; Yang, W.; Chen, S.; Yao, J.; Shao, Z.; Chen, X. Size-controllable dual drug-loaded silk fibroin nanospheres through a facile formation process. J. Mater. Chem. B 2018, 6, 1179–1186. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Yan, H. Chlorambucil loaded in mesoporous polymeric microspheres as oral sustained release formulations with enhanced hydrolytic stability. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 564–569. [Google Scholar] [CrossRef]
- Tawagi, E.; Ganesh, T.; Cheng, H.M.; Santerre, J.P. Synthesis of degradable-polar-hydrophobic-ionic co-polymeric microspheres by membrane emulsion photopolymerization: In vitro and in vivo studies. Acta Biomater. 2019, 89, 279–288. [Google Scholar] [CrossRef]
- Li, Z.; Ma, S.; Zhang, G.; Wang, D.; Zhou, F. Soft/Hard-Coupled Amphiphilic Polymer Nanospheres for Water Lubrication. ACS Appl. Mater. Interfaces 2018, 10, 9178–9187. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Scott, E.A. On the advancement of polymeric bicontinuous nanospheres toward biomedical applications. Nanoscale Horiz. 2019, 4, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Xing, J.; Ding, X.; Zhang, S.; Shen, Y.; Yu, B. Preparation of porous sulfonated poly(styrene-divinylbenzene) microspheres and its application in hydrophilic and chiral separation. Talanta 2020, 210, 120586. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.; Kozlova, A.; Pavlov, A.M.; Atkin, V.; Verkhovskii, R.; Kamyshinsky, R.; Demina, P.; Vasiliev, A.L.; Venig, S.B.; Bukreeva, T.V. Novel type of hollow hydrogel microspheres with magnetite and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, G.; Li, X.; Lu, W.; Peng, Z. Self-standing hollow porous AuPt nanospheres and their enhanced electrocatalytic performance. J. Colloid Interface Sci. 2019, 554, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, H.; Geng, K.; Shen, J.; Feng, X.; Xu, P.; Duan, Y.; Li, Y.; Wu, R.; Gou, Z.; et al. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 2021, 272, 120783. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Manga, Y.B.; Ostrikov, K.K.; Chiang, W.H.; Pandey, A.; Lekha, R.; Nyambat, B.; Chuang, E.Y.; Chen, C.H. Microplasma Cross-Linked Graphene Oxide-Gelatin Hydrogel for Cartilage Reconstructive Surgery. ACS Appl. Mater. Interfaces 2020, 12, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Huynh, C.T.; Liu, F.; Cheng, Y.; Coughlin, K.A.; Alsberg, E. Thiol-Epoxy “Click” Chemistry to Engineer Cytocompatible PEG-Based Hydrogel for siRNA-Mediated Osteogenesis of hMSCs. ACS Appl. Mater. Interfaces 2018, 10, 25936–25942. [Google Scholar] [CrossRef]
- Chou, H.Y.; Tsai, H.C. Development of hydrogels with thermal-healing properties using a network of polyvinyl alcohol and boron nitride composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111364. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Feng, Z.; Guo, W.; Yang, D.; Gao, S.; Li, Y.; Shen, S.; Yuan, Z.; Huang, B.; Zhang, Y.; et al. PCL-MECM-Based Hydrogel Hybrid Scaffolds and Meniscal Fibrochondrocytes Promote Whole Meniscus Regeneration in a Rabbit Meniscectomy Model. ACS Appl. Mater. Interfaces 2019, 11, 41626–41639. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, Y.; Zou, L.; Yan, S.; Zhu, H.; Zhang, K.; Liu, W.; He, D.; Yin, J. A bilayered scaffold with segregated hydrophilicity-hydrophobicity enables reconstruction of goat hierarchical temporomandibular joint condyle cartilage. Acta Biomater. 2021, 121, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 17018. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Steiger, C.; Lin, S.; Parada, G.A.; Liu, J.; Chan, H.F.; Yuk, H.; Phan, N.V.; Collins, J.; Tamang, S.; et al. Ingestible hydrogel device. Nat. Commun. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, Y.N.; Wei, Y.; Zhao, L.; Tao, L. Self-Adapting Hydrogel to Improve the Therapeutic Effect in Wound-Healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef]
- Wu, C.H.; Sun, M.K.; Kung, Y.; Wang, Y.C.; Chen, S.L.; Shen, H.H.; Chen, W.S.; Young, T.H. One injection for one-week controlled release: In vitro and in vivo assessment of ultrasound-triggered drug release from injectable thermoresponsive biocompatible hydrogels. Ultrason. Sonochem. 2020, 62, 104875. [Google Scholar] [CrossRef]
- Xu, P.; Xu, H.; Yang, Y.; Wang, X.; An, W.; Hu, Y.; Xu, S. A nonswellable gradient hydrogel with tunable mechanical properties. J. Mater. Chem. B 2020, 8, 2702–2708. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, Z.; Liu, C.; Hu, Q.; Cai, X.; Xiao, J.; Cheng, Y. A pH-responsive hydrogel with potent antibacterial activity against both aerobic and anaerobic pathogens. Biomater. Sci. 2019, 7, 581–584. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Roth-Konforti, M.E.; Comune, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Shabat, D.; Adler-Abramovich, L. UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery. Macromol. Rapid Commun. 2018, 39, e1800588. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.D.; Babo, P.S.; Costa-Almeida, R.; Domingues, R.M.A.; Mendes, B.B.; Paz, E.; Freitas, P.; Rodrigues, M.T.; Granja, P.L.; Gomes, M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomedicine 2018, 14, 2375–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talló, K.; Bosch, M.; Pons, R.; Cocera, M.; López, O. Preparation and characterization of a supramolecular hydrogel made of phospholipids and oleic acid with a high water content. J. Mater. Chem. B 2020, 8, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Liu, L.; He, G.; Zhang, T.; Yang, M.; Cai, J.; Fan, L.; Tao, S. Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692–1698. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Grizić, D.; Lamprecht, A. Microparticle preparation by a propylene carbonate emulsification-extraction method. Int. J. Pharm. 2018, 544, 213–221. [Google Scholar] [CrossRef]
- Niu, R.; Yang, Y.; Wang, S.; Zhou, X.; Luo, S.; Zhang, C.; Wang, Y. Chitosan microparticle-based immunoaffinity chromatography supports prepared by membrane emulsification technique: Characterization and application. Int. J. Biol. Macromol. 2019, 131, 1147–1154. [Google Scholar] [CrossRef]
- Xia, Y.; Yuan, M.; Chen, M.; Li, J.; Ci, T.; Ke, X. Liquid jet breakup: A new method for the preparation of poly lactic-co-glycolic acid microspheres. Eur. J. Pharm. Biopharm. 2019, 137, 140–147. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Wei, H.; Li, W.; Chen, H.; Wen, X.; He, J.; Li, J. Simultaneous Diels-Alder click reaction and starch hydrogel microsphere production via spray drying. Carbohydr. Polym. 2020, 241, 116351. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeong, S.H.; Lee, Y.B. Preparation and In Vitro/In Vivo Characterization of Polymeric Nanoparticles Containing Methotrexate to Improve Lymphatic Delivery. Int. J. Mol. Sci. 2019, 20, 3312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021, 121, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Ning, C.; Li, M.; Zhao, G.; Jiang, W.; Ding, J.; Chen, X. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials 2018, 181, 378–391. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. B 2020, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, B.; Wei, M.; Huang, Y.; Zhou, Y.; Zhang, Q.; Du, L. Prussian blue nanosphere-embedded in situ hydrogel for photothermal therapy by peritumoral administration. Acta Pharm. Sin. B 2019, 9, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.A.; Duy Le, T.M.; Ho, H.G.V.; To, T.C.T.; Nguyen, V.V.L.; Huynh, D.P.; Lee, D.S. A novel injectable pH-temperature sensitive hydrogel containing chitosan-insulin electrosprayed nanosphere composite for an insulin delivery system in type I diabetes treatment. Biomater. Sci. 2020, 8, 3830–3843. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Li, S.; Xing, L.; Du, S.; Yuan, G.; Li, J.; Zhou, T.; Xiong, D.; Tan, H.; et al. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef]
- Simão, A.R.; Fragal, V.H.; Lima, A.M.O.; Pellá, M.C.G.; Garcia, F.P.; Nakamura, C.V.; Tambourgi, E.B.; Rubira, A.F. pH-responsive hybrid hydrogels: Chondroitin sulfate/casein trapped silica nanospheres for controlled drug release. Int. J. Biol. Macromol. 2020, 148, 302–315. [Google Scholar] [CrossRef]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Ma, Z.; Song, W.; He, Y.; Li, H. Multilayer Injectable Hydrogel System Sequentially Delivers Bioactive Substances for Each Wound Healing Stage. ACS Appl. Mater. Interfaces 2020, 12, 29787–29806. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, S.Y.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Kim, M.H.; Kim, H.J.; Lee, J.; Lee, K.; et al. Monopotassium phosphate-reinforced in situ forming injectable hyaluronic acid hydrogels for subcutaneous injection. Int. J. Biol. Macromol. 2020, 163, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hu, J.; Qu, Z.; Tao, R.; Wang, G.; Liu, W. Niacin Metal-Organic Framework-Laden Self-Healing Hydrogel for Wound Healing. J. Biomed. Nanotechnol. 2020, 16, 1719–1726. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Y.; Jiang, J.; Yan, Y.; Fan, D.; Yang, X.; Zuo, Y.; Li, Y.; Gu, H.; Li, J. Development of an Antibacterial Bone Graft by Immobilization of Levofloxacin Hydrochloride-Loaded Mesoporous Silica Microspheres on a Porous Scaffold Surface. J. Biomed. Nanotechnol. 2019, 15, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Garakani, S.; Davachi, S.M.; Bagher, Z.; Heraji Esfahani, A.; Jenabi, N.; Atoufi, Z.; Khanmohammadi, M.; Abbaspourrad, A.; Rashedi, H.; Jalessi, M. Fabrication of chitosan/polyvinylpyrrolidone hydrogel scaffolds containing PLGA microparticles loaded with dexamethasone for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 356–370. [Google Scholar] [CrossRef]
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Pérez-Caballer, A.; San Román, J.; Vázquez-Lasa, B. Injectable hydrogel-based drug delivery system for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110702. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-co-Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
- Liao, J.; Han, R.; Wu, Y.; Qian, Z. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Gionet-Gonzales, M.; Vorwald, C.E.; Bohannon, L.K.; Clark, K.; Galuppo, L.D.; Leach, J.K. Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials 2019, 197, 119–128. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Chen, J.; Xie, Z.; Huang, K.; Wang, G.; Yang, Y.; Ni, W.; Chen, Z.; Shi, P.; et al. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann. Rheum. Dis. 2019, 78, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Song, E.K.; Jeon, J.; Jang, D.G.; Kim, H.E.; Sim, H.J.; Kwon, K.Y.; Medina-Ruiz, S.; Jang, H.J.; Lee, A.R.; Rho, J.G.; et al. ITGBL1 modulates integrin activity to promote cartilage formation and protect against arthritis. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Asghar, S.; Litherland, G.J.; Lockhart, J.C.; Goodyear, C.S.; Crilly, A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology 2020, 59, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Varela-Eirín, M.; Varela-Vázquez, A.; Guitián-Caamaño, A.; Paíno, C.L.; Mato, V.; Largo, R.; Aasen, T.; Tabernero, A.; Fonseca, E.; Kandouz, M.; et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 2018, 9, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culvenor, A.G.; Øiestad, B.E.; Hart, H.F.; Stefanik, J.J.; Guermazi, A.; Crossley, K.M. Prevalence of knee osteoarthritis features on magnetic resonance imaging in asymptomatic uninjured adults: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Saeedi Garakani, S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Hashemi Motlagh, G. Injectable PNIPAM/Hyaluronic acid hydrogels containing multipurpose modified particles for cartilage tissue engineering: Synthesis, characterization, drug release and cell culture study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res. Ther. 2021, 23, 50. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A. A hydrogel/particle composite with gradient in oxygen releasing microparticle for oxygenation of the cartilage-to-bone interface: Modeling and experimental viewpoints. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111522. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Liu, J.; Zhang, Y.; Yang, B.; Wan, Y.; Wu, J. Dual Network Hydrogels Incorporated with Bone Morphogenic Protein-7-Loaded Hyaluronic Acid Complex Nanoparticles for Inducing Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Pharmaceutics 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Gaur, J.K.; Pandey, S.K.; Bobji, M.S.; Srivastava, C. High-Strength, Strongly Bonded Nanocomposite Hydrogels for Cartilage Repair. ACS Appl. Mater. Interfaces 2021, 13, 24505–24523. [Google Scholar] [CrossRef] [PubMed]
- Abou-ElNour, M.; Soliman, M.E.; Skouras, A.; Casettari, L.; Geneidi, A.S.; Ishak, R.A.H. Microparticles-in-Thermoresponsive/Bioadhesive Hydrogels as a Novel Integrated Platform for Effective Intra-articular Delivery of Triamcinolone Acetonide. Mol. Pharm. 2020, 17, 1963–1978. [Google Scholar] [CrossRef]
- Harre, U.; Schett, G. Cellular and molecular pathways of structural damage in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, D.T.; Wheeler, T.A.; van der Meulen, M.C.H.; Singh, A. Injectable mechanical pillows for attenuation of load-induced post-traumatic osteoarthritis. Regen. Biomater. 2019, 6, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Visage, C.L.; Tassin, J.F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; An, R.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Tough hydrophobic association hydrogels with self-healing and reforming capabilities achieved by polymeric core-shell nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ma, Y.; Tan, H.; Jia, Y.; Zou, S.; Guo, S.; Zhao, M.; Huang, H.; Ling, Z.; Chen, Y.; et al. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 67–74. [Google Scholar] [CrossRef]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, itc1–itc16. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Yin, N.; Tan, X.; Liu, H.; He, F.; Ding, N.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale 2020, 12, 8546–8562. [Google Scholar] [CrossRef]
- Yin, N.; Guo, X.; Sun, R.; Liu, H.; Tang, L.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. Intra-articular injection of indomethacin-methotrexate in situ hydrogel for the synergistic treatment of rheumatoid arthritis. J. Mater. Chem. B 2020, 8, 993–1007. [Google Scholar] [CrossRef]
- Tang, Q.; Lim, T.; Shen, L.Y.; Zheng, G.; Wei, X.J.; Zhang, C.Q.; Zhu, Z.Z. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials 2021, 268, 120605. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chuah, Y.J.; He, P.; Wang, D.A. Engineering a multiphasic, integrated graft with a biologically developed cartilage-bone interface for osteochondral defect repair. J. Mater. Chem. B 2019, 7, 6515–6525. [Google Scholar] [CrossRef]
- Asgari, N.; Bagheri, F.; Eslaminejad, M.B.; Ghanian, M.H.; Sayahpour, F.A.; Ghafari, A.M. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem. Cell Res. Ther. 2020, 11, 289. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Wang, B.; Huang, Y.; Qu, Y.; Peng, J.; Qian, Z. Injectable Alginate Hydrogel Cross-Linked by Calcium Gluconate-Loaded Porous Microspheres for Cartilage Tissue Engineering. ACS Omega 2017, 2, 443–454. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Wang, S.; Yang, C.; Li, Y.; Cao, Y. Hydroxyapatite Nanoparticle-Crosslinked Peptide Hydrogels for Three-Dimensional Culture and Differentiation of MC3T3-E1 Osteoblasts. J. Biomed. Nanotechnol. 2019, 15, 2351–2362. [Google Scholar] [CrossRef]
- Nasiri, N.; Hosseini, S.; Alini, M.; Khademhosseini, A.; Baghaban Eslaminejad, M. Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment. Drug Discov. Today 2019, 24, 2212–2224. [Google Scholar] [CrossRef]
- Kouhi, M.; Varshosaz, J.; Hashemibeni, B.; Sarmadi, A. Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111114. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef]
- Wu, X.; Stroll, S.I.; Lantigua, D.; Suvarnapathaki, S.; Camci-Unal, G. Eggshell particle-reinforced hydrogels for bone tissue engineering: An orthogonal approach. Biomater. Sci. 2019, 7, 2675–2685. [Google Scholar] [CrossRef]
- Naghizadeh, Z.; Karkhaneh, A.; Nokhbatolfoghahaei, H.; Farzad-Mohajeri, S.; Rezai-Rad, M.; Dehghan, M.M.; Aminishakib, P.; Khojasteh, A. Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: In vitro and in vivo study. J. Cell. Physiol. 2021, 236, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yuan, L.; Li, J.; Wang, Z.; Chen, J.; Guo, C.; Mo, X.; Yan, Z. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110705. [Google Scholar] [CrossRef] [PubMed]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.W.; Gunes, K.; Eren-Ozcan, G.G.; et al. An alginate-poly(acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: Biodegradability, biocompatibility and protein adsorption. Int. J. Biol. Macromol. 2021, 172, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Subramanian, A.; Sethuraman, S. Injectable glycosaminoglycan-protein nano-complex in semi-interpenetrating networks: A biphasic hydrogel for hyaline cartilage regeneration. Carbohydr. Polym. 2017, 175, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W.; et al. Development of Magnetic Nanocomposite Hydrogel with Potential Cartilage Tissue Engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Huang, Z.; Zhao, P.; Liang, Q.; Liu, Y.; Duan, L.; Liu, W.; Zhu, F.; Bian, L.; et al. Magnetic Enhancement of Chondrogenic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 2200–2207. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Li, K.; Xu, J.; Liu, M.; Fan, Y. The Effect of Magnetic Poly(lactic-co-glycolic acid) Microsphere-Gelatin Hydrogel on the Growth of Pre-Osteoblasts Under Static Magnetic Field. J. Biomed. Nanotechnol. 2020, 16, 1658–1666. [Google Scholar] [CrossRef]
- Lee, S.J.; Yan, D.; Zhou, X.; Cui, H.; Esworthy, T.; Hann, S.Y.; Keidar, M.; Zhang, L.G. Integrating cold atmospheric plasma with 3D printed bioactive nanocomposite scaffold for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110844. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef]
- Deng, X.Q.; Chao, N.N.; Ding, W.; Qin, T.W.; Wang, W.; Zhang, Y.; Luo, J.C. Production and Characterization of Composite Chitosan Hydrogel Containing Extracellular Matrix Particles for Tissue Engineering Applications. J. Biomed. Nanotechnol. 2019, 15, 756–768. [Google Scholar] [CrossRef]
- Almarza, A.J.; Brown, B.N.; Arzi, B.; Ângelo, D.F.; Chung, W.; Badylak, S.F.; Detamore, M. Preclinical Animal Models for Temporomandibular Joint Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, S.; Zhang, J.; Wang, L.; Wang, K.; Svensson, P. Temporal summation and motor function modulation during repeated jaw movements in patients with temporomandibular disorder pain and healthy controls. Pain 2017, 158, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zheng, L.; Fan, Y.; Zhang, J.; Xu, R.; Xie, J.; Zhou, X. Parathyroid hormone ameliorates temporomandibular joint osteoarthritic-like changes related to age. Cell Prolif. 2020, 53, e12755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Healthc. Mater. 2019, 8, e1801236. [Google Scholar] [CrossRef]
- Luo, P.; Jiang, C.; Ji, P.; Wang, M.; Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019, 10, 216. [Google Scholar] [CrossRef] [Green Version]

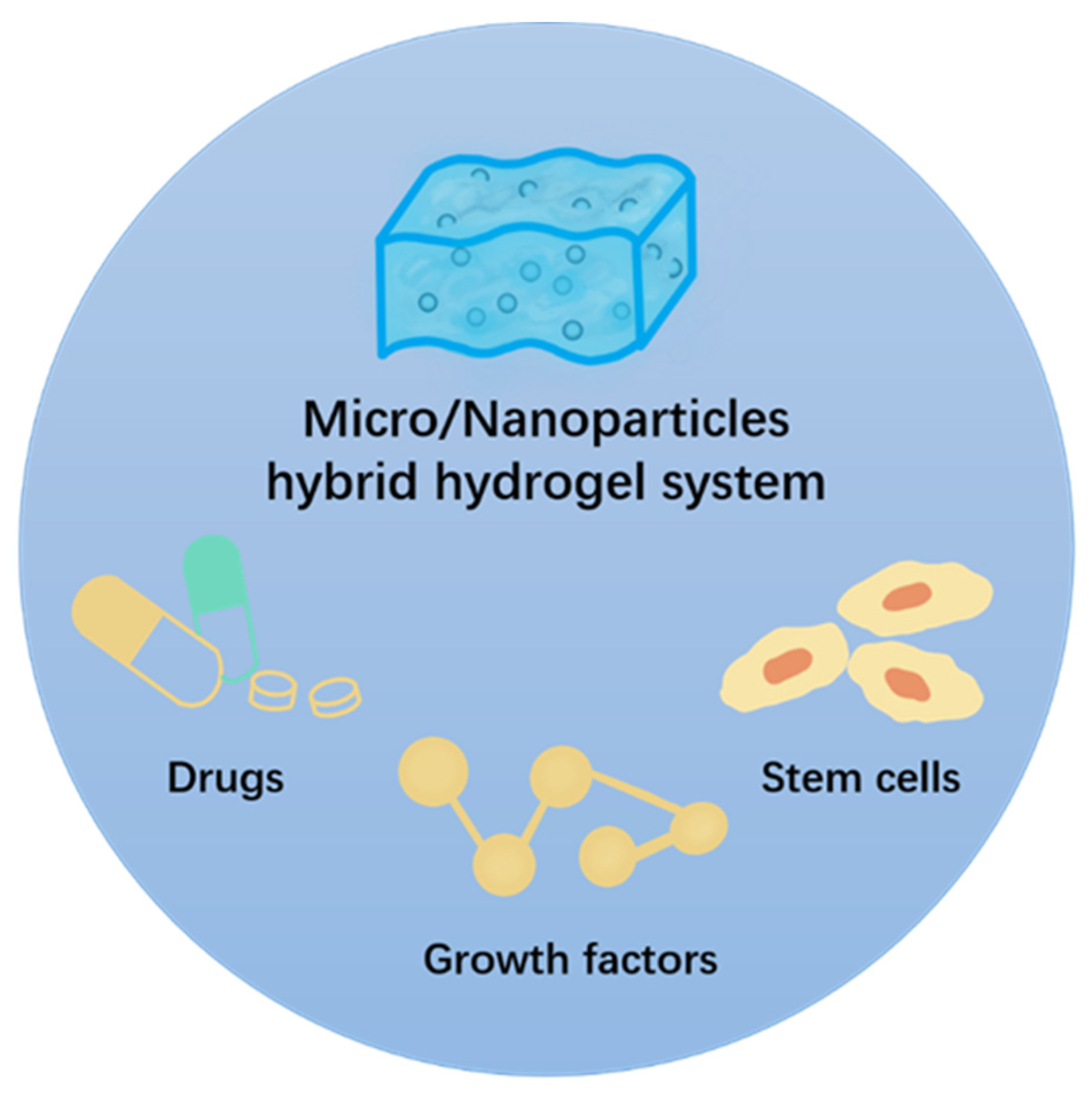
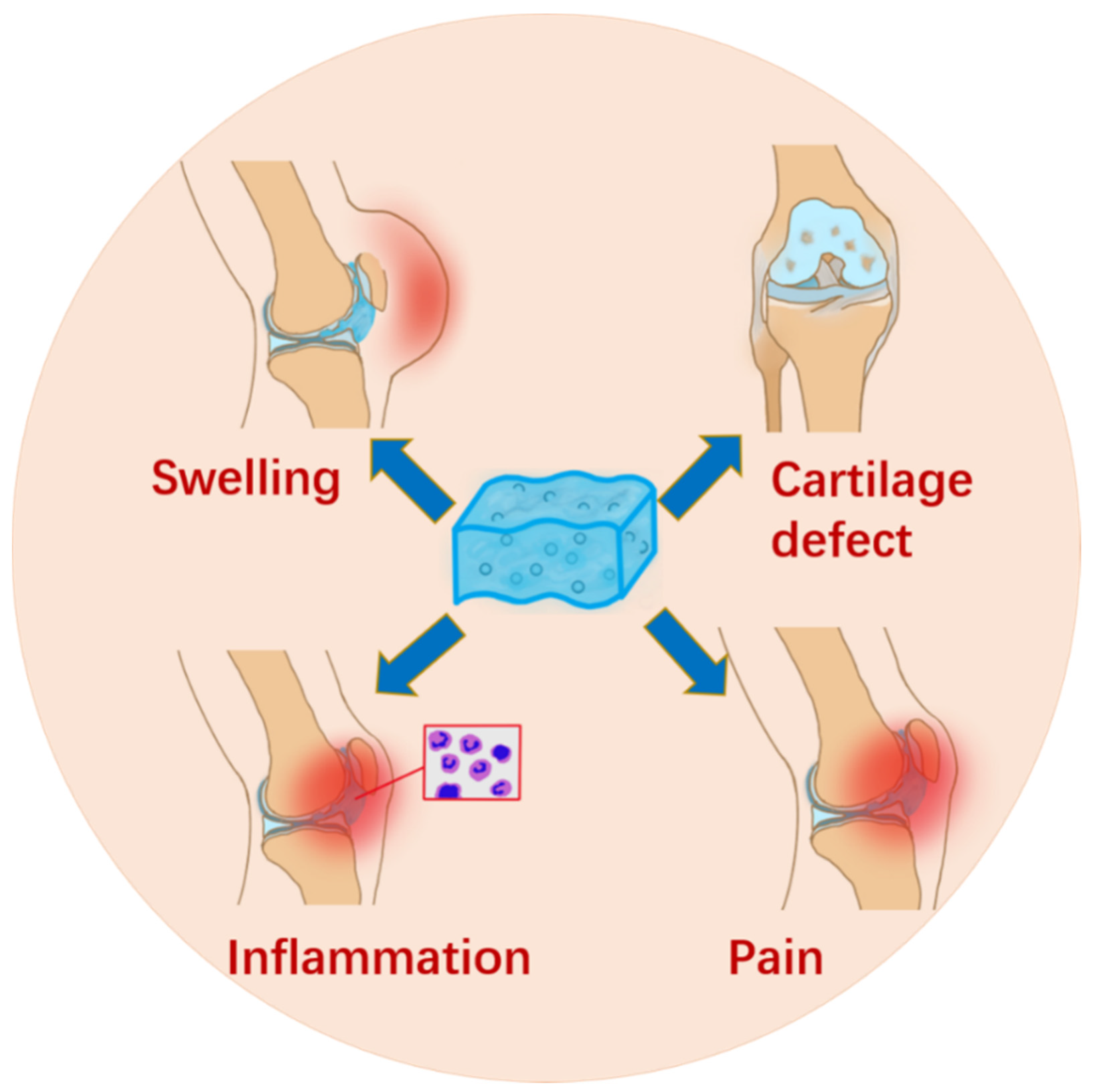

| Micro-/Nanoparticles | Hydrogel | Property | Stowage | Application | Effect | Ref. |
|---|---|---|---|---|---|---|
| Chitosan-acrylic acid-coated PLGA (ACH-PLGA) micro-/nanoparticles | PNIPAM/hyaluronic acid | injectable, thermosensitive, biocompatibility | melatonin | cartilage tissue engineering | sustained drug release | [111] |
| Hydroxyapatite (HAp) microparticles | RGD-alginate | injectable, viscoelastic | Sr | bone repair | osteogenic, anti-osteoclastogenic and immunomodulatory | [112] |
| Chitosan (CS) nanoparticles | silk fibroin (SF) | biocompatibility | TGFβ1@CS, BMP-2@SF | repair knee joint cartilage defects | release TGF-β1 and BMP-2, promoted chondrogenic ability of BMSCs | [113] |
| Polylactic acid (PLA) microparticles | hydrogel of functionalized pectin and fibroin | \ | calcium peroxide | a promoting step for regeneration of cartilage-to-bone interface | oxygenation of the cartilage-to-bone interface | [114] |
| Hyaluronic acid (HA)/chitosan-poly(dioxanone)(CH-PDO) complex nanoparticles | ALG-POL/SF dual network | elastic, tough and strong | bone morphogenic protein-7 (BMP-7) | cartilage tissue engineering | controlled release of BMP-7 | [115] |
| CNT nanoparticles | polyacrylamide (PAM) | bioactivity and cytocompatibility | TiO2 | cartilage repair | cartilage replacement | [116] |
| Poly(lactic acid) (PLA)/methoxy-polyethylene glycol-poly(δdecalactone) (mPEG−PDL) microparticles | poly(PEGMA) Copolymer | thermoresponsive, bioadhesive | triamcinolone acetonide (TA) | RA | anti-inflammatory | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Wu, Y.; Shan, Y.; Zhang, X.; Liao, J. Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases. Gels 2021, 7, 155. https://doi.org/10.3390/gels7040155
Han X, Wu Y, Shan Y, Zhang X, Liao J. Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases. Gels. 2021; 7(4):155. https://doi.org/10.3390/gels7040155
Chicago/Turabian StyleHan, Xu, Yongzhi Wu, Yue Shan, Xu Zhang, and Jinfeng Liao. 2021. "Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases" Gels 7, no. 4: 155. https://doi.org/10.3390/gels7040155
APA StyleHan, X., Wu, Y., Shan, Y., Zhang, X., & Liao, J. (2021). Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases. Gels, 7(4), 155. https://doi.org/10.3390/gels7040155






