Advanced Methods for the Characterization of Supramolecular Hydrogels
Abstract
:1. Introduction
2. Spectroscopy
2.1. NMR Spectroscopy
2.2. Absorption Spectroscopy
2.3. Raman Spectroscopy
2.4. Circular Dichroism (CD)
3. Diffraction
3.1. X-ray Diffraction (XRD)
3.1.1. Wide-Angle X-ray Diffraction (WAXD)
3.1.2. Small-Angle X-ray Scattering (SAXS)
3.2. Neutron Diffraction
Small-Angle Neutron Scattering (SANS)
4. Microscopy
4.1. Electron Microscopy
4.1.1. Transmission Electron Microscopy (TEM)
4.1.2. Scanning Electron Microscopy (SEM)
4.1.3. Cryo-EM
4.2. Fluorescence Microscopy
Super-Resolution Microscopy (SRM)
4.3. Scanning Probe Microscopy (SPM)
Atomic Force Microscopy (AFM)
5. Mechanical and Surface Characterization
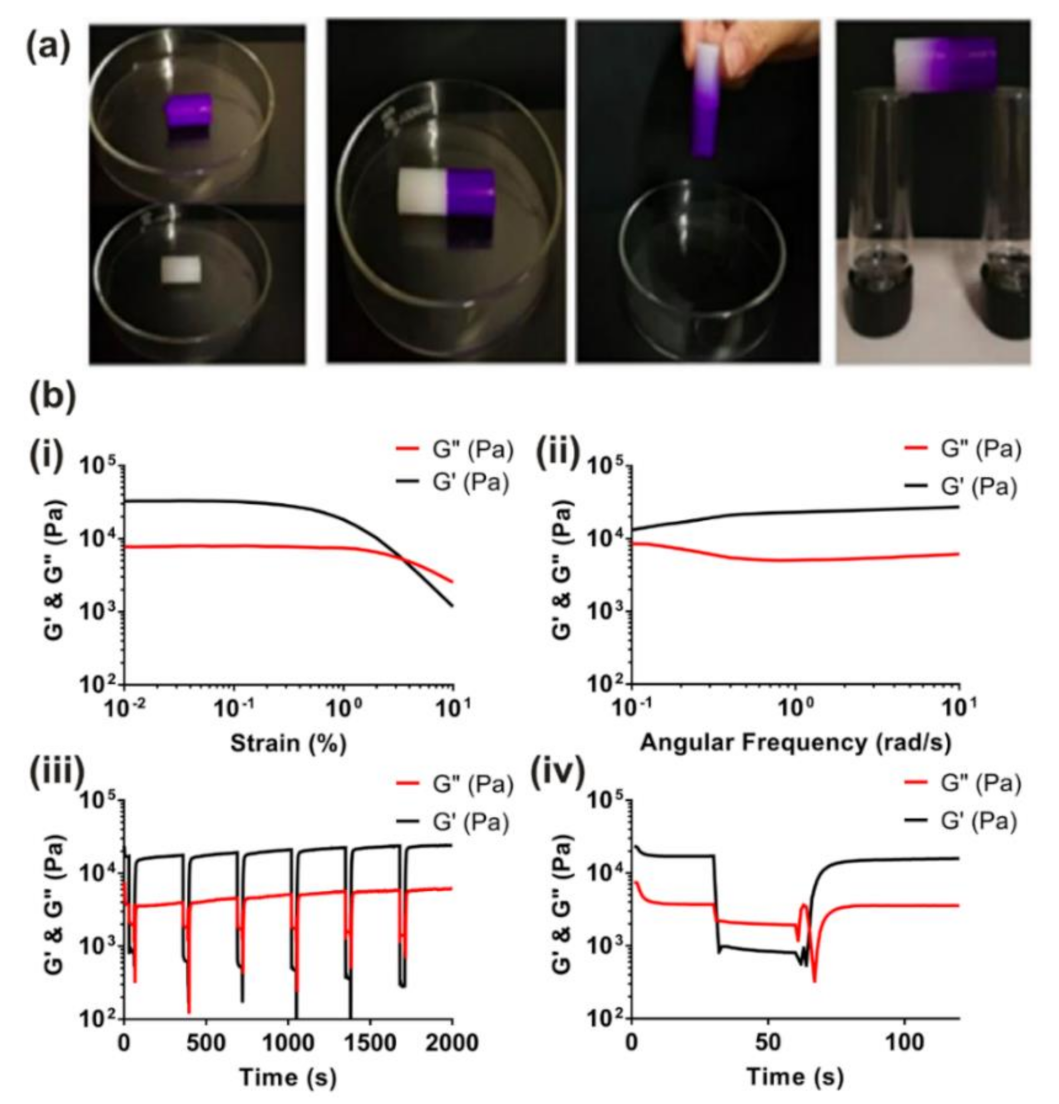
5.1. Rheology
5.2. Shear-Thinning and Self-Healing Characterization
5.3. Micro-rheology
5.4. Other Mechanical Testing
5.5. Lubrication Characterization
5.6. Computational Modeling of Rheological Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-CD | α-cyclodextrin |
| AFM | Atomic force microscopy |
| ATR | Attenuated total reflection |
| β-CD | β-cyclodextrin |
| CD | Circular dichroism |
| cryo-EM | Cryogenic electron microscopy |
| cryo-TEM | Cryogenic transmission electron microscopy |
| Dex | Dexamethasone sodium phosphate |
| DFA | Dimer fatty acid |
| DFT | Density functional theory |
| DLS | Dynamic light scattering |
| DMA | N,N-dimethylacrylamide |
| DWS | Diffusing-wave spectroscopy |
| Fmoc | N-fluorenylmethoxycarbonyl |
| FIB | Focused ion beam |
| FLIM | Fluorescence lifetime imaging microscopy |
| FT-IR | Fourier-transform infrared |
| FOSA | 2-(N-ethylperfluorooctane sulfonamido)ethyl acrylate |
| Hep-MPEG | Heparin-conjugated poly(ethylene glycol) methyl ether |
| LAOS | Large-amplitude oscillatory shear |
| LVE | Linear-viscoelastic regime |
| MAS | Magic-angle spinning |
| NMR | Nuclear magnetic resonance |
| PCL | Polycaprolactone |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene oxide) |
| PMPC | Poly(2-methacryloyloxyethyl phosphorylcholine) |
| PL188 | Poloxamer 188 |
| PL407 | Poloxamer 407 |
| RQC | Residual quadrupolar coupling |
| RVC | Ropivacaine |
| SANS | Small-angle neutron scattering |
| SAOS | Small-amplitude oscillatory shear |
| SAXS | Small-angle X-ray scattering |
| SEM | Scanning electron microscopy |
| SERS | Surface-enhanced Raman scattering |
| SIM | Structured illumination microscopy |
| SMLM | Single-molecule localization microscopy |
| SPM | Scanning probe microscopy |
| SRM | Super-resolution microscopy |
| STD | Saturation transfer difference |
| STED | Stimulated emission depletion |
| TEM | Transmission electron microscopy |
| UV–vis | Ultraviolet–visible |
| WAXD | Wide-angle X-ray diffraction |
| XRD | X-ray diffraction |
| 3D | Three-dimensional |
| 5-FU | 5-fluorouracil |
References
- Flory, P.J. Introductory Lecture. Faraday Discuss. Chem. Soc. 1974, 57, 7. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Lew, A.; Ortony, J. Beyond Covalent Crosslinks: Applications of Supramolecular Gels. Gels 2018, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Chichester, UK, 2009; ISBN 978-0-470-51233-3. [Google Scholar]
- Yan, X.; Chen, Y.-R.; Song, Y.-F.; Ye, J.; Yang, M.; Xu, B.-B.; Zhang, J.; Wang, X.; Yu, J.-K. Advances in the Application of SupraMolecular Hydrogels for Stem Cell Delivery and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 847. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromol. Biosci. 2019, 19, 1800259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saboktakin, M.R.; Tabatabaei, R.M. Supramolecular Hydrogels as Drug Delivery Systems. Int. J. Biol. Macromol. 2015, 75, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of Supramolecular Gels. Chem. Soc. Rev. 2013, 42, 6697. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, Applicability and Advanceability. Adv. Mater. 2019, 31, 1806204. [Google Scholar] [CrossRef]
- Dou, X.-Q.; Feng, C.-L. Amino Acids and Peptide-Based Supramolecular Hydrogels for Three-Dimensional Cell Culture. Adv. Mater. 2017, 29, 1604062. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host–Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, Y.; Zhu, Y.; Hao, L.; Chen, Y.; An, G.; Wu, H.; Shi, X.; Mao, C. A Rapidly Self-Healing Host-Guest Supramolecular Hydrogel with High Mechanical Strength and Excellent Biocompatibility. Angew. Chem. Int. Ed. 2018, 57, 9008–9012. [Google Scholar] [CrossRef]
- Rutgeerts, L.A.J.; Soultan, A.H.; Subramani, R.; Toprakhisar, B.; Ramon, H.; Paderes, M.C.; De Borggraeve, W.M.; Patterson, J. Robust Scalable Synthesis of a Bis-Urea Derivative Forming Thixotropic and Cytocompatible Supramolecular Hydrogels. Chem. Commun. 2019, 55, 7323–7326. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.M.; Skala, L.P.; Plank, T.N.; Hyman, B.J.; Manjunatha Reddy, G.N.; Marsh, A.; Brown, S.P.; Davis, J.T. A G4 K+ Hydrogel Stabilized by an Anion. J. Am. Chem. Soc. 2014, 136, 12596–12599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamon, F.; Blaszkiewicz, C.; Buchotte, M.; Banaszak-Léonard, E.; Bricout, H.; Tilloy, S.; Monflier, E.; Cézard, C.; Bouteiller, L.; Len, C.; et al. Synthesis and Characterization of a New Photoinduced Switchable β-Cyclodextrin Dimer. Beilstein J. Org. Chem. 2014, 10, 2874–2885. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, S.; Balog, S.; Kilbinger, A.F.M.; Casalini, T. The Influence of Substituents on Gelation and Stacking Order of Oligoaramid – Based Supramolecular Networks. Soft Matter 2019, 15, 7250–7261. [Google Scholar] [CrossRef]
- Fitremann, J.; Lonetti, B.; Fratini, E.; Fabing, I.; Payré, B.; Boulé, C.; Loubinoux, I.; Vaysse, L.; Oriol, L. A Shear-Induced Network of Aligned Wormlike Micelles in a Sugar-Based Molecular Gel. From Gelation to Biocompatibility Assays. J. Colloid Interface Sci. 2017, 504, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Basavalingappa, V.; Bera, S.; Xue, B.; O’Donnell, J.; Guerin, S.; Cazade, P.-A.; Yuan, H.; Haq, E.; Silien, C.; Tao, K.; et al. Diphenylalanine-Derivative Peptide Assemblies with Increased Aromaticity Exhibit Metal-like Rigidity and High Piezoelectricity. ACS Nano 2020, 14, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Wang, X.; Gelain, F.; Zhang, S. Biological Designer Self-Assembling Peptide Nanofiber Scaffolds Significantly Enhance Osteoblast Proliferation, Differentiation and 3-D Migration. PLoS ONE 2007, 2, e190. [Google Scholar] [CrossRef]
- Gottarelli, G.; Lena, S.; Masiero, S.; Pieraccini, S.; Spada, G.P. The Use of Circular Dichroism Spectroscopy for Studying the Chiral Molecular Self-Assembly: An Overview. Chirality 2008, 20, 471–485. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Shapiro, Y.E. Structure and Dynamics of Hydrogels and Organogels: An NMR Spectroscopy Approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Brown, S.P. Advanced Solid-State NMR Methods for Characterising Structure and Self-Assembly in Supramolecular Chemistry, Polymers and Hydrogels. Curr. Opin. Colloid Interface Sci. 2018, 33, 86–98. [Google Scholar] [CrossRef]
- Ramalhete, S.M.; Nartowski, K.P.; Sarathchandra, N.; Foster, J.S.; Round, A.N.; Angulo, J.; Lloyd, G.O.; Khimyak, Y.Z. Supramolecular Amino Acid Based Hydrogels: Probing the Contribution of Additive Molecules Using NMR Spectroscopy. Chem.-Eur. J. 2017, 23, 8014–8024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjunatha Reddy, G.N.; Peters, G.M.; Tatman, B.P.; Rajan, T.S.; Kock, S.M.; Zhang, J.; Frenguelli, B.G.; Davis, J.T.; Marsh, A.; Brown, S.P. Magic-Angle Spinning NMR Spectroscopy Provides Insight into the Impact of Small Molecule Uptake by G-Quartet Hydrogels. Mater. Adv. 2020, 1, 2236–2247. [Google Scholar] [CrossRef]
- Wallace, M.; Iggo, J.A.; Adams, D.J. Probing the Surface Chemistry of Self-Assembled Peptide Hydrogels Using Solution-State NMR Spectroscopy. Soft Matter 2017, 13, 1716–1727. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.; Peters, T. NMR Spectroscopy Techniques for Screening and Identifying Ligand Binding to Protein Receptors. Angew. Chem. Int. Ed. 2003, 42, 864–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.N.; Zeng, H.; Ji, X.; Xie, T.; Yan, X.; Wu, Z.L.; Huang, F. Reversible Ion-Conducting Switch in a Novel Single-Ion Supramolecular Hydrogel Enabled by Photoresponsive Host–Guest Molecular Recognition. Adv. Mater. 2019, 31, 1807328. [Google Scholar] [CrossRef]
- Stone, D.A.; Hsu, L.; Stupp, S.I. Self-Assembling Quinquethiophene–Oligopeptide Hydrogelators. Soft Matter 2009, 5, 1990. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Kumar, Y.P.; Dash, J. Supramolecular Hydrogel Inspired from DNA Structures Mimics Peroxidase Activity. ACS Biomater. Sci. Eng. 2017, 3, 2358–2365. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, L.; Wang, J.; Wang, J.; Ma, J.; Yuan, Z.; Lincoln, S.F.; Guo, X. Photo-Reversible Supramolecular Hydrogels Assembled by α-Cyclodextrin and Azobenzene Substituted Poly (Acrylic Acid) s: Effect of Substitution Degree, Concentration, and Tethered Chain Length: Photo-Reversible Supramolecular Hydrogels. Macromol. Mater. Eng. 2016, 301, 191–198. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, R.; Lei, L.; Li, X. Self-Assembly of Succinated Paclitaxel into Supramolecular Hydrogel for Local Cancer Chemotherapy. J. Biomed. Nanotechnol. 2018, 14, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Z.; Xiong, T.; Zhao, W.; Jiang, R.; Chen, H.; Li, X. Calcium Ion Coordinated Dexamethasone Supramolecular Hydrogel as Therapeutic Alternative for Control of Non-Infectious Uveitis. Acta Biomater. 2017, 61, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Chan, K.L.A. Applications of ATR-FTIR Spectroscopic Imaging to Biomedical Samples. Biochim. Biophys. Acta BBA-Biomembr. 2006, 1758, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- More, M.P.; Chitalkar, R.V.; Bhadane, M.S.; Dhole, S.D.; Patil, A.G.; Patil, P.O.; Deshmukh, P.K. Development of Graphene-Drug Nanoparticle Based Supramolecular Self Assembled PH Sensitive Hydrogel as Potential Carrier for Targeting MDR Tuberculosis. Mater. Technol. 2019, 34, 324–335. [Google Scholar] [CrossRef]
- Braun, G.A.; Pogostin, B.H.; Pucetaite, M.; Londergan, C.H.; Åkerfeldt, K.S. Deuterium-Enhanced Raman Spectroscopy for Histidine PKa Determination in a PH-Responsive Hydrogel. Biophys. J. 2020, 119, 1701–1705. [Google Scholar] [CrossRef]
- Haynes, C.L.; McFarland, A.D.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2005, 77, 338A–346A. [Google Scholar] [CrossRef] [Green Version]
- Miljanić, S.; Frkanec, L.; Biljan, T.; Meić, Z.; Žinić, M. Surface-Enhanced Raman Scattering on Molecular Self-Assembly in Nanoparticle-Hydrogel Composite. Langmuir 2006, 22, 9079–9081. [Google Scholar] [CrossRef]
- Woody, R.W. Circular dichroism. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 246, pp. 34–71. ISBN 978-0-12-182147-0. [Google Scholar]
- West, M.W.; Wang, W.; Patterson, J.; Mancias, J.D.; Beasley, J.R.; Hecht, M.H. De Novo Amyloid Proteins from Designed Combinatorial Libraries. Proc. Natl. Acad. Sci. USA 1999, 96, 11211–11216. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Gu, H.; Zhang, Y.; Wang, L.; Xu, B. Small Molecule Hydrogels Based on a Class of Antiinflammatory Agents. Chem. Commun. 2004, 208–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An Injectable Dipeptide–Fullerene Supramolecular Hydrogel for Photodynamic Antibacterial Therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Etter, M.; Dinnebier, R.E. A Century of Powder Diffraction: A Brief History: A Century of Powder Diffraction: A Brief History. Z. Anorg. Allg. Chem. 2014, 640, 3015–3028. [Google Scholar] [CrossRef]
- Rupp, B. Biomolecular Crystallography: Principles, Practice and Application to Structural Biology; Garland Science: New York, NY, USA, 2010. [Google Scholar]
- Modern Diffraction Methods; Mittemeijer, E.J.; Welzel, U.S. (Eds.) Garland Science: New York, USA, 2013; ISBN 978-3-527-64990-7. [Google Scholar]
- You, Y.; Xing, R.; Zou, Q.; Shi, F.; Yan, X. High-Tolerance Crystalline Hydrogels Formed from Self-Assembling Cyclic Dipeptide. Beilstein J. Nanotechnol. 2019, 10, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Tu, K.; Zhang, L.-M. Bioactive Supramolecular Hydrogel with Controlled Dual Drug Release Characteristics. Biomacromolecules 2010, 11, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, Z.; Liang, R.; Ma, Y.; Huang, W.; Jiang, R.; Shi, S.; Chen, H.; Li, X. Fabrication of a Micellar Supramolecular Hydrogel for Ocular Drug Delivery. Biomacromolecules 2016, 17, 798–807. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Geng, R.; Zhang, X.; Wang, F.; Jiao, T.; Yang, J.; Bai, Z.; Peng, Q. A Facile Preparation Method for New Two-Component Supramolecular Hydrogels and Their Performances in Adsorption, Catalysis, and Stimuli-Response. RSC Adv. 2019, 9, 22551–22558. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Farahani, A.D.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Unraveling the Self-Assembly Modes in Multicomponent Supramolecular Gels Using Single-Crystal X-Ray Diffraction. Chem. Mater. 2020, 32, 3517–3527. [Google Scholar] [CrossRef]
- Iwashita, N. X-ray Powder Diffraction. In Materials Science and Engineering of Carbon; Elsevier: Oxford, UK, 2016; pp. 7–25. ISBN 978-0-12-805256-3. [Google Scholar]
- Graewert, M.A.; Svergun, D.I. Impact and Progress in Small and Wide Angle X-Ray Scattering (SAXS and WAXS). Curr. Opin. Struct. Biol. 2013, 23, 748–754. [Google Scholar] [CrossRef]
- Lavčević, M.L.; Turković, A. Small-Angle X-Ray Scattering and Wide-Angle X-Ray Diffraction on Thermally Annealed Nanostructured TiO2 Films. Thin Solid Film. 2002, 419, 105–113. [Google Scholar] [CrossRef]
- Hub, J.S. Interpreting Solution X-Ray Scattering Data Using Molecular Simulations. Curr. Opin. Struct. Biol. 2018, 49, 18–26. [Google Scholar] [CrossRef]
- Hastings, J.B.; Thomlinson, W.; Cox, D.E. Synchrotron X-Ray Powder Diffraction. J. Appl. Crystallogr. 1984, 17, 85–95. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Zhang, L. Fabrication and Characterization of Novel Macroporous Cellulose–Alginate Hydrogels. Polymer 2009, 50, 5467–5473. [Google Scholar] [CrossRef]
- László, K.; Guillermo, A.; Fluerasu, A.; Moussaïd, A.; Geissler, E. Microphase Structure of Poly(N-Isopropylacrylamide) Hydrogels As Seen by Small- and Wide-Angle X-Ray Scattering and Pulsed Field Gradient NMR. Langmuir 2010, 26, 4415–4420. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.; Leong, K.W. Injectable Drug-Delivery Systems Based on Supramolecular Hydrogels Formed by Poly (Ethylene Oxide) s and ?-Cyclodextrin. J. Biomed. Mater. Res. 2003, 65A, 196–202. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Chen, Q.; Zhang, B.; Song, J. Hofmeister Anion-Induced Tunable Rheology of Self-Healing Supramolecular Hydrogels. Nanoscale Res. Lett. 2019, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.K.D.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; de Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 Binary Thermosensitive Hydrogels as Delivery Systems for Infiltrative Local Anesthesia: Physico-Chemical Characterization and Pharmacological Evaluation. Mater. Sci. Eng. C 2016, 68, 299–307. [Google Scholar] [CrossRef]
- Hura, G.L.; Menon, A.L.; Hammel, M.; Rambo, R.P.; Poole II, F.L.; Tsutakawa, S.E.; Jenney Jr, F.E.; Classen, S.; Frankel, K.A.; Hopkins, R.C.; et al. Robust, High-Throughput Solution Structural Analyses by Small Angle X-Ray Scattering (SAXS). Nat. Methods 2009, 6, 606–612. [Google Scholar] [CrossRef]
- Bacon, G.E.; Lonsdale, K. Neutron Diffraction. Rep. Prog. Phys. 1953, 16, 1–61. [Google Scholar] [CrossRef]
- Zaccai, G.; Jacrot, B. Small Angle Neutron Scattering. Annu. Rev. Biophys. Bioeng. 1983, 12, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.-S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef]
- Mortensen, K.; Talmon, Y. Cryo-TEM and SANS Microstructural Study of Pluronic Polymer Solutions. Macromolecules 1995, 28, 8829–8834. [Google Scholar] [CrossRef]
- Lorson, T.; Jaksch, S.; Lübtow, M.M.; Jüngst, T.; Groll, J.; Lühmann, T.; Luxenhofer, R. A Thermogelling Supramolecular Hydrogel with Sponge-Like Morphology as a Cytocompatible Bioink. Biomacromolecules 2017, 18, 2161–2171. [Google Scholar] [CrossRef]
- Wiener, C.G.; Wang, C.; Liu, Y.; Weiss, R.A.; Vogt, B.D. Nanostructure Evolution during Relaxation from a Large Step Strain in a Supramolecular Copolymer-Based Hydrogel: A SANS Investigation. Macromolecules 2017, 50, 1672–1680. [Google Scholar] [CrossRef]
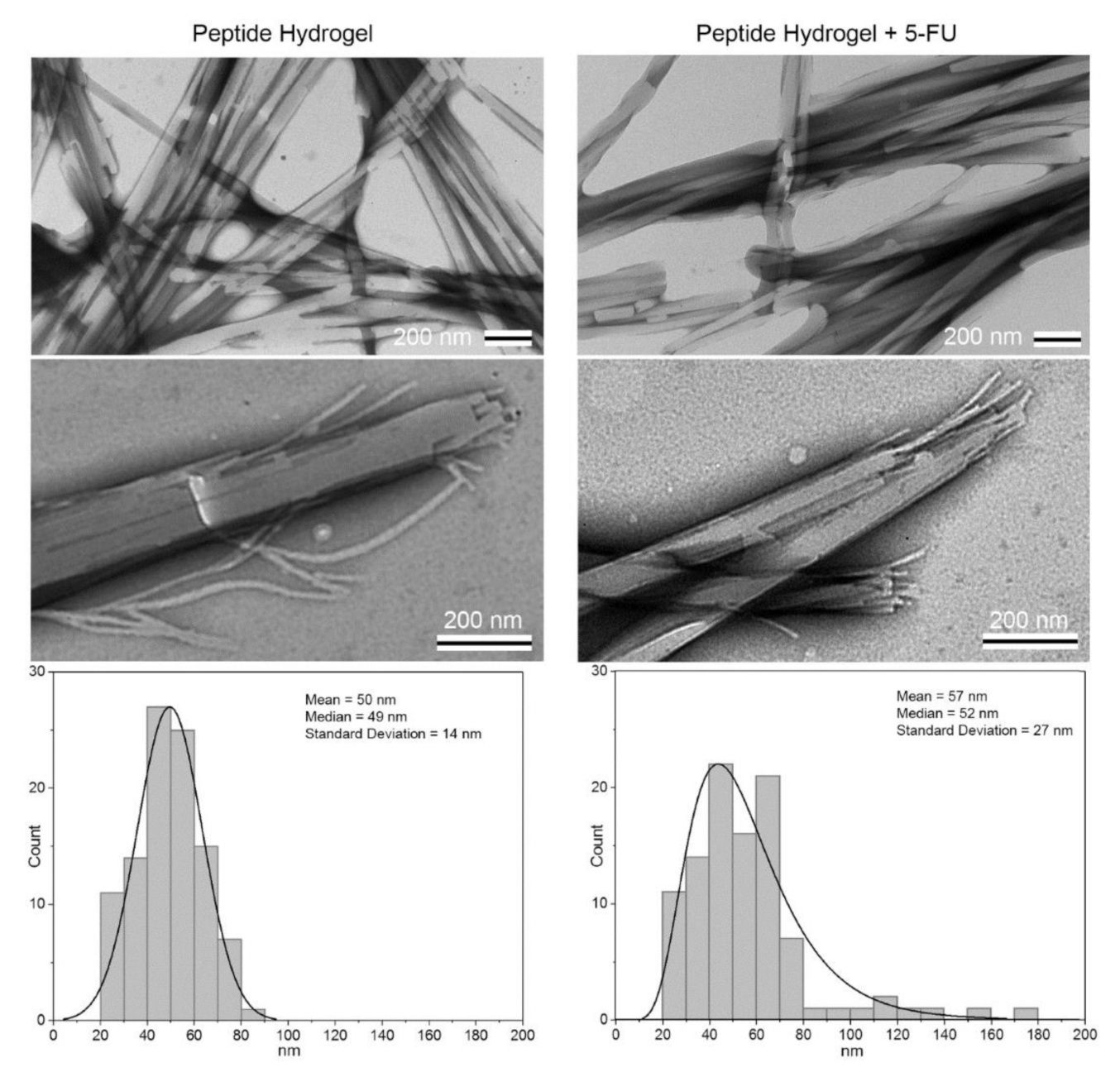
- Parisi, E.; Garcia, A.; Marson, D.; Posocco, P.; Marchesan, S. Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels 2019, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Yu, X.; Wu, H.; Li, J.; Lv, H.; Yang, X. Nonswellable and Tough Supramolecular Hydrogel Based on Strong Micelle Cross-Linkings. Biomacromolecules 2019, 20, 3399–3407. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Wu, Y.; Zhang, J.; Zhu, Y.; Wang, J. A Multifunctional Supramolecular Hydrogel: Preparation, Properties and Molecular Assembly. Soft Matter 2018, 14, 566–573. [Google Scholar] [CrossRef]
- Tang, F.; Feng, H.; Du, Y.; Xiao, Y.; Dan, H.; Zhao, H.; Chen, Q. Developing a Self-Healing Supramolecular Nucleoside Hydrogel Based on Guanosine and Isoguanosine. Chem. Asian J. 2018, 13, 1962–1971. [Google Scholar] [CrossRef]
- Olderøy, M.Ø.; Lilledahl, M.B.; Beckwith, M.S.; Melvik, J.E.; Reinholt, F.; Sikorski, P.; Brinchmann, J.E. Biochemical and Structural Characterization of Neocartilage Formed by Mesenchymal Stem Cells in Alginate Hydrogels. PLoS ONE 2014, 9, e91662. [Google Scholar] [CrossRef] [Green Version]
- Milne, J.L.S.; Borgnia, M.J.; Bartesaghi, A.; Tran, E.E.H.; Earl, L.A.; Schauder, D.M.; Lengyel, J.; Pierson, J.; Patwardhan, A.; Subramaniam, S. Cryo-Electron Microscopy-A Primer for the Non-Microscopist. FEBS J. 2013, 280, 28–45. [Google Scholar] [CrossRef]
- da Silva, L.C.E.; Borges, A.C.; de Oliveira, M.G.; de Farias, M.A. Visualization of Supramolecular Structure of Pluronic F127 Micellar Hydrogels Using Cryo-TEM. MethodsX 2020, 7, 101084. [Google Scholar] [CrossRef]
- Clarke, D.E.; Parmenter, C.D.J.; Scherman, O.A. Tunable Pentapeptide Self-Assembled β-Sheet Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 7709–7713. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.W.; Conchello, J.-A. Fluorescence Microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Kisley, L. Fluorescence Microscopy of Biophysical Protein Dynamics in Nanoporous Hydrogels. J. Appl. Phys. 2019, 126, 081101. [Google Scholar] [CrossRef] [Green Version]
- Vandaele, J.; Louis, B.; Liu, K.; Camacho, R.; Kouwer, P.H.J.; Rocha, S. Structural Characterization of Fibrous Synthetic Hydrogels Using Fluorescence Microscopy. Soft Matter 2020, 16, 4210–4219. [Google Scholar] [CrossRef]
- Shi, Y.; Summers, P.A.; Kuimova, M.K.; Azevedo, H.S. Unravelling the Enzymatic Degradation Mechanism of Supramolecular Peptide Nanofibers and Its Correlation with Their Internal Viscosity. Nano Lett. 2020, 20, 7375–7381. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-Resolution Microscopy Demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
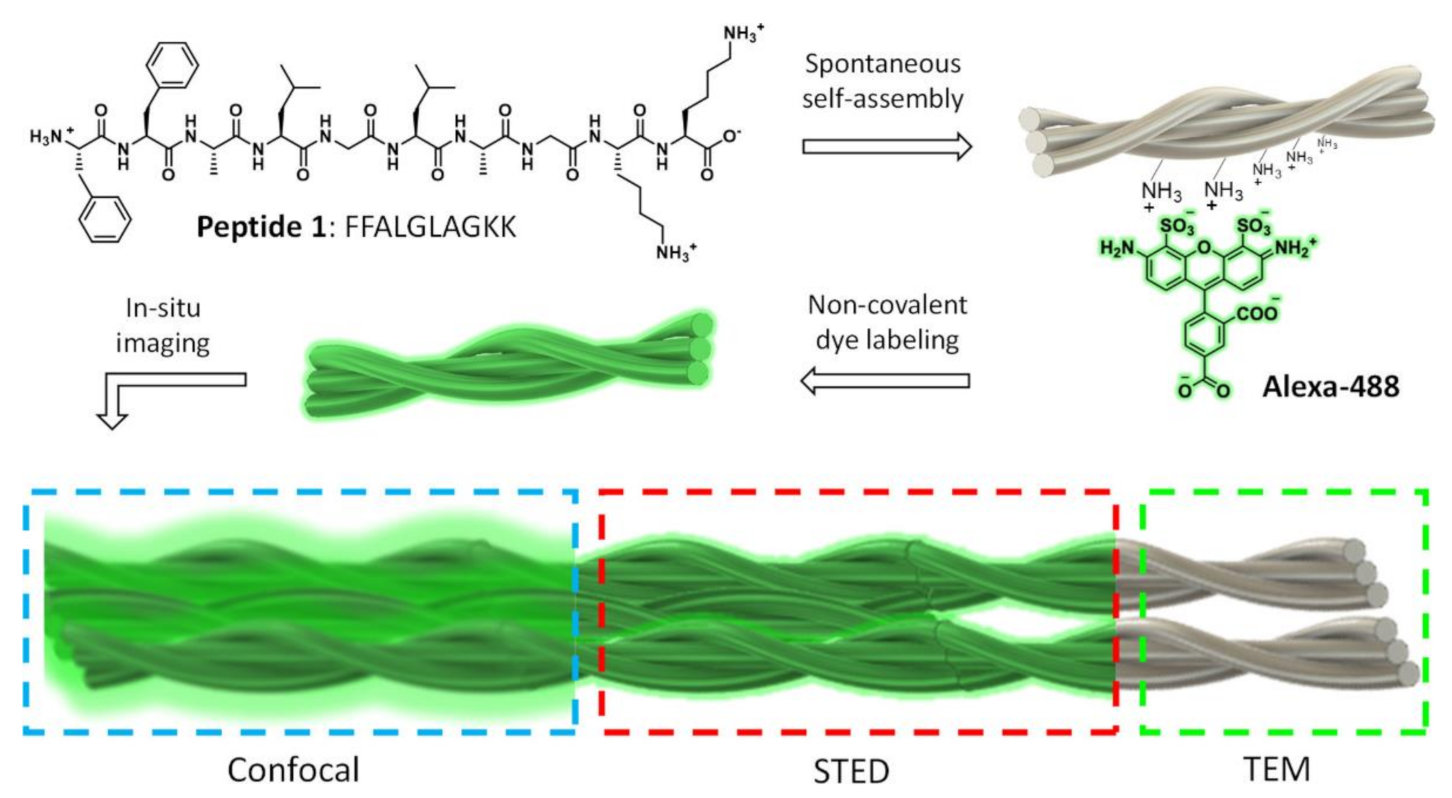
- Kumar, M.; Son, J.; Huang, R.H.; Sementa, D.; Lee, M.; O’Brien, S.; Ulijn, R.V. In Situ, Noncovalent Labeling and Stimulated Emission Depletion-Based Super-Resolution Imaging of Supramolecular Peptide Nanostructures. ACS Nano 2020, 14, 15056–15063. [Google Scholar] [CrossRef]
- Pujals, S.; Feiner-Gracia, N.; Delcanale, P.; Voets, I.; Albertazzi, L. Super-Resolution Microscopy as a Powerful Tool to Study Complex Synthetic Materials. Nat. Rev. Chem. 2019, 3, 68–84. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.B.; Lee, S.-L. Supramolecular Chemistry: Host–Guest Molecular Complexes. Molecules 2021, 26, 3995. [Google Scholar] [CrossRef]
- Paiva, J.S.; Jorge, P.A.S.; Rosa, C.C.; Cunha, J.P.S. Optical Fiber Tips for Biological Applications: From Light Confinement, Biosensing to Bioparticles Manipulation. Biochim. Biophys. Acta BBA-Gen. Subj. 2018, 1862, 1209–1246. [Google Scholar] [CrossRef]
- Hermann, R.J.; Gordon, M.J. Nanoscale Optical Microscopy and Spectroscopy Using Near-Field Probes. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.J.; West, P. Atomic Force Microscopy; Oxford University Press: Oxford, UK; New York, NY, USA, 2010; ISBN 978-0-19-957045-4. [Google Scholar]
- Roy, S.; Javid, N.; Frederix, P.W.J.M.; Lamprou, D.A.; Urquhart, A.J.; Hunt, N.T.; Halling, P.J.; Ulijn, R.V. Dramatic Specific-Ion Effect in Supramolecular Hydrogels. Chem.-A Eur. J. 2012, 18, 11723–11731. [Google Scholar] [CrossRef]
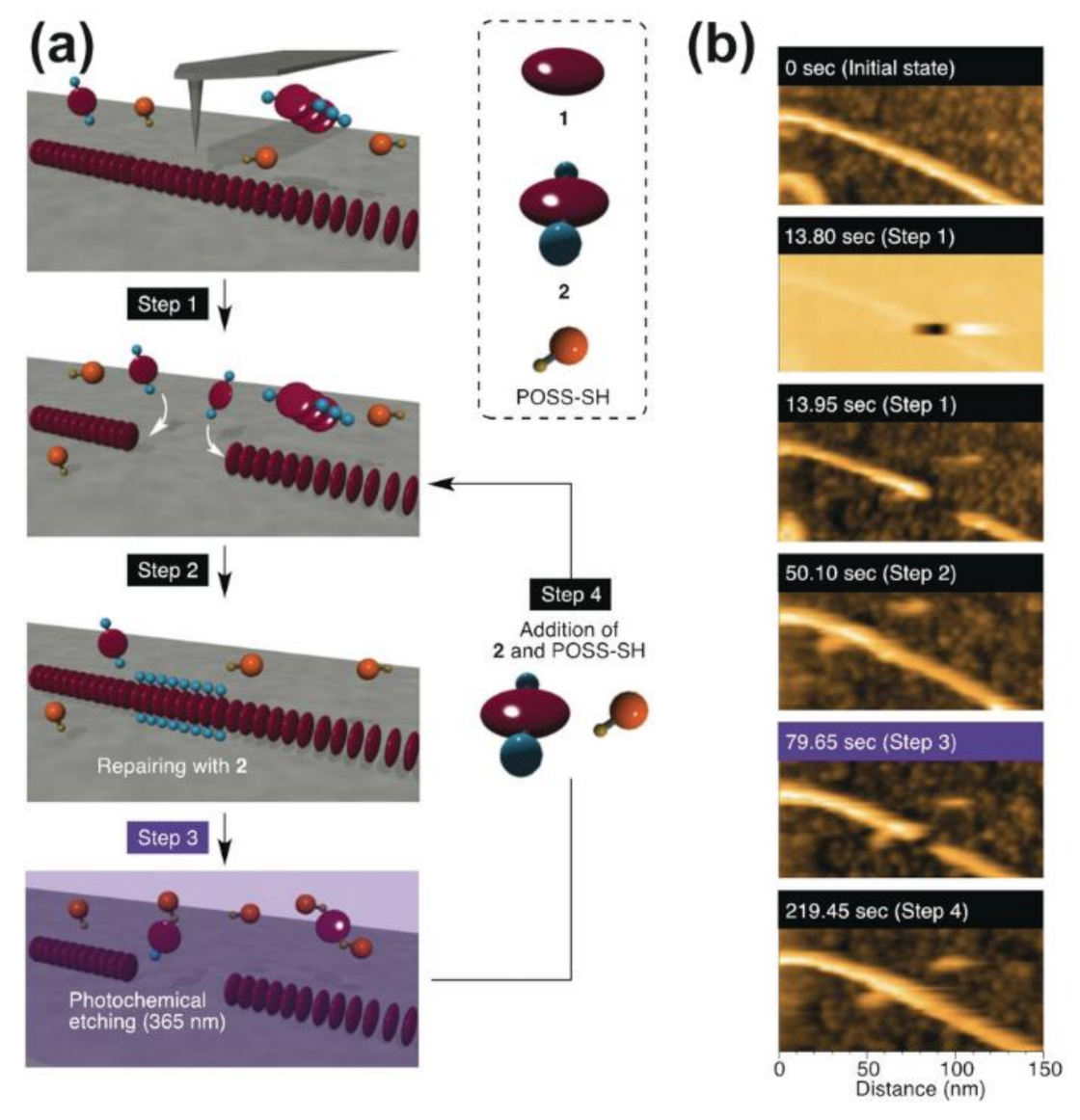
- Fukui, T.; Uchihashi, T.; Sasaki, N.; Watanabe, H.; Takeuchi, M.; Sugiyasu, K. Direct Observation and Manipulation of Supramolecular Polymerization by High-Speed Atomic Force Microscopy. Angew. Chem. Int. Ed. 2018, 57, 15465–15470. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.J.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef] [Green Version]
- EzEldeen, M.; Toprakhisar, B.; Murgia, D.; Smisdom, N.; Deschaume, O.; Bartic, C.; Van Oosterwyck, H.; Pereira, R.V.S.; Opdenakker, G.; Lambrichts, I.; et al. Chlorite Oxidized Oxyamylose Differentially Influences the Microstructure of Fibrin and Self Assembling Peptide Hydrogels as Well as Dental Pulp Stem Cell Behavior. Sci. Rep. 2021, 11, 5687. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, M.D.A.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the Elastic Modulus of Soft Culture Surfaces and Three-Dimensional Hydrogels Using Atomic Force Microscopy. Nat. Protoc. 2021, 16, 2418–2449. [Google Scholar] [CrossRef]
- Nalam, P.C.; Gosvami, N.N.; Caporizzo, M.A.; Composto, R.J.; Carpick, R.W. Nano-Rheology of Hydrogels Using Direct Drive Force Modulation Atomic Force Microscopy. Soft Matter 2015, 11, 8165–8178. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3D-Printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A Protocol for Rheological Characterization of Hydrogels for Tissue Engineering Strategies: A Protocol for Rheological Characterization of Hydrogels. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Megone, W.; Roohpour, N.; Gautrot, J.E. Impact of Surface Adhesion and Sample Heterogeneity on the Multiscale Mechanical Characterisation of Soft Biomaterials. Sci. Rep. 2018, 8, 6780. [Google Scholar] [CrossRef]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The Influence of Swelling on Elastic Properties of Polyacrylamide Hydrogels. Front. Mater. 2020, 7, 212. [Google Scholar] [CrossRef]
- Yuan, Q.; Lu, X.; Khayat, K.H.; Feys, D.; Shi, C. Small Amplitude Oscillatory Shear Technique to Evaluate Structural Build-up of Cement Paste. Mater. Struct. 2017, 50, 112. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent Advances in Shear-thinning and Self-healing Hydrogels for Biomedical Applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Yang, J.H.; Du, X.J.; Xu, F.; Zrinyi, M.; Osada, Y.; Li, F.; Chen, Y.M. Dextran-Based Self-Healing Hydrogels Formed by Reversible Diels-Alder Reaction under Physiological Conditions. Macromol. Rapid Commun. 2013, 34, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Krajina, B.A.; Tropini, C.; Zhu, A.; DiGiacomo, P.; Sonnenburg, J.L.; Heilshorn, S.C.; Spakowitz, A.J. Dynamic Light Scattering Microrheology Reveals Multiscale Viscoelasticity of Polymer Gels and Precious Biological Materials. ACS Cent. Sci. 2017, 3, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Samaniuk, J.R.; Vermant, J. Micro and Macrorheology at Fluid–Fluid Interfaces. Soft Matter 2014, 10, 7023–7033. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Indei, T.; Koga, T.; Narita, T. Physical Gelation of Supramolecular Hydrogels Cross-Linked by Metal-Ligand Interactions: Dynamic Light Scattering and Microrheological Studies. Polymer 2017, 128, 363–372. [Google Scholar] [CrossRef]
- Cicuta, P.; Donald, A.M. Microrheology: A Review of the Method and Applications. Soft Matter 2007, 3, 1449. [Google Scholar] [CrossRef]
- Frith, W.J.; Donald, A.M.; Adams, D.J.; Aufderhorst-Roberts, A. Gels Formed from Amino-Acid Derivatives, Their Novel Rheology as Probed by Bulk and Particle Tracking Rheological Methods. J. Non-Newton. Fluid Mech. 2015, 222, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Priyanka; Kumar, A. Smart Soft Supramolecular Hybrid Hydrogels Modulated by Zn2+/Ag NPs with Unique Multifunctional Properties and Applications. Dalton Trans. 2020, 49, 15095–15108. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.-M. Triple Redox/Temperature Responsive Diselenide-Containing Homopolypeptide Micelles and Supramolecular Hydrogels Thereof. J. Polym. Sci. Part Polym. Chem. 2018, 56, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Shi, X.; Zhang, J.; Zhu, Y.; Wang, J. Self-Assembled PH-Responsive Supramolecular Hydrogel for Hydrophobic Drug Delivery. RSC Adv. 2018, 8, 31581–31587. [Google Scholar] [CrossRef] [Green Version]
- Kakuta, T.; Takashima, Y.; Harada, A. Highly Elastic Supramolecular Hydrogels Using Host–Guest Inclusion Complexes with Cyclodextrins. Macromolecules 2013, 46, 4575–4579. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zheng, J.; Tong, D.; Zhao, N.; Ma, C.; Xiao, H.; Zhang, J.; Huang, Y.; Chen, T. Stretchable Supramolecular Hydrogels with Triple Shape Memory Effect. Chem. Sci. 2016, 7, 6715–6720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 4th ed.; Butterworth-Heinemann/Elsevier: Oxford, UK, 2014; ISBN 978-0-12-397776-2. [Google Scholar]
- Shoaib, T.; Espinosa-Marzal, R.M. Advances in Understanding Hydrogel Lubrication. Colloids Interfaces 2020, 4, 54. [Google Scholar] [CrossRef]
- Gombert, Y.; Simič, R.; Roncoroni, F.; Dübner, M.; Geue, T.; Spencer, N.D. Structuring Hydrogel Surfaces for Tribology. Adv. Mater. Interfaces 2019, 6, 1901320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, J.; Jin, H.; Wang, S.; Song, W. Bioinspired Supramolecular Lubricating Hydrogel Induced by Shear Force. J. Am. Chem. Soc. 2018, 140, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.E.; Parkes, M.; Puetzer, J.L.; Chapman, R.; Stevens, M.M.; Cann, P.; Jeffers, J.R.T. A Low Friction, Biphasic and Boundary Lubricating Hydrogel for Cartilage Replacement. Acta Biomater. 2018, 65, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-Inspired, Lipid-Based Boundary-Lubricated Hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Van Lommel, R.; De Borggraeve, W.M.; De Proft, F.; Alonso, M. Computational Tools to Rationalize and Predict the Self-Assembly Behavior of Supramolecular Gels. Gels 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Du, B.; Stadler, F.J. A Novel Approach to Analyze the Rheological Properties of Hydrogels with Network Structure Simulation. J. Polym. Res. 2018, 25, 4. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.d. Structure–Property Relations in Linear Viscoelasticity of Supramolecular Hydrogels. RSC Adv. 2021, 11, 16860–16880. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels 2021, 7, 158. https://doi.org/10.3390/gels7040158
Denzer BR, Kulchar RJ, Huang RB, Patterson J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels. 2021; 7(4):158. https://doi.org/10.3390/gels7040158
Chicago/Turabian StyleDenzer, Bridget R., Rachel J. Kulchar, Richard B. Huang, and Jennifer Patterson. 2021. "Advanced Methods for the Characterization of Supramolecular Hydrogels" Gels 7, no. 4: 158. https://doi.org/10.3390/gels7040158
APA StyleDenzer, B. R., Kulchar, R. J., Huang, R. B., & Patterson, J. (2021). Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels, 7(4), 158. https://doi.org/10.3390/gels7040158





