Preparation of Agarose Fluorescent Hydrogel Inserted by POSS and Its Application for the Identification and Adsorption of Fe3+
Abstract
:1. Introduction
2. Results and Discussion
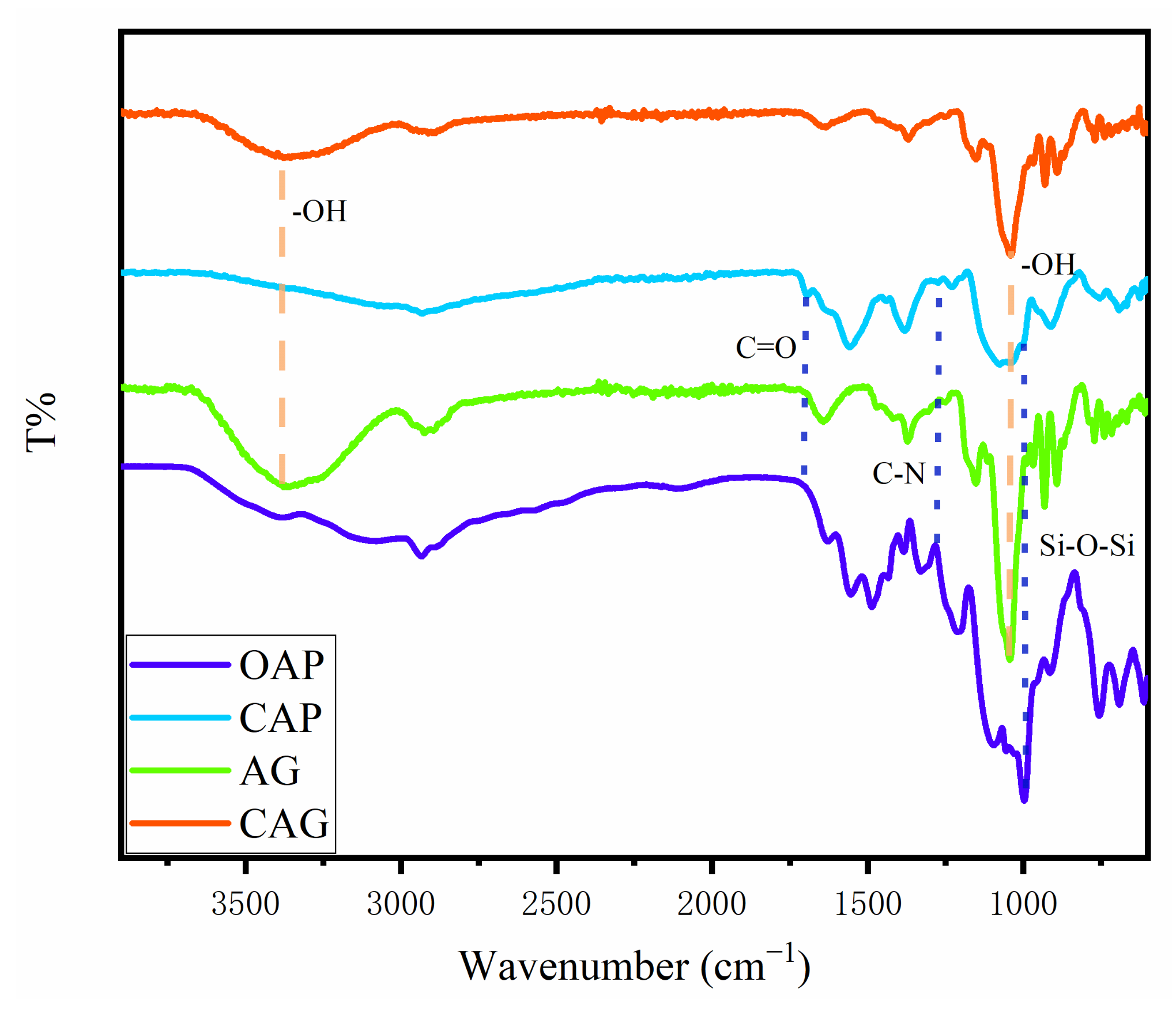
2.1. Structural Analysis
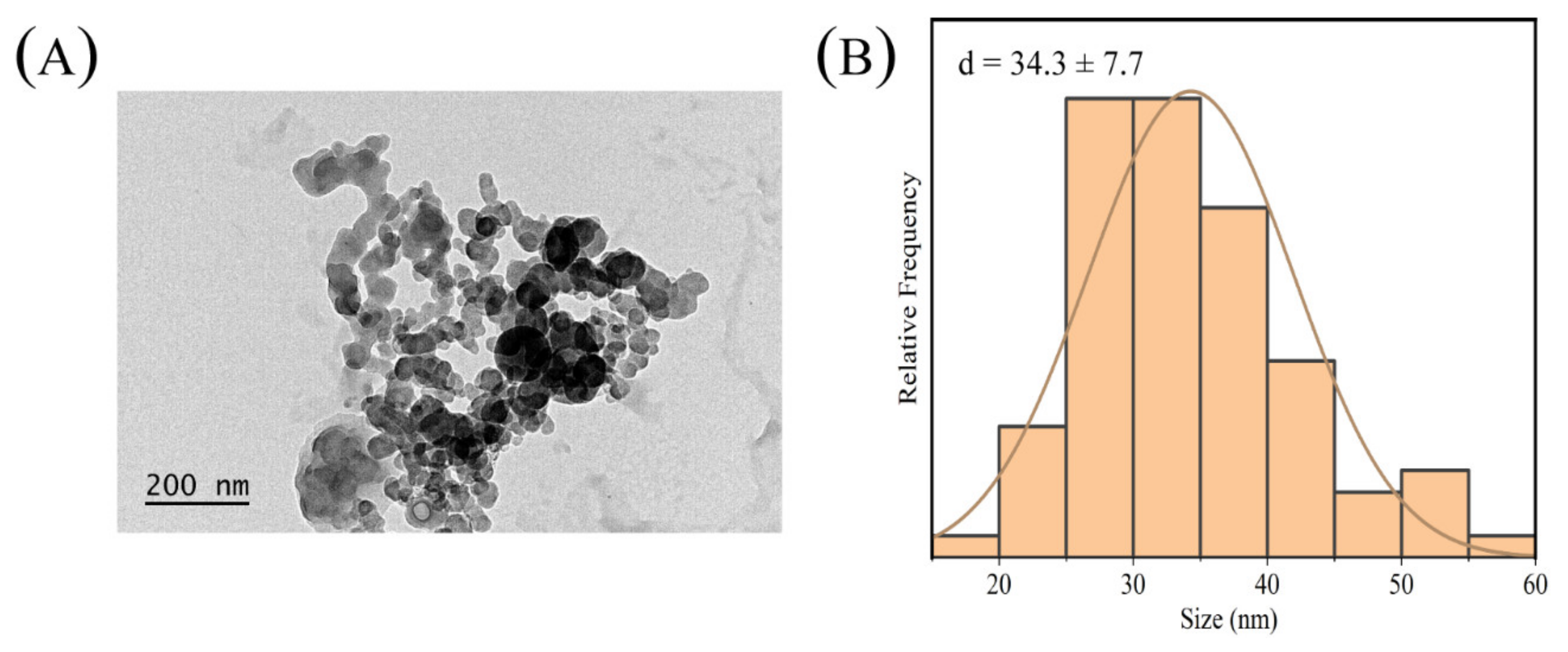
2.2. Morphology Analysis
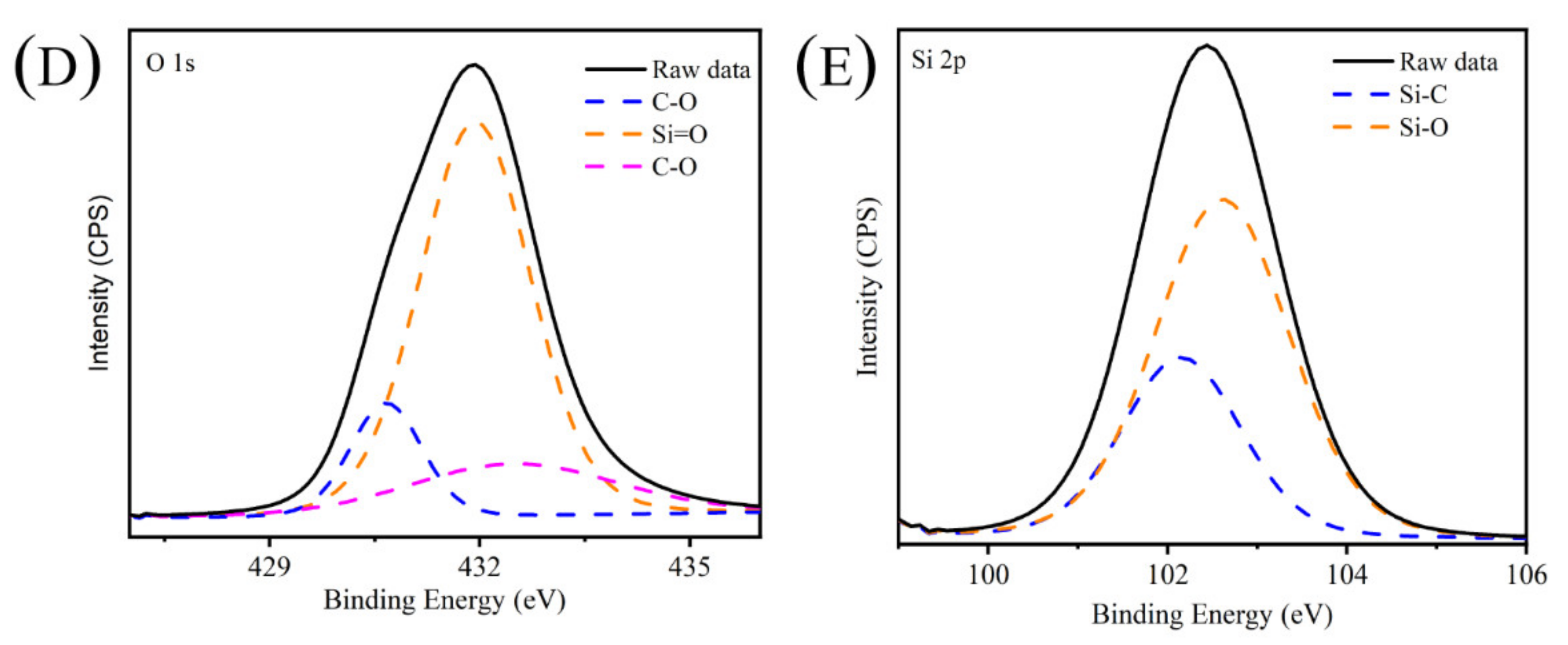
2.3. X-ray Photoelectron Spectrometry Analysis of CAP
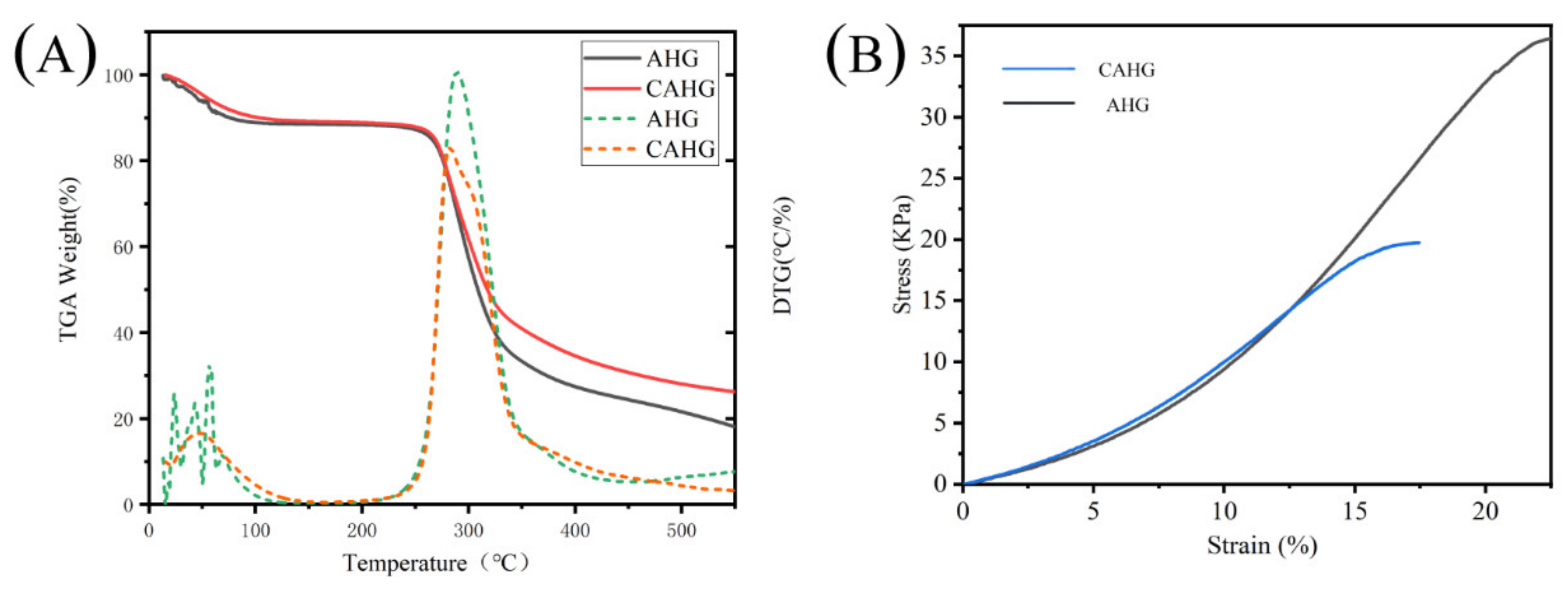
2.4. Thermal Stability and Mechanical Properties of Agarose Hydrogel
2.5. AIE Effect Verification of CAP
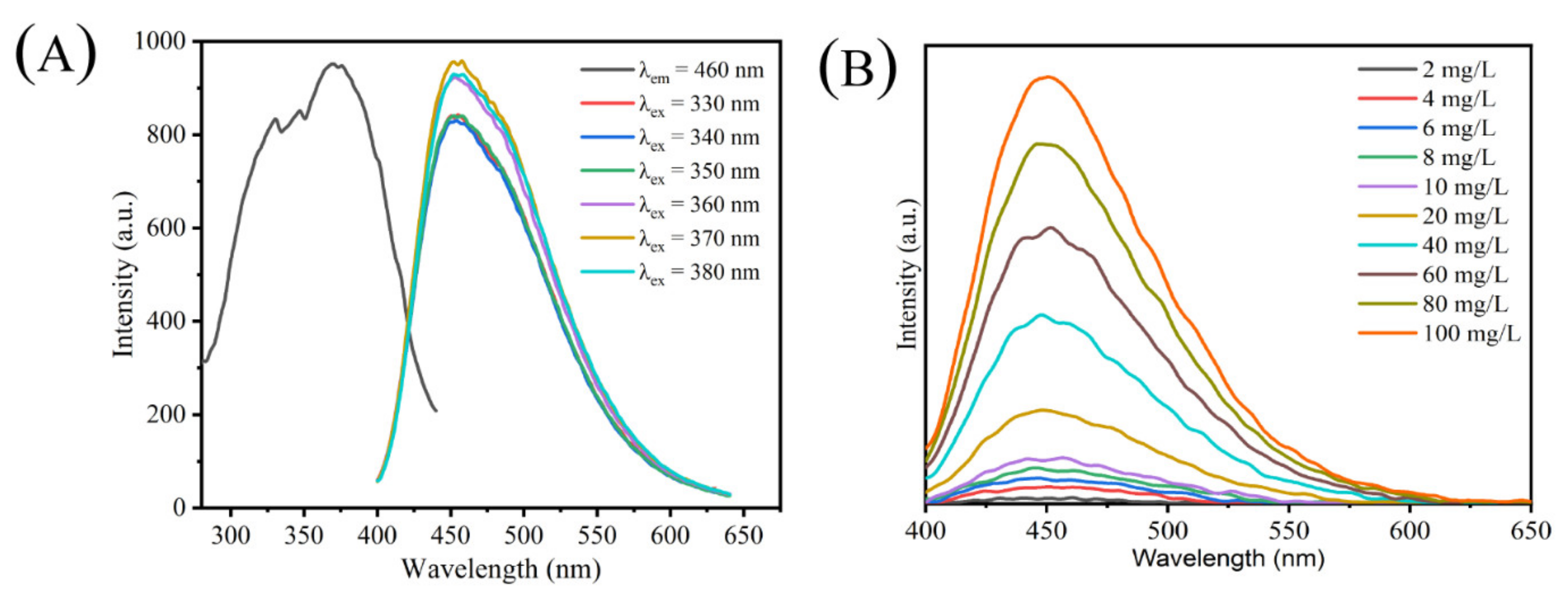
2.6. Photophysical Properties of CAP
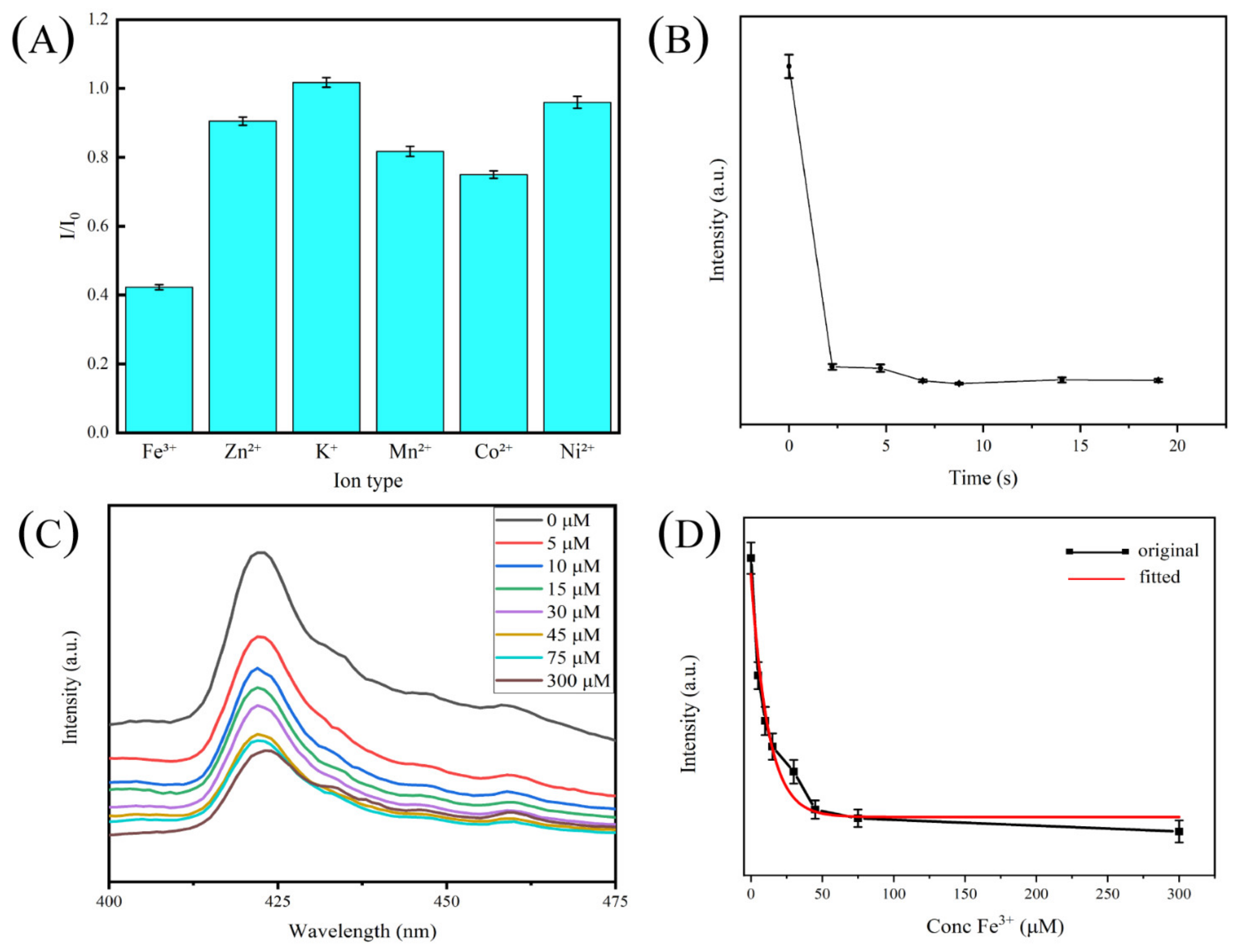
2.7. Ion Detection
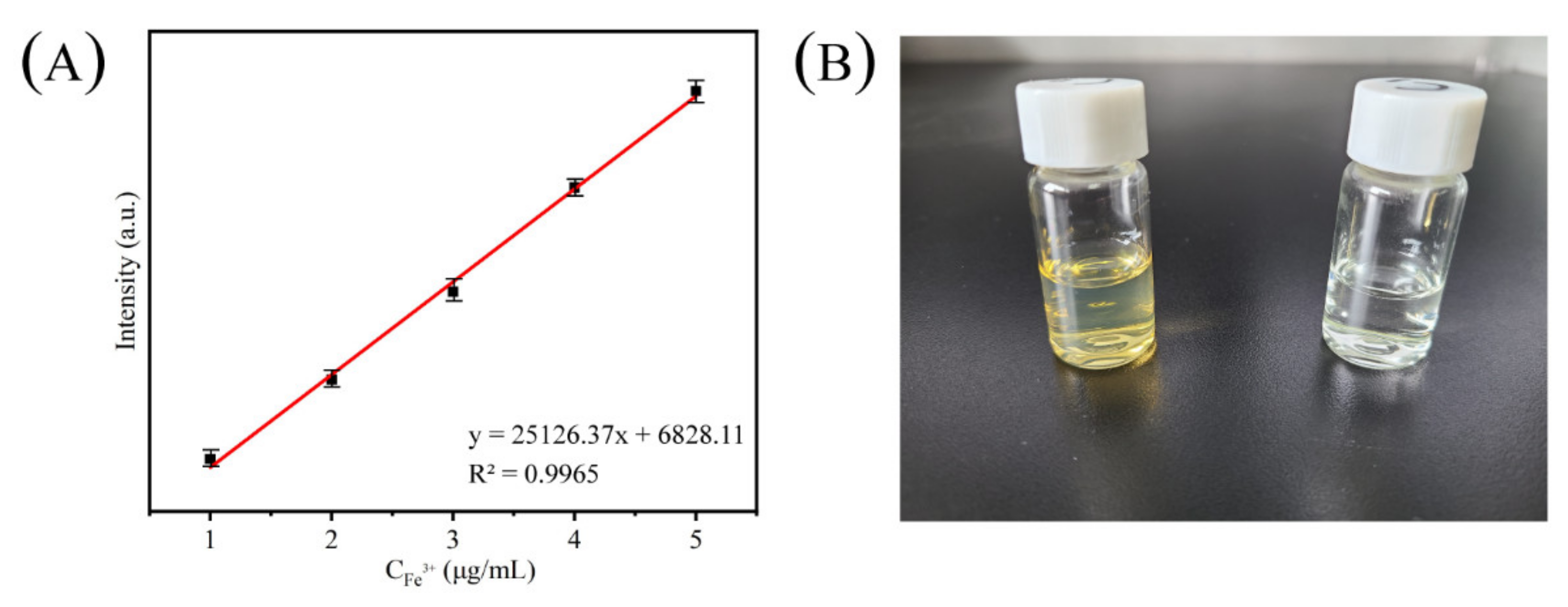
2.7.1. Ion Selectivity and Fe3+ Adsorption
2.7.2. Ion Adsorption
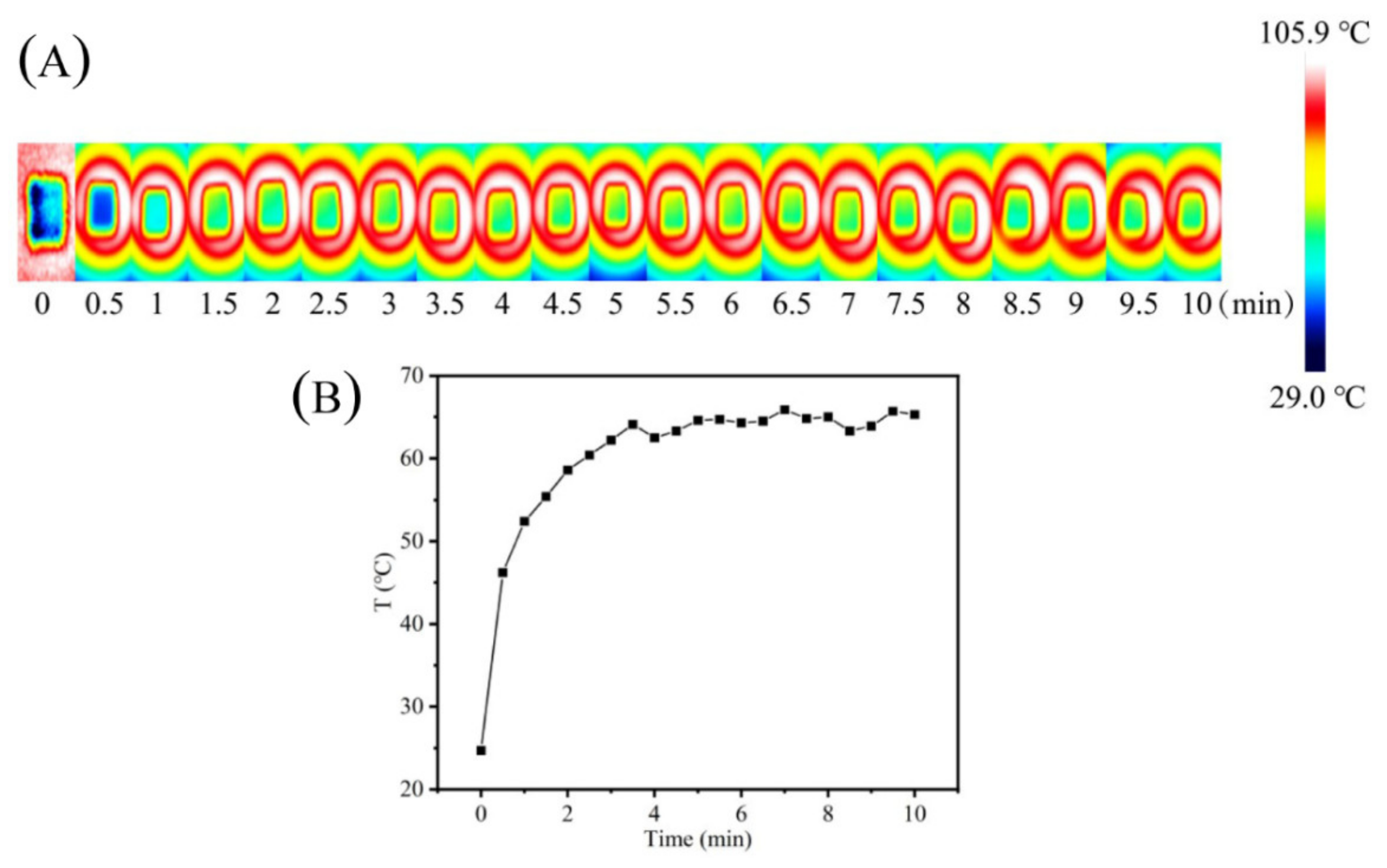
2.7.3. Evaluation of Photothermal Properties of CAHG-Fe3+
3. Conclusions
4. Materials and Methods
4.1. Preparation of POSS
4.2. Preparation of Agarose Hydrogel
4.3. Characterization
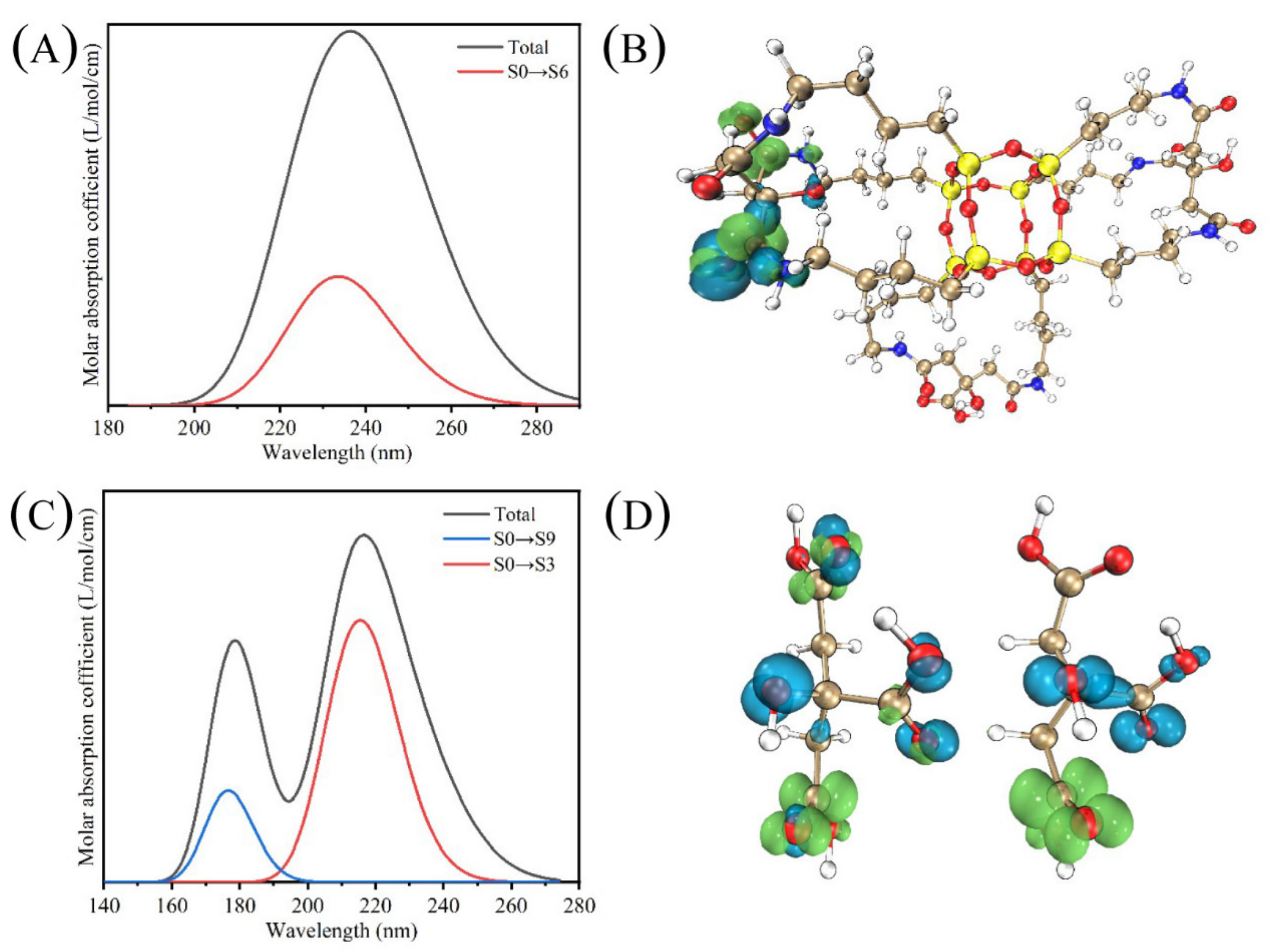
4.4. Theoretical Calculation of CAP
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurvet, I.; Juganson, K.; Vija, H.; Sihtmae, M.; Blinova, I.; Syvertsen-Wiig, G.; Kahru, A. Toxicity of nine (doped) rare earth metal oxides and respective individual metals to aquatic microorganisms Vibrio fischeri and Tetrahymena thermophila. Materials 2017, 10, 754. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, S.; Zheng, X.; Li, H.; Chen, Q.; Qin, K.; Ding, Y.; Wei, Y. Carbon dots derived from Fusobacterium nucleatum for intracellular determination of Fe3+ and bioimaging both in vitro and in vivo. Anal. Methods 2021, 13, 1121–1131. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, E.M.; Byun, H.; Chang, H.K.; Jeong, K.; Aman, Z.M.; Choi, Y.S.; Park, J.; Shin, H. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials 2018, 165, 105–120. [Google Scholar] [CrossRef]
- Ando, M.; Tsuchiya, M.; Itai, S.; Murayama, T.; Kurashina, Y.; Heo, Y.J.; Onoe, H. Janus hydrogel microbeads for glucose sensing with pH calibration. Sensors 2021, 21, 4829. [Google Scholar] [CrossRef]
- Nawaz, H.; Zhang, X.; Chen, S.; You, T.; Xu, F. Recent studies on cellulose-based fluorescent smart materials and their applications: A comprehensive review. Carbohydr. Polym. 2021, 267, 118135. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liu, T.; Lv, F.; Qian, Z. Real-time fluorescence tracking of protoporphyrin incorporated thermosensitive hydrogel and its drug release in vivo. ACS Appl. Mater. Interfaces 2016, 8, 5104–5113. [Google Scholar] [CrossRef]
- Zhang, Y.; Rossi, F.; Papa, S.; Violatto, M.B.; Bigini, P.; Sorbona, M.; Redaelli, F.; Veglianese, P.; Hilborn, J.; Ossipov, D.A. Non-invasive in vitro and in vivo monitoring of degradation of fluorescently labeled hyaluronan hydrogels for tissue engineering applications. Acta Biomater. 2016, 30, 188–198. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, W.; Yang, Z.; Yang, Y.; Li, Z.; Zhang, S.; Zhang, B.; Dong, F.; Lv, J.; Han, B.; et al. Functional copolymers married with lanthanide (III) ions: A win-win pathway to fabricate rare earth fluorescent materials with multiple applications. ACS Appl. Mater. Interfaces 2021, 13, 5539–5550. [Google Scholar] [CrossRef]
- Fan, D.; Fei, X.; Tian, J.; Zhi, H.; Xu, L.; Wang, X.; Wang, Y. Synthesis and investigation of a novel luminous hydrogel. Polym. Chem. 2016, 7, 3766–3772. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.X.; Sun, L.; Yang, J.H.; Zrínyi, M.; Chen, Y.M. Dual-physical cross-linked tough and photoluminescent hydrogels with good biocompatibility and antibacterial activity. Macromol. Rapid Commun. 2017, 38, 1600788. [Google Scholar] [CrossRef]
- Cui, F.; Sun, J.; Ji, J.; Yang, X.; Wei, K.; Xu, H.; Gu, Q.; Zhang, Y.; Sun, X. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 2021, 406, 124330. [Google Scholar] [CrossRef]
- Huang, H.; Ge, H.; Ren, Z.; Huang, Z.; Xu, M.; Wang, X. Controllable synthesis of biocompatible fluorescent carbon dots from cellulose hydrogel for the specific detection of Hg2+. Front. Bioeng. Biotechnol. 2021, 9, 3. [Google Scholar] [CrossRef]
- Sun, L.; Mo, Z.; Li, Q.; Zheng, D.; Qiu, X.; Pan, X. Facile synthesis and performance of pH/temperature dual-response hydrogel containing lignin-based carbon dots. Int. J. Biol. Macromol. 2021, 175, 516–525. [Google Scholar] [CrossRef]
- Whitaker, D.J.; Huang, Z.; Longbottom, B.W.; Sala, R.L.; Wu, G.; Scherman, O.A. Supramolecular hydrogels prepared from fluorescent alkyl pyridinium acrylamide monomers and CB. Polym. Chem. 2021, 12, 519–525. [Google Scholar] [CrossRef]
- Zou, L.; Ding, R.; Li, X.; Miao, H.; Xu, J.; Pan, G. Typical fluorescent sensors exploiting molecularly imprinted hydrogels for environmentally and medicinally important analytes detection. Gels 2021, 7, 67. [Google Scholar] [CrossRef]
- Vazquez-Victorio, G.; Rodriguez-Hernandez, A.; Cano-Jorge, M.; Monroy-Romero, A.X.; Macias-Silva, M.; Hautefeuille, M. Fabrication of adhesive substrate for incorporating hydrogels to investigate the influence of stiffness on cancer CELL Behavior. Methods Mol. Biol. 2021, 2174, 277–297. [Google Scholar]
- Xie, S.; Chen, Y.; Guo, Z.; Luo, Y.; Tan, H.; Xu, L.; Xu, J.; Zheng, J. Agar/carbon dot crosslinked polyacrylamide double-network hydrogels with robustness, self-healing, and stimulus-response fluorescence for smart anti-counterfeiting. Mater. Chem. Front. 2021, 5, 5442–5451. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, Z.; Zhao, H.; Liang, R.; Wang, C.; Wang, D.; Li, J. Preparation of PVA fluorescent gel and luminescence of europium sensitized by terbium (III). Polymers 2020, 12, 893. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing cellulose hydrogels from non-woody biomass. Carbohydr. Polym. 2021, 264, 118036. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Yin, J.; Wang, R.; Shi, M.; Liu, P.; Qin, Z.; Xing, R.; Jiao, T. Self-assembled natural biomacromolecular fluorescent hydrogels with tunable red edge effects. Colloids Surf. Physicochem. Eng. Asp. 2021, 612, 125993. [Google Scholar] [CrossRef]
- An, J.; Chen, R.; Chen, M.; Hu, Y.; Lyu, Y.; Liu, Y. An ultrasensitive turn-on ratiometric fluorescent probes for detection of Ag+ based on carbon dots/SiO2 and gold nanoclusters. Sens. Actuators B Chem. 2021, 329, 129097. [Google Scholar] [CrossRef]
- Adivi, F.G.; Hashemi, P. Removal of histamine from biological samples by functionalized Fe3O4@Agarose@Silica nanoparticles and its fast determination by ion mobility spectrometry. Colloids Surf. B Biointerfaces 2021, 203, 111171. [Google Scholar]
- Huang, J.; Wang, Y.L.; Yu, X.D.; Zhou, Y.N.; Chu, L.Q. Enhanced fluorescence of carboxymethyl chitosan via metal ion complexation in both solution and hydrogel states. Int. J. Biol. Macromol. 2020, 152, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Su, Z.; Zhang, K.; Ren, J.; Sun, R.; Wang, X. All-biomass fluorescent hydrogels based on biomass carbon dots and alginate/nanocellulose for biosensing. ACS Appl. Biomater. 2018, 1, 1398–1407. [Google Scholar] [CrossRef]
- Cheng, R.; Xiang, Y.; Guo, R.; Li, L.; Zou, G.; Fu, C.; Hou, H.; Ji, X. Structure and interface modification of carbon dots for electrochemical energy application. Small 2021, 17, 2102091. [Google Scholar] [CrossRef]
- Humaera, N.A.; Fahri, A.N.; Armynah, B.; Tahir, D. Natural source of carbon dots from part of a plant and its applications: A review. Luminescence 2021, 36, 1354–1364. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Jiang, X.-M.; Gong, G.-F.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Pillararene-based AIEgens: Research progress and appealing applications. Chem. Commun. 2021, 57, 284–301. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Qin, A.; Tang, B.Z. AIE polymers in sensing, imaging and theranostic applications. Mater. Chem. Front. 2021, 5, 4073–4088. [Google Scholar] [CrossRef]
- Wang, F.; Mu, Q.; Peng, D.; Zhang, F.; Li, J.; Zhao, N.; Yu, Y.; Li, B. Synthesis of functionalized polyhedral oligomeric silsesquioxane and its application in silicone. Chem. Ind. Eng. Prog. 2017, 36, 1324–1332. [Google Scholar]
- Wang, M.; Chi, H.; Joshy, K.S.; Wang, F. Progress in the synthesis of bifunctionalized polyhedral oligomeric silsesquioxane. Polymers 2019, 11, 2098. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Xing, W.; Yang, Y.; Da, H.; Fei, Y.; Hong, S.; Wu, D. Facile preparation of pH-responsive AIE-active POSS dendrimers for the detection of trivalent metal cations and acid gases. Polym. Chem. 2016, 7, 6432–6436. [Google Scholar] [CrossRef]
- Liu, M.; Lu, X.; Hu, L.; Liu, C.; Li, Y.; Zhang, W.; Ding, C.; Gu, J.; Cao, F. Hydrothermal synthesis of a highly photoluminescent molecule from citric acid and cysteamine for the efficient detection of Au3+ in aqueous solution. Opt. Mater. 2019, 96, 109359. [Google Scholar] [CrossRef]
- Wen, Q.-L.; Pu, Z.-F.; Yang, Y.-J.; Wang, J.; Wu, B.-C.; Hu, Y.-L.; Liu, P.; Ling, J.; Cao, Q. Hyaluronic acid as a material for the synthesis of fluorescent carbon dots and its application for selective detection of Fe3+ ion and folic acid. Microchem. J. 2020, 159, 105364. [Google Scholar] [CrossRef]
- Sun, Q.K.; Qiu, X.; Lu, Y.S.; Xu, X.; Wang, H.H.; Xue, S.F.; Yang, W.J. AIE-active 9,10-bis(alkylarylvinyl)anthracences with pendent diethoxylphosphorylmethyl groups as solution-processable efficient EL luminophores. J. Mater. Chem. C 2017, 5, 9157–9164. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Dang, S.; Wang, Z.; Liu, J.; Fu, J.; Shi, L. Lanthanide complex-functionalized polyhedral oligomeric silsesquioxane with multicolor emission covered from 450 nm to 1700 nm. New J. Chem. 2016, 40, 209–216. [Google Scholar] [CrossRef]
- Andarini, M.; Mokhtarom, M.; Yamin, B.M.; Amin, M.C.I.M.; Lazim, A.M. Preparation of Ferum Oxide Nanoparticles (FeNPs) from ferocenium salt using polyacrylamidc (P(Am) as template). Sains Malays. 2019, 48, 1913–1918. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Wang, H.; Luo, J.; Song, G.; Tang, G. Temperature buffering capacity of silk hydrogel: A useful packaging material. Mater. Lett. 2018, 211, 110–113. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Li, Y.; Li, Z.; Song, T.; Liu, X.; Hao, J. Thermal stability and mechanical properties of hybrid materials based on nitrocellulose grafted by aminopropylisobutyl polyhedral oligomeric silsesquioxane. Polimery 2017, 62, 576–587. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Ye, J.; Zhao, P.; Xiao, M. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Bodzin, D.J.; Haddad, T.S.; Boatz, J.A.; Mabry, J.M.; Mitchell, C.; Bowers, M. Structural investigation of encapsulated fluoride in polyhedral oligomeric silsesquioxane cages using ion mobility mass spectrometry and molecular mechanics. Chem. Mater. 2008, 20, 4299–4309. [Google Scholar] [CrossRef]
- Parambadath, S.; Mathew, A.; Park, S.; Ha, C. Pentane-1,2-dicarboxylic acid functionalized spherical MCM-41: A simple and highly selective heterogeneous ligand for the adsorption of Fe3+ from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1918–1927. [Google Scholar] [CrossRef]
- Khalfa, L.; Cervera, M.; Souissi-najjar, S.; Bagane, M. Removal of Fe (III) from synthetic wastewater into raw and modified clay: Experiments and models fitting. Sep. Sci. Technol. 2017, 56, 708–718. [Google Scholar] [CrossRef]
- Al-Anber, M. Removal of high-level Fe3+ from aqueous solution using natural inorganic materials: Bentonite (NB) and quartz (NQ). Desalination 2010, 250, 885–891. [Google Scholar] [CrossRef]
- Jusoh, A.B.; Cheng, W.H.; Low, W.M.; Megat, M. Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 2005, 182, 347–353. [Google Scholar] [CrossRef]
- Shavandi, M.A.; Haddadian, Z.; Ismail, M.; Abdullah, N.; Abidin, Z. Removal of Fe (III), Mn (II) and Zn (II) from palm oil mill effluent (POME) by natural zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 750–759. [Google Scholar] [CrossRef]
- Duan, Y.; Freyburger, A.; Kunz, W.; Zollfrank, C. Lignin/chitin films and their adsorption characteristics for heavy metal ions. ACS Sustain. Chem. Eng. 2018, 6, 65–73. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; Oliveira, M.; Rambo, C.; Franscescon, F.; Freitas, L.; Muneron, M.; Fiori, M.; Oliveira, J.; Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Pu, Y.-J.; Yuan, H.; Yang, M.; He, B.; Gu, Z.-W. Synthesis of peptide dendrimers with polyhedral oligomeric silsesquioxane cores via click chemistry. Chin. Chem. Lett. 2013, 24, 917–920. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Physics, A.S. Lanthanides in solar energy conversion. In Handbook on the Physics & Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2014; Volume 44, pp. 169–281. [Google Scholar]
- Xu, Q.; Li, Z.; Li, H.R. Water-soluble luminescent hybrid composites consisting of oligosilsesquioxanes and lanthanide complexes and their sensing ability for Cu2+. Chem. A Eur. J. 2016, 22, 3037–3043. [Google Scholar] [CrossRef]
- Huang, L.B.; Cheng, L.H.; Yu, H.Q.; Zhang, J.S.; Zhou, L.; Sun, J.S.; Zhong, H.Y.; Li, X.P.; Tian, Y.; Zheng, Y.F.; et al. Electrospinning preparation and optical transition properties of Eu (DBM) (3) Phen/PS fluorescent composite fibers. Opt. Commun. 2012, 285, 1476–1480. [Google Scholar] [CrossRef]
- Yue, Y.Z.; Liang, Y.; Wang, H.; Feng, L.L.; Feng, S.Y.; Lu, H.F. Photophysical properties of sol-gel derived luminescent silicone hybrids synthesized via facile amino-ene reaction. Photochem. Photobiol. 2013, 89, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Caiut, J.; Ribeiro, S.J.L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H. Synthesis and luminescence properties of water dispersible Eu3+ doped boehmite nanoparticles. Nanotechnology 2007, 18, 455605. [Google Scholar] [CrossRef]



| Sample | First Stage | Second Stage | Weight | ||
|---|---|---|---|---|---|
| T (°C) | Weight Loss (%) | T (°C) | Weight Loss (%) | Remaining after 550 °C (%) | |
| AHG | 125.1 | 12.4 | 433.6 | 63.3 | 18.1 |
| CAHG | 142.6 | 10.8 | 470.5 | 59.3 | 26.2 |
| Transitions | f | E (eV) | Contribution |
|---|---|---|---|
| S0 → S6 | 0.0092 | 5.3082 | 33.6280% |
| S0 → S2 | 0.0058 | 5.0560 | 17.3790% |
| S0 → S1 | 0.0056 | 4.9711 | 13.1280% |
| S0 → S8 | 0.0035 | 5.4415 | 10.3130% |
| Ion | Fe3+ |
|---|---|
| Initial concentration (M) | 9.23 × 10−6 |
| Residual concentration (M) | 6.77 × 10−6 |
| Adsorption rate (%) | 27 |
| qt (mg/g) | 26.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Li, M.; Li, Y.; Zhang, Z.; Wang, D.; Wang, C.; Li, J. Preparation of Agarose Fluorescent Hydrogel Inserted by POSS and Its Application for the Identification and Adsorption of Fe3+. Gels 2021, 7, 173. https://doi.org/10.3390/gels7040173
Fu Z, Li M, Li Y, Zhang Z, Wang D, Wang C, Li J. Preparation of Agarose Fluorescent Hydrogel Inserted by POSS and Its Application for the Identification and Adsorption of Fe3+. Gels. 2021; 7(4):173. https://doi.org/10.3390/gels7040173
Chicago/Turabian StyleFu, Zhengquan, Ming Li, Yuanhang Li, Zhiyuan Zhang, Di Wang, Chengyu Wang, and Jian Li. 2021. "Preparation of Agarose Fluorescent Hydrogel Inserted by POSS and Its Application for the Identification and Adsorption of Fe3+" Gels 7, no. 4: 173. https://doi.org/10.3390/gels7040173
APA StyleFu, Z., Li, M., Li, Y., Zhang, Z., Wang, D., Wang, C., & Li, J. (2021). Preparation of Agarose Fluorescent Hydrogel Inserted by POSS and Its Application for the Identification and Adsorption of Fe3+. Gels, 7(4), 173. https://doi.org/10.3390/gels7040173







