Synthesis and Characterization of ZnO(MgO)-CaO-SiO2-P2O5 Bioglass Obtained by Sol-Gel Method in Presence of Surfactant Agent
Abstract
:1. Introduction
- −
- from a medical point of view, inside the body, Na+ is equimolarly replaced by K+, which can lead to various dysfunctions (e.g., heart rhythm disturbance, insulin synthesis) [22]; on the other hand, Zn2+ can lead to improved antibacterial and anti-inflammatory activity, and the presence of Mg2+ can improve angiogenesis and osteogenesis;
- −
- regarding the synthesis route, given that in the composition of the glasses Na2O acts as fondant (reducing the melting temperature of the precursors), in this case this is no longer necessary because the sol-gel synthesis method involves low temperatures [19].
2. Results and Discussions
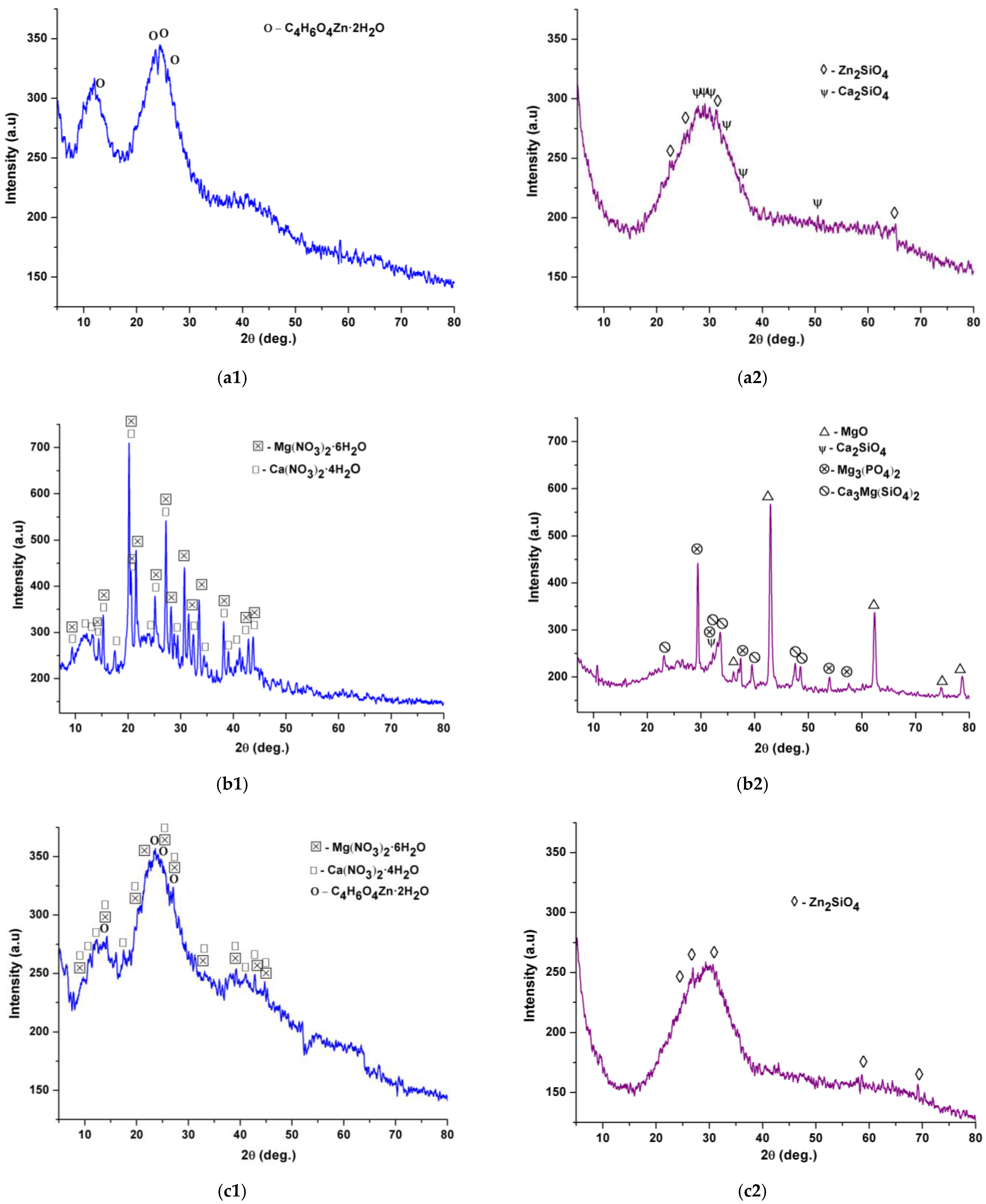
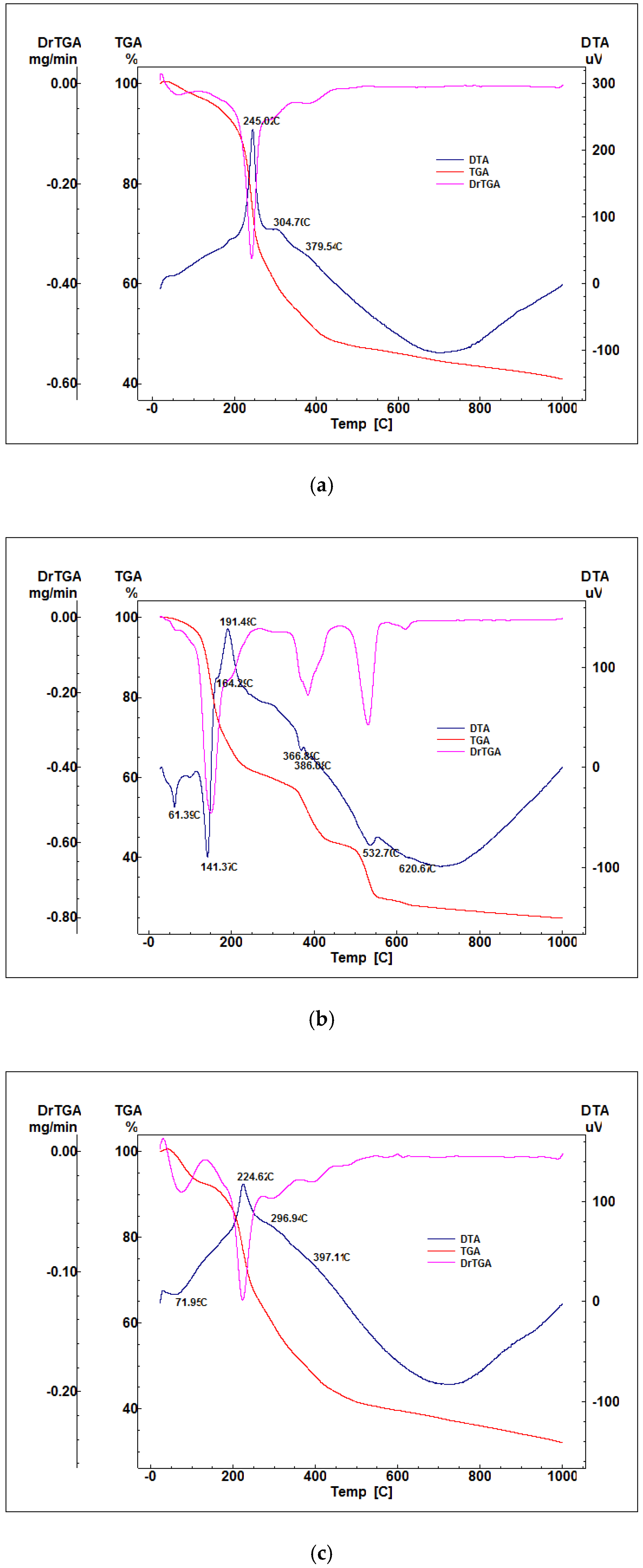
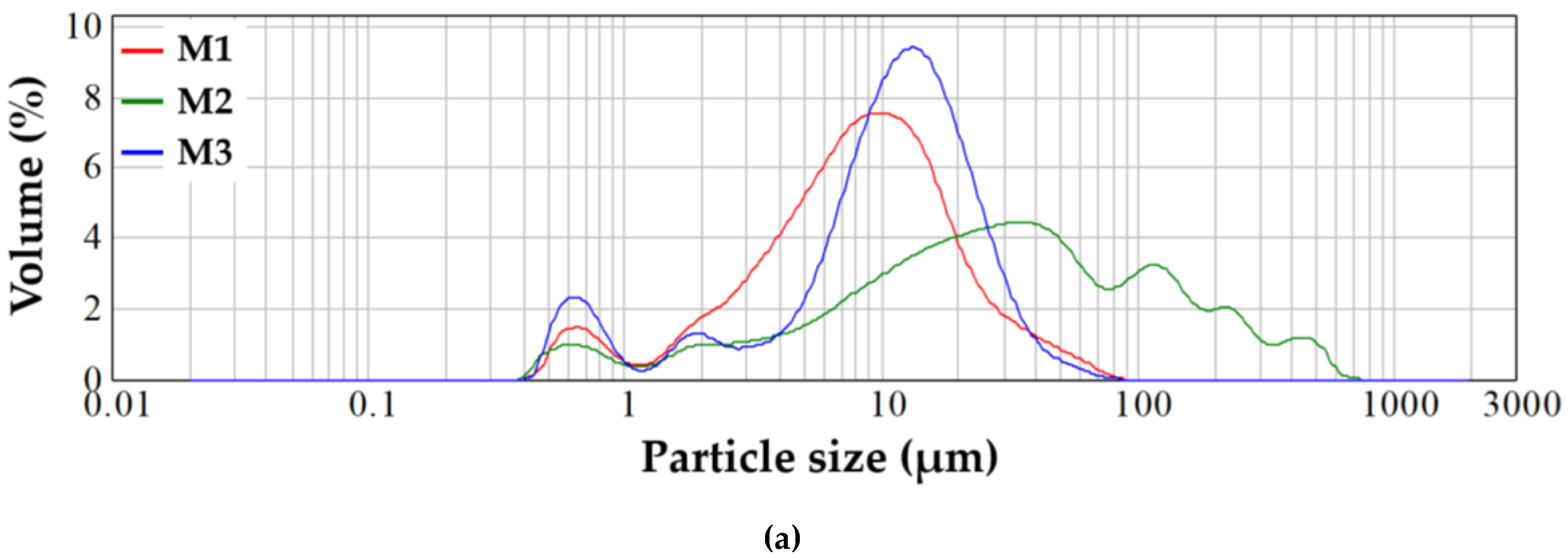
2.1. Characterization of Precursor Powders
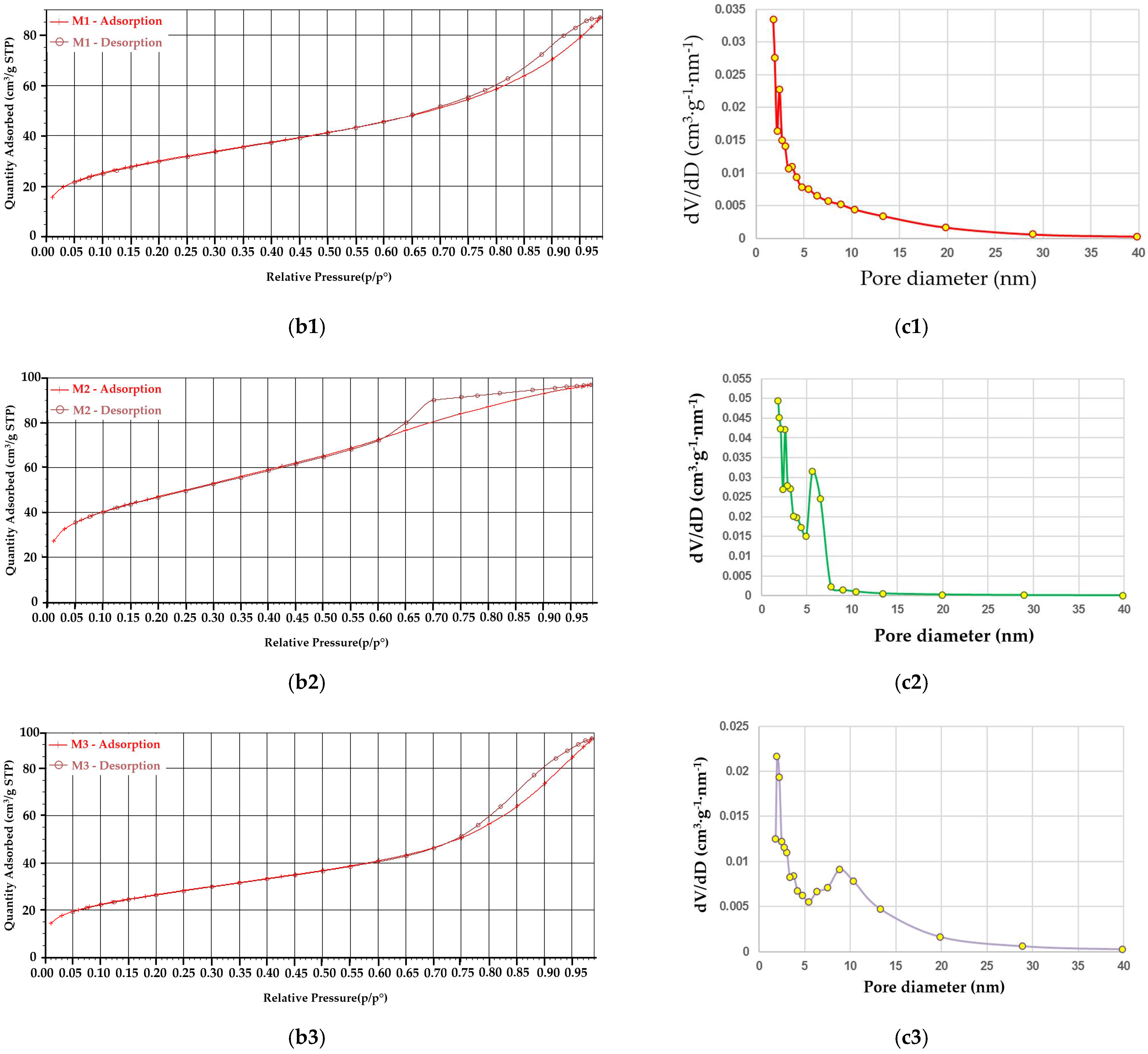
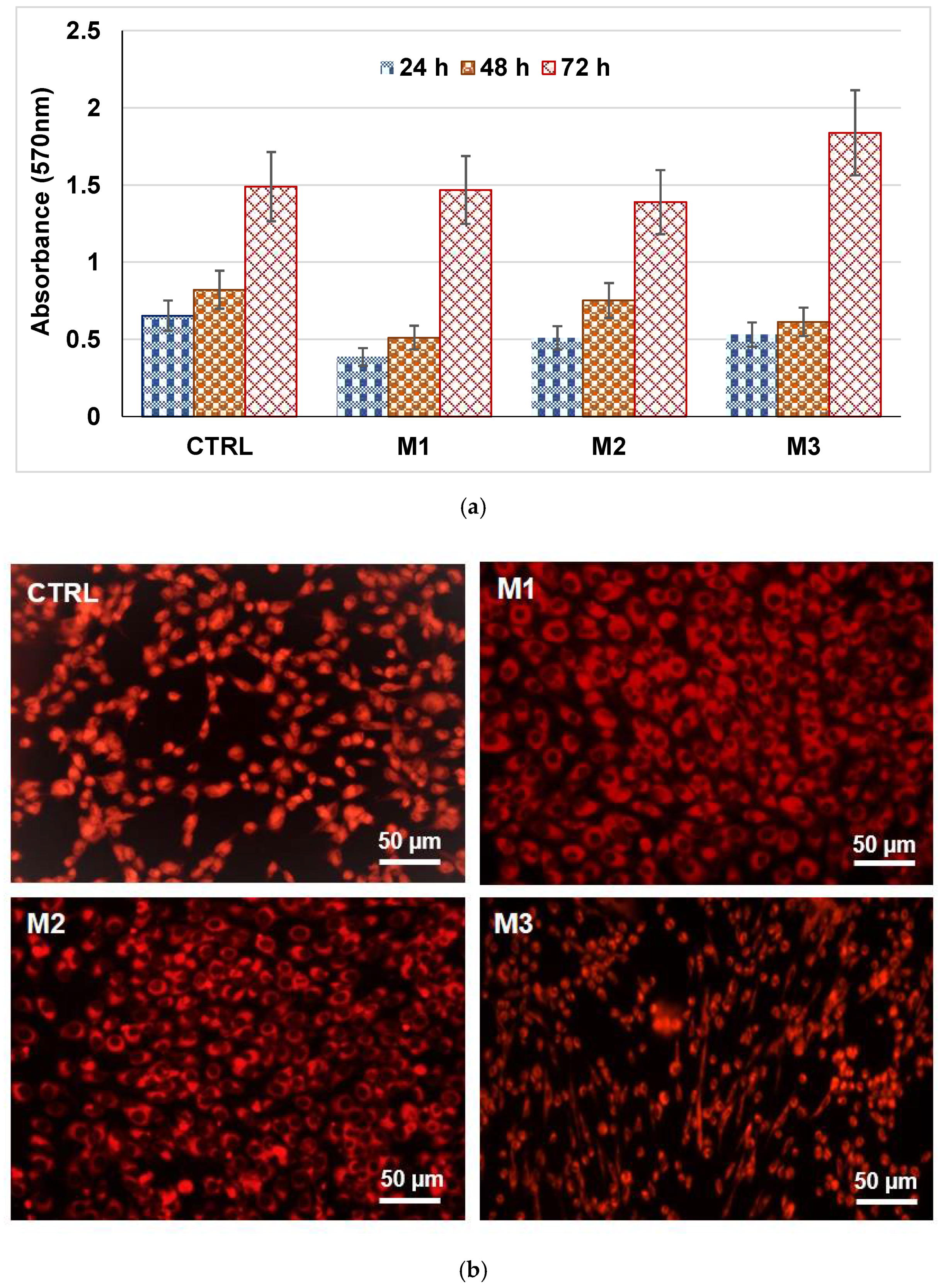
2.2. Characterization of Glass Powders
3. Conclusions
4. Materials and Methods
4.1. Materials
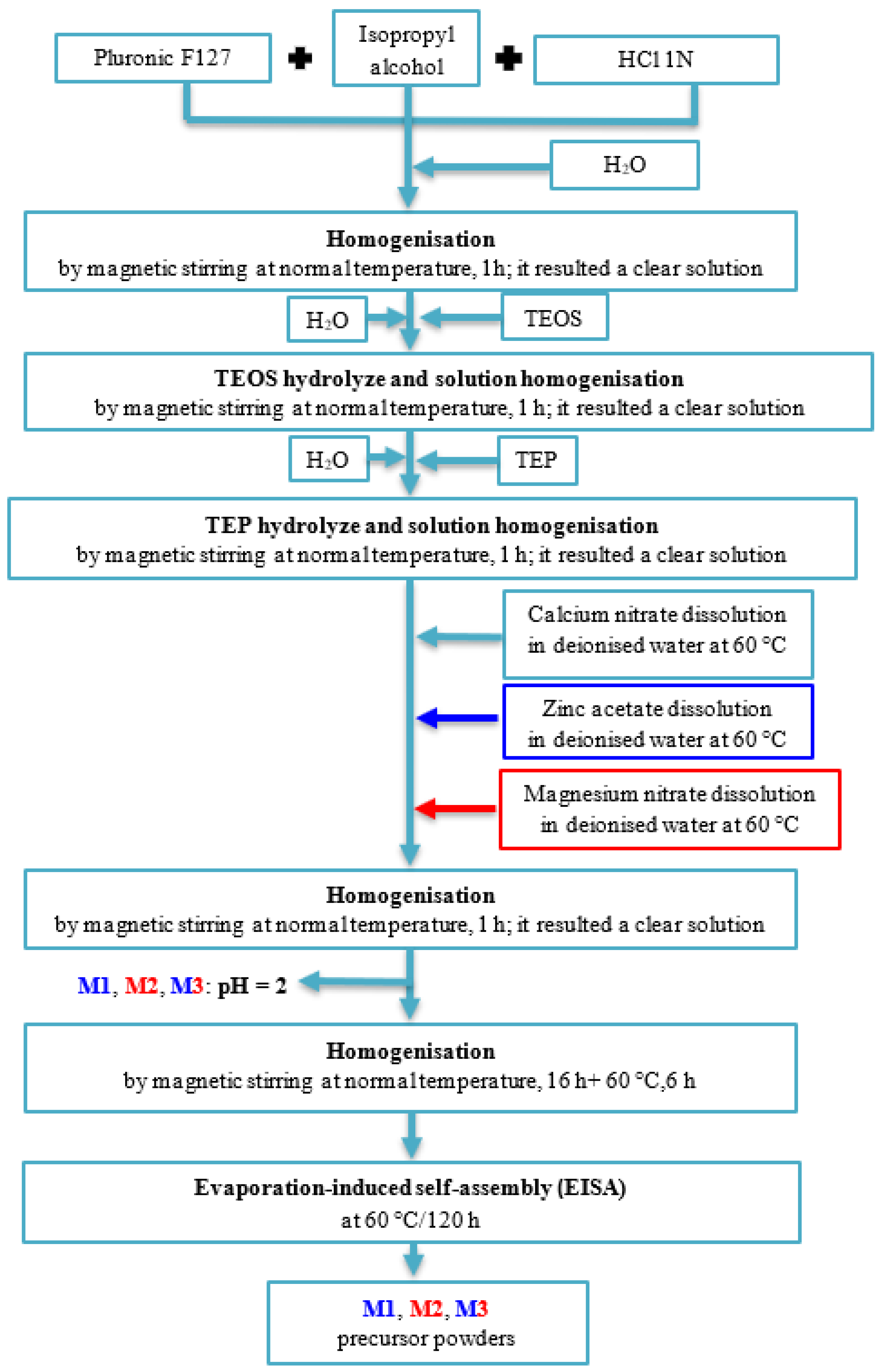
4.2. Synthesis of Vitreous Powders
4.3. Characterization of Powders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harbi, N.A.; Mohammed, H.; Hadeethi, Y.A.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, V.M.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed. Glas. 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Kumar, A.; Murugavel, S.; Aditya, A.; Boccaccini, A.R. Mesoporous 45S5 bioactive glass: Synthesis,: In vitro dissolution and biomineralization behavior. J. Mater. Chem. B 2017, 5, 8786–8798. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A guided walk through the world of mesoporous bioactive glasses (MBGs): Fundamentals, processing, and applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chemie. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Vern, E.; Baino, F. Comparison between bioactive sol-gel and melt-derived glasses/glass-ceramics based on the Multicomponent. Materials 2020, 13, 540. [Google Scholar] [CrossRef] [Green Version]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Wu, C.; Fang, Y.; Yang, J.; Wang, S. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous Mesoporous Mater. 2011, 143, 311–319. [Google Scholar] [CrossRef]
- Wu, C.; Miron, R.; Sculean, A.; Kaskel, S.; Doert, T.; Schulze, R.; Zhang, Y. Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds. Biomaterials 2011, 32, 7068–7078. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation, In vitro bioactivity and drug release property of well-ordered mesoporous 58S bioactive glass. J. Non. Cryst. Solids. 2008, 354, 1338–1341. [Google Scholar] [CrossRef]
- Peitl, O.; Zanotto, E.D.; Hench, L.L. Highly bioactive P2O5-Na2O-CaO-SiO2glass-ceramics. J. Non. Cryst. Solids. 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Peitl, O.; Zanotto, E.D.; Serbena, F.C.; Hench, L.L. Compositional and microstructural design of highly bioactive P2O5-Na2O-CaO-SiO2 glass-ceramics. Acta Biomater. 2012, 8, 321–332. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Vallet-Regi, M.; Heikkilä, J. Use of Bioactive Glasses as Bone Substitutes in Orthopedics and Traumatology, 2nd ed.; Elsevier Ltd.: New York, NY, USA, 2018. [Google Scholar]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non. Cryst. Solids. 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of bioactive glasses for cardiac and pulmonary tissue engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Cerruti, M.; Sahai, N. Silicate biomaterials for orthopaedic and dental implants. Rev. Mineral. Geochem. 2006, 64, 283–313. [Google Scholar] [CrossRef]
- Wu, X.; Walsh, K.; Hoff, B.L.; Unal, G.C. Mineralization of biomaterials for bone tissue engineering. Bioengineering 2020, 7, 132. [Google Scholar] [CrossRef]
- Lu, X.; Kolzow, J.; Chen, R.R.; Du, J. Effect of solution condition on hydroxyapatite formation in evaluating bioactivity of B2O3 containing 45S5 bioactive glasses. Bioact. Mater. 2019, 4, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.N.; Bahir, M.M.; Mene, R.U.; Mahabole, M.P.; Khairnar, R.S. Study of nanobiomaterial hydroxyapatite in simulated body fluid: Formation and growth of apatite. Mater. Sci. Eng. B 2010, 168, 224–230. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Cǎproiu, M.T.; Drǎghici, C.; Chifiriuc, M.C.; Drǎcea, N.O. Synthesis, characterization and antimicrobial activity evaluation of some new derivatives of 6,11-dihydrodibenzob, ethiepin 5,5-dioxide. Rev. Chim. 2009, 60, 137–141. [Google Scholar]
- Limban, C.; Chifiriuc, M.C. Antibacterial activity of new dibenzoxepinone oximes with fluorine and trifluoromethyl group substituents. Int. J. Mol. Sci. 2011, 12, 6432. [Google Scholar] [CrossRef] [Green Version]
- Stoica, A.O.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Grumezescu, A.M.; Popa, M.; Chifiriuc, M.C. Preparation and characterization of undoped and cobalt doped ZnO for antimicrobial use. Int. J. Pharm. 2016, 510, 430–438. [Google Scholar] [CrossRef]


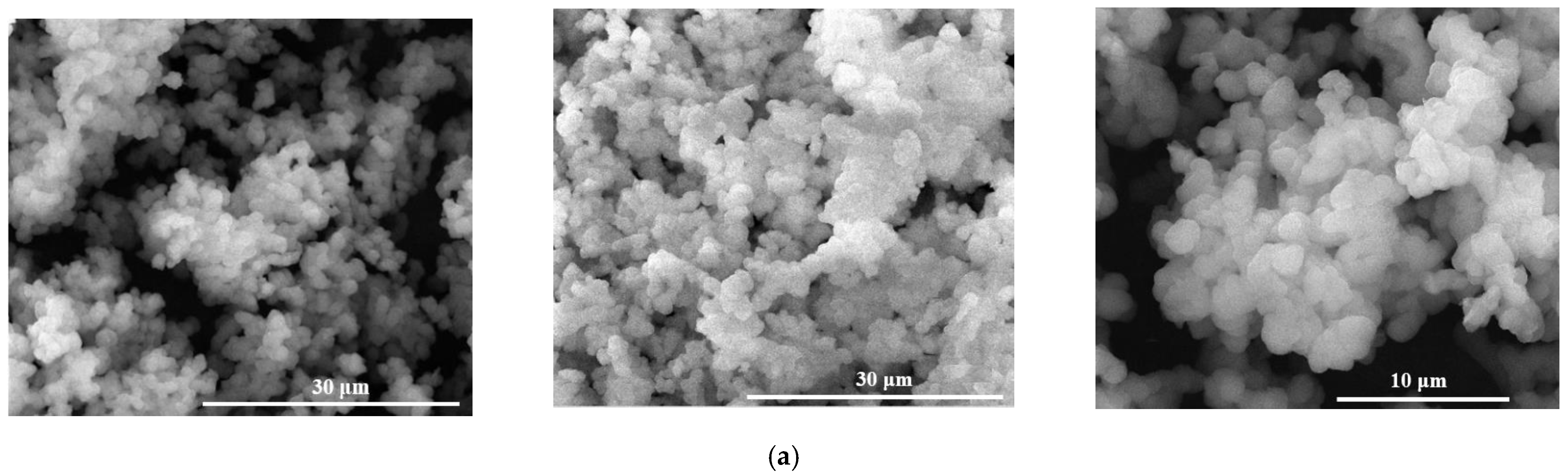


| Powders Code | Oxidic Composition (wt%) | ||||
|---|---|---|---|---|---|
| SiO2 | CaO | P2O5 | ZnO | MgO | |
| M1 | 53 | 20 | 4 | 23 | - |
| M2 | 53 | 20 | 4 | - | 23 |
| M3 | 53 | 20 | 4 | 11.5 | 11.5 |
| Powders Code/ Dispersional Characteristic | M1 | M2 | M3 |
|---|---|---|---|
| SBET (cm2/g) | 106.49 | 164.92 | 93.87 |
| d (µm) | 8.37 | 29.30 | 11.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiţulică, C.-D.; Damian-Buda, A.-I.; Cucuruz, A.; Voicu, G. Synthesis and Characterization of ZnO(MgO)-CaO-SiO2-P2O5 Bioglass Obtained by Sol-Gel Method in Presence of Surfactant Agent. Gels 2021, 7, 187. https://doi.org/10.3390/gels7040187
Ghiţulică C-D, Damian-Buda A-I, Cucuruz A, Voicu G. Synthesis and Characterization of ZnO(MgO)-CaO-SiO2-P2O5 Bioglass Obtained by Sol-Gel Method in Presence of Surfactant Agent. Gels. 2021; 7(4):187. https://doi.org/10.3390/gels7040187
Chicago/Turabian StyleGhiţulică, Cristina-Daniela, Andrada-Ioana Damian-Buda, Andreia Cucuruz, and Georgeta Voicu. 2021. "Synthesis and Characterization of ZnO(MgO)-CaO-SiO2-P2O5 Bioglass Obtained by Sol-Gel Method in Presence of Surfactant Agent" Gels 7, no. 4: 187. https://doi.org/10.3390/gels7040187





