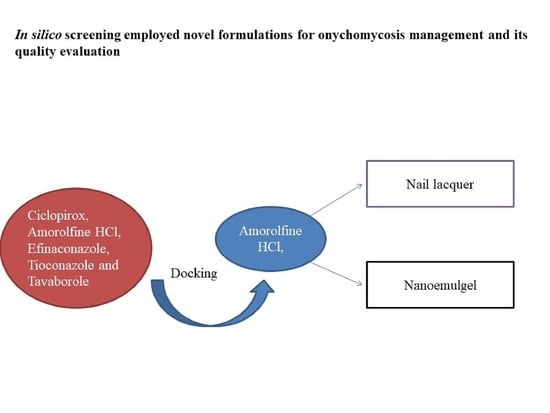
In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Docking Study
2.2. Analytical Method Development
2.3. Development of Nail Lacquer
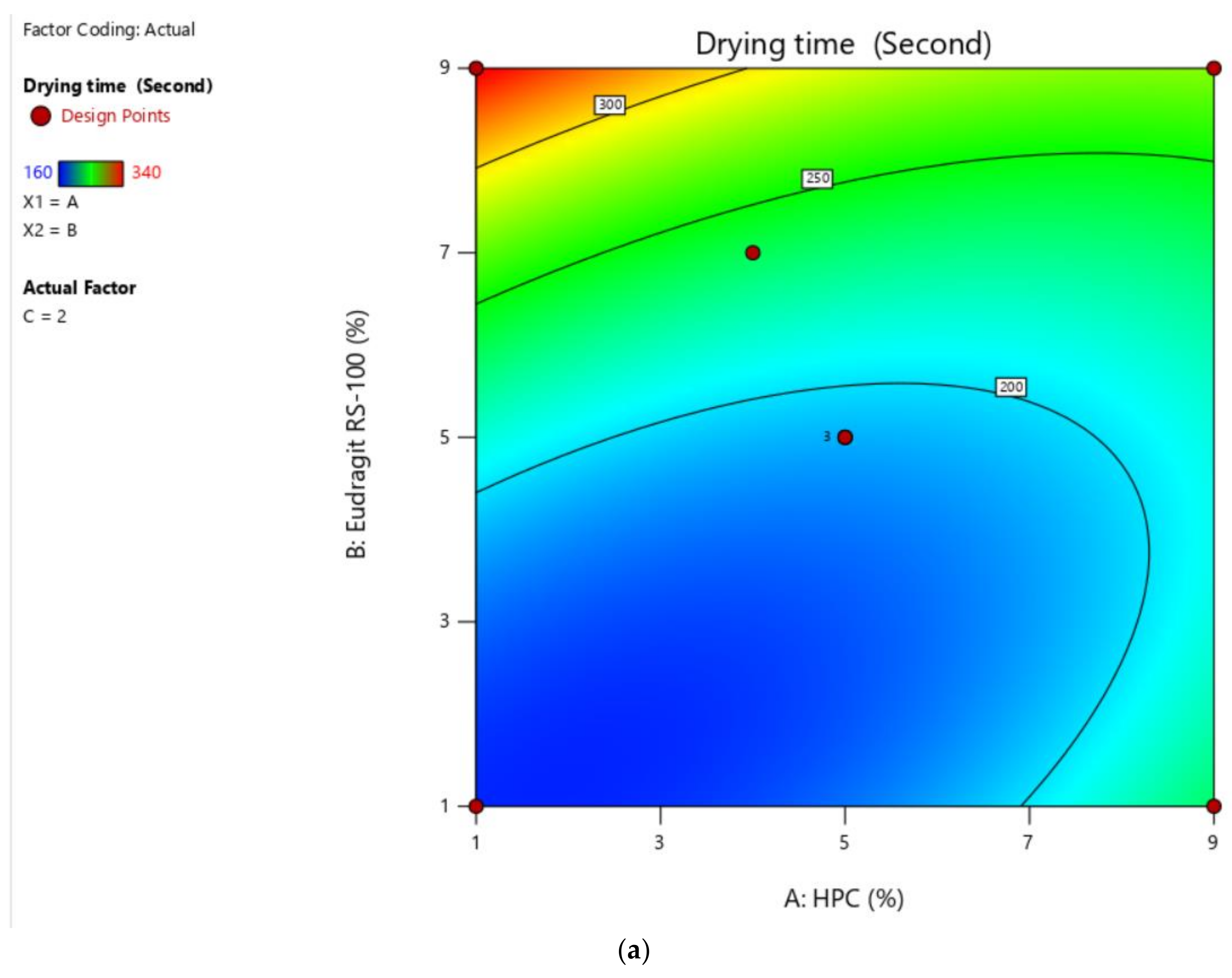
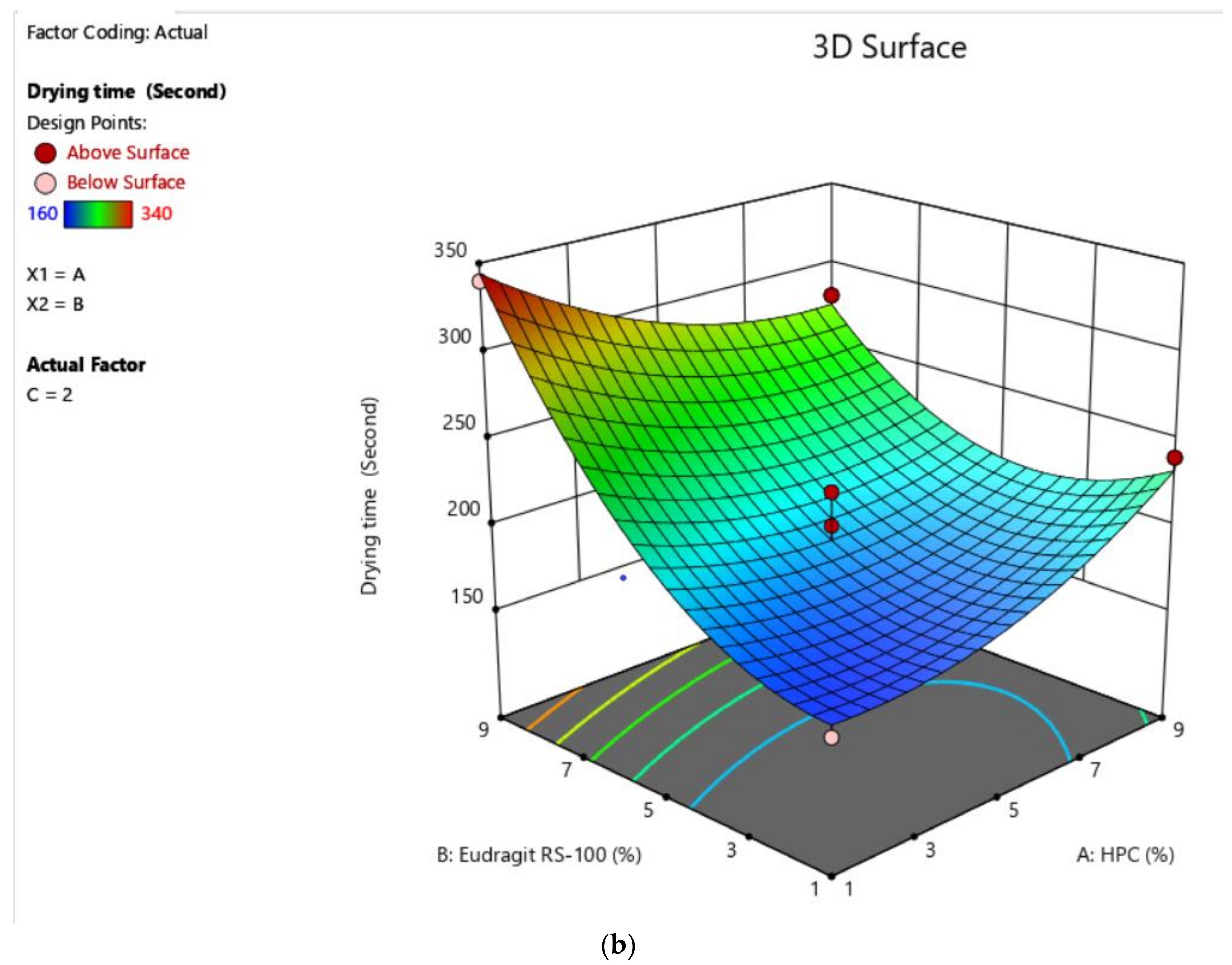
2.3.1. Selection of Solvent System Ratio and Optimization Polymer Concentration by Application of Box–Behnken Designs (BBD) to Check the Drying Time and Non-Volatile Content
2.3.2. Preparation of Nail Lacquer
2.4. Evaluation of Nail Lacquer
2.4.1. Drying Time
2.4.2. Non-Volatile Content
2.4.3. Water-Resistance Capacity
2.4.4. Blush Test for Nail Lacquer
2.4.5. In Vitro Drug Release Study
2.4.6. Transungual Permeation Study
2.5. Development of Nanoemulgel
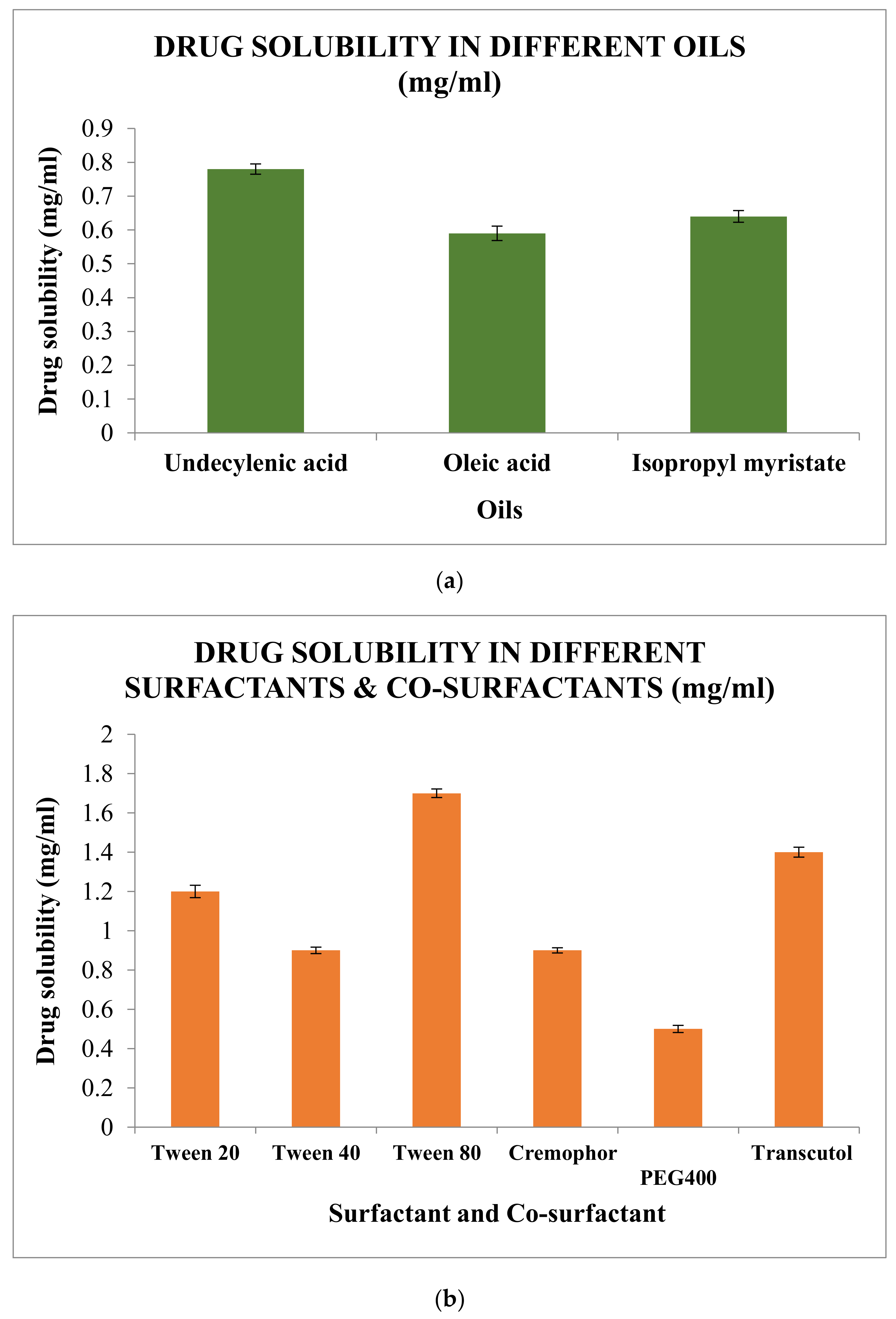
2.5.1. Screening of Excipients
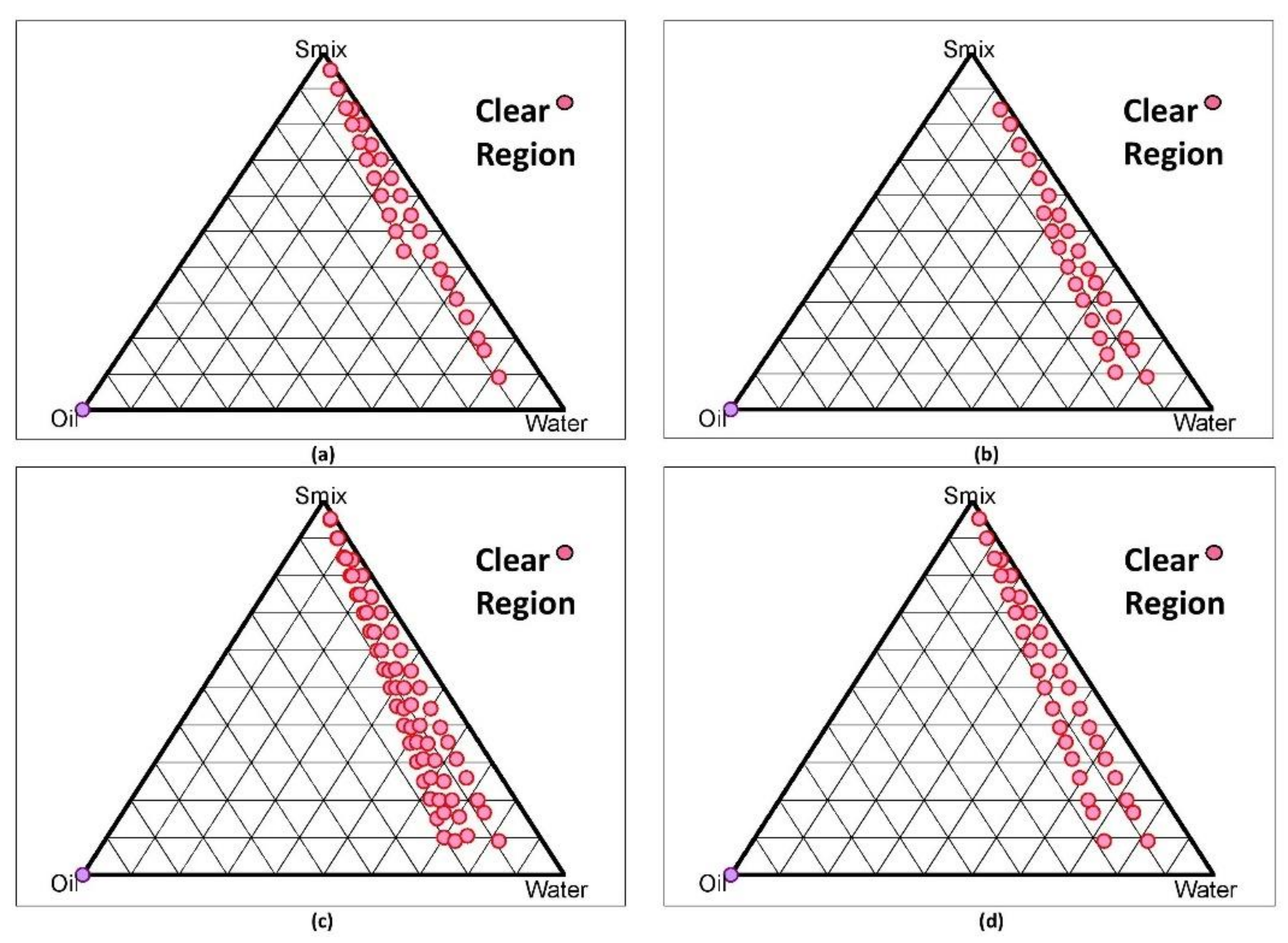
2.5.2. Development of Nanoemulsion (NE) (o/w type) by Construction of
Pseudo-Ternary Phase Diagram
2.5.3. Thermodynamic Stability Studies
2.6. Characterization of Stable Nanoemulsion
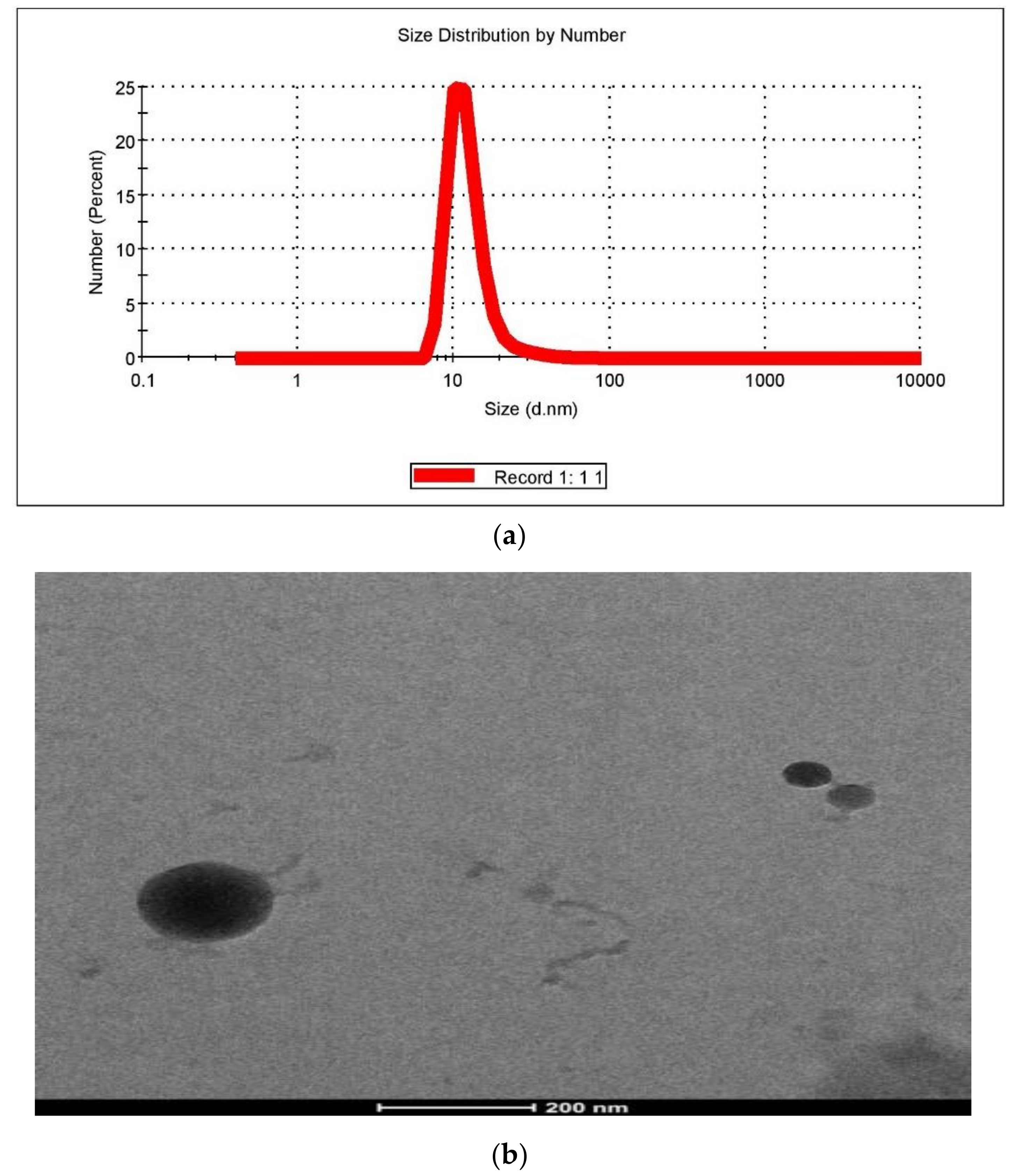
2.6.1. Particle Size and Polydispersity Index (PDI)
2.6.2. Zeta Potential
2.6.3. Morphology/TEM
2.6.4. pH
2.7. Development of Nanoemulgel
2.8. Evaluation of Nanoemulgel
2.8.1. Spreadability Study
2.8.2. Extrudability Study
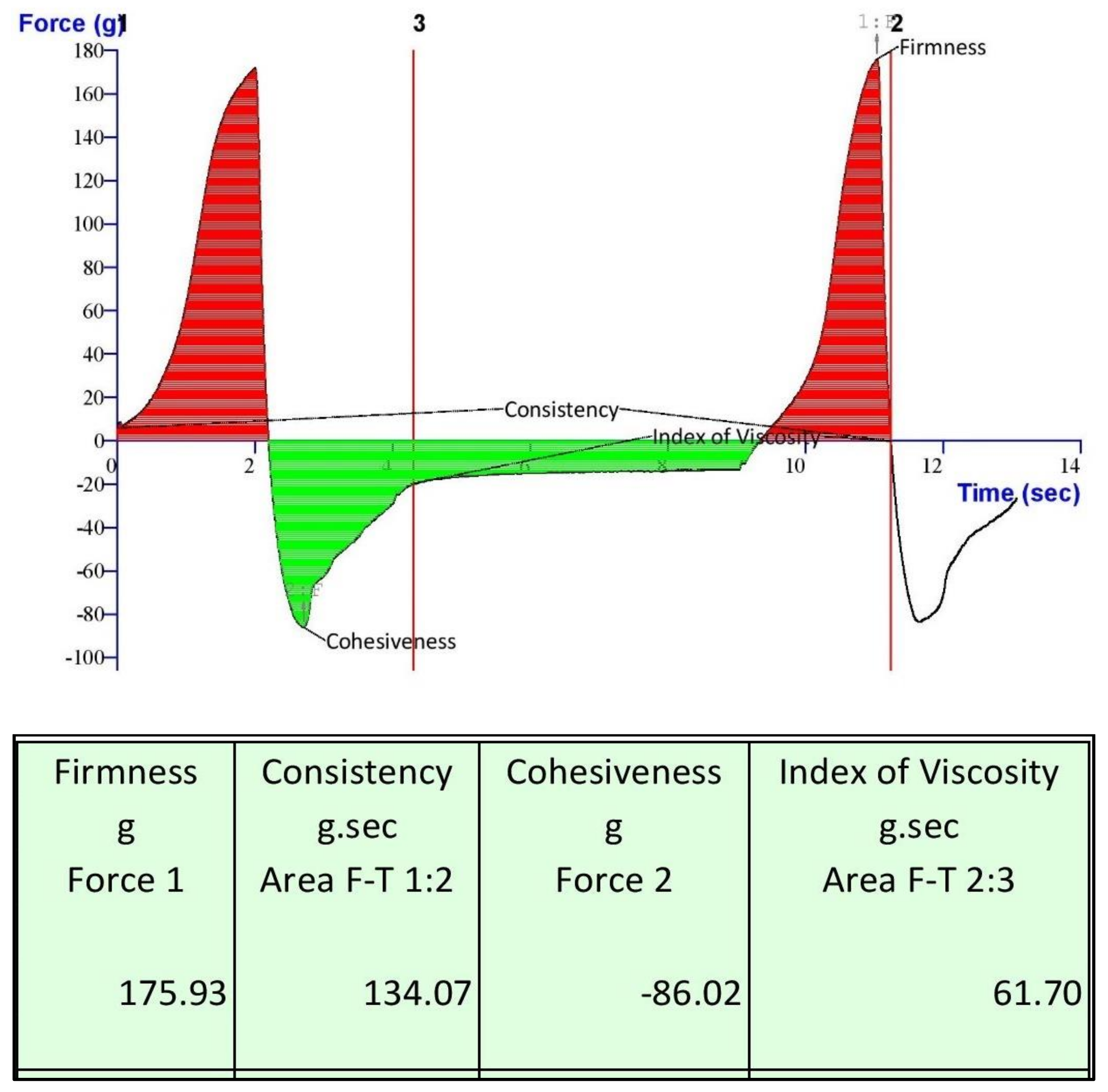
2.8.3. Gel Texture Analysis and Stability study
2.8.4. In Vitro Drug Release Study
2.8.5. Transungual Permeation Study
3. Conclusions
4. Materials
5. Method
5.1. Molecular Docking Study
5.2. Analytical Method Development
5.3. Development of Nail Lacquer
5.3.1. Selection of Solvent System Ratio and Optimization Polymer Concentration by Application of BBD to Check the Drying Time and Non-Volatile Content
5.3.2. Preparation of Nail Lacquer
5.4. Evaluation of Nail Lacquer
5.4.1. Drying Time
5.4.2. Non-Volatile Content
5.4.3. Water-Resistance Capacity
5.4.4. Blush Test for Nail Lacquer
5.4.5. In Vitro Release Study
5.4.6. Transungual Permeation Study
5.5. Development of Nanoemulgel
5.5.1. Screening of Excipients
5.5.2. Development of Nanoemulsion (NE) (Oil in Water Type) by Constructing Pseudo-Ternary Phase Diagram
5.5.3. Thermodynamic Stability Studies
5.6. Characterization of Stable Nanoemulsion
5.6.1. Particle Size and Polydispersity Index (PDI)
5.6.2. Zeta Potential
5.6.3. Morphology/TEM
5.6.4. pH
5.7. Development of Nanoemulgel (NEG)
5.8. Evaluation of Nanoemulgel
5.8.1. Spreadability Study
5.8.2. Extrudability Study
5.8.3. Gel Texture Analysis and Stability Study
5.8.4. In Vitro Drug Release Study
5.8.5. Transungual Permeation Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vlahovic, T.C. Onychomycosis: Evaluation, Treatment Options, Managing Recurrence, and Patient Outcomes. Clin. Podiatr. Med. Surg. 2016, 33, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N. Recent Advances in Therapies for Onychomycosis and Its Management. F1000Research 2019, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Iqbal, Z.; Ahmad, S.; Kohli, K.; Farooq, U.; Padhi, S.; Kabir, M.; Panda, A.K. Menopausal Remediation and Quality of Life (QoL) Improvement: Insights and Perspectives. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, S.; Cantrell, W.; Elewski, B.E. Onychomycosis and Diabetes. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.K.; Peterson, G.M.; Naunton, M.; Bushell, M.; Kosari, S.; Baby, K.E.; Thomas, J. Challenges and Opportunities in the Management of Onychomycosis. J. Fungi 2018, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and Prevention of Recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% Solution in the Treatment of Toenail Onychomycosis: Two Phase III Multicenter, Randomized, Double-Blind Studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Bseiso, E.A.; Nasr, M.; Sammour, O.A.; Abd El Gawad, N.A. Novel Nail Penetration Enhancer Containing Vesicles “NPEVs” for Treatment of Onychomycosis. Drug Deliv. 2016, 23, 2813–2819. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A Review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Dhamoon, R.K.; Popli, H.; Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef]
- Jain, P.; Taleuzzaman, M.; Kala, C.; Kumar Gupta, D.; Ali, A.; Aslam, M. Quality by Design (Qbd) Assisted Development of Phytosomal Gel of Aloe Vera Extract for Topical Delivery. J. Liposome Res. 2020, 31, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Basha, B.N.; Prakasam, K.; Goli, D. Formulation and Evaluation of Gel Containing Fluconazole-Antifungal Agent. Int. J. Drug Dev. Res. 2011, 3, 119–127. [Google Scholar]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Satpathi, P.; Banerjee, D.; Maiti, A.; Sengupta, M.; Mohata, A. Onychomycosis in Eastern India -Study in a Peripheral Tertiary Care Centre. J. Pak. Assoc. Dermatol. 2013, 23, 14–19. [Google Scholar]
- Alessandrini, A.; Starace, M.; Bruni, F.; Piraccini, B.M. An Open Study to Evaluate Effectiveness and Tolerability of a Nail Oil Composed of Vitamin E and Essential Oils in Mild to Moderate Distal Subungual Onychomycosis. Ski. Appendage Disord. 2020, 6, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Bonvin, D.; Hofmann, H.; Mionić Ebersold, M. Fungicidal PMMA-Undecylenic Acid Composites. Int. J. Mol. Sci. 2018, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Bellet, J.S.; Daniel, C.R. Home remedies for onychomycosis. In Onychomycosis; Rigopoulos, D., Elewski, B., Richert, B., Eds.; John Wiley & Sons: Chichester, UK, 2018; pp. 207–212. ISBN 978-1-119-22651-2. [Google Scholar]
- Van der Steen, M.; Stevens, C.V. Undecylenic Acid: A Valuable and Physiologically Active Renewable Building Block from Castor Oil. ChemSusChem 2009, 2, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Rehder, P.; Nguyen, T.T. A New Concept in the Topical Treatment of Onychomycosis with Cyanoacrylate, Undecylenic Acid, and Hydroquinone. Foot Ankle Spec. 2008, 1, 93–96. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Luan, J.; Zheng, F.; Yang, X.; Yu, A.; Zhai, G. Nanostructured Lipid Carriers for Oral Delivery of Baicalin: In Vitro and In Vivo Evaluation. Colloids Surf. Physicochem. Eng. Asp. 2015, 466, 154–159. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic Surfactant Vesicular Systems for Effective Drug Delivery—An Overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Atrux-Tallau, N.; Lasselin, J.; Han, S.-H.; Delmas, T.; Bibette, J. Quantitative Analysis of Ligand Effects on Bioefficacy of Nanoemulsion Encapsulating Depigmenting Active. Colloids Surf. B Biointerfaces 2014, 122, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, N.; Tikoo, K.; Sinha, V.R. Development of Mirtazapine Loaded Solid Lipid Nanoparticles for Topical Delivery: Optimization, Characterization and Cytotoxicity Evaluation. Int. J. Pharm. 2020, 586, 119439. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, H.; Deshpande, A.; Deshpande, S. Kinetic modeling and dissolution profiles comparison: An overview. Int. J. Pharm. Bio. Sci. 2013, 10, 728–773. [Google Scholar]
- Bhuptani, R.S.; Deshpande, K.M.; Patravale, V.B. Transungual Permeation: Current Insights. Drug Deliv. Transl. Res. 2016, 6, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-Activity Relationship (SAR) Study and Design Strategies of Nitrogen-Containing Heterocyclic Moieties for Their Anticancer Activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef] [PubMed]
- Molecular Modeling, Synthesis, and Evaluation of 1-(4-Methoxybenzofuran-5-Yl)-3-Phenylpropane-1, 3-Dione for Its Anxiolytic Potentiality. Available online: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=EsG-lKYAAAAJ&citation_for_view=EsG-lKYAAAAJ:2osOgNQ5qMEC (accessed on 11 September 2021).
- Haider, M.R.; Ahmad, K.; Siddiqui, N.; Ali, Z.; Akhtar, M.J.; Fuloria, N.; Fuloria, S.; Ravichandran, M.; Yar, M.S. Novel 9-(2-(1-Arylethylidene)Hydrazinyl)Acridine Derivatives: Target Topoisomerase 1 and Growth Inhibition of HeLa Cancer Cells. Bioorganic Chem. 2019, 88, 102962. [Google Scholar] [CrossRef]
- Al, P.A.K. et Antipsychotic Potential and Molecular Modelling of 1-(4-Methoxybenzofuran-5-Yl)-3-Phenylpropane-1, 3-Dione. J. Gujarat Res. Soc. 2019, 21, 362–369. [Google Scholar]
- Kerai, L.V.; Hilton, S.; Murdan, S. UV-Curable Gel Formulations: Potential Drug Carriers for the Topical Treatment of Nail Diseases. Int. J. Pharm. 2015, 492, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Šveikauskaitė, I.; Pockevičius, A.; Briedis, V. Potential of Chemical and Physical Enhancers for Transungual Delivery of Amorolfine Hydrochloride. Materials 2019, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.D.; Tran, P.H.L. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design Expert Assisted Nanoformulation Design for Co-Delivery of Topotecan and Thymoquinone: Optimization, in Vitro Characterization and Stability Assessment. J. Mol. Liq. 2017, 242, 382–394. [Google Scholar] [CrossRef]
- PubMed. Application of Response Surface Methodology for Design and Optimization of Reservoir-Type Transdermal Patch of Simvastatin. Available online: https://pubmed.ncbi.nlm.nih.gov/26452533/ (accessed on 11 September 2021).
- Akhtar, N.; Sharma, H.; Pathak, K. Onychomycosis: Potential of Nail Lacquers in Transungual Delivery of Antifungals. Scientifica 2016, 2016, 1387936. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.M.S.; Ribeiro, R.C.A.; Pinheiro, G.K.L.O.; Pinheiro, F.I.; Oliveira, W.N.; Souza, L.B.F.C.; Silva, A.L.; Amaral-Machado, L.; Alencar, É.N.; Chaves, G.M.; et al. Polishing the Therapy of Onychomycosis Induced by Candida spp.: Amphotericin B-Loaded Nail Lacquer. Pharmaceutics 2021, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Sharma, V.; Pathak, K. Matrix Based System of Isotretinoin as Nail Lacquer to Enhance Transungal Delivery across Human Nail Plate. Int. J. Pharm. 2015, 478, 268–277. [Google Scholar] [CrossRef]
- ScienceDirect. Efinaconazole Nail Lacquer for the Transungual Drug Delivery: Formulation, Optimization, Characterization and in Vitro Evaluation. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1773224720312879 (accessed on 11 September 2021).
- Sigma-Aldrich. Dialysis Tubing Cellulose Membrane Avg. Flat Width 25 Mm (1.0 in.). Available online: http://www.sigmaaldrich.com/ (accessed on 11 September 2021).
- Hassan, N.; Singh, M.; Sulaiman, S.; Jain, P.; Sharma, K.; Nandy, S.; Dudeja, M.; Ali, A.; Iqbal, Z. Molecular Docking-Guided Ungual Drug-Delivery Design for Amelioration of Onychomycosis. ACS Omega 2019, 4, 9583–9592. [Google Scholar] [CrossRef]
- Hooda, A.; Sradhanjali, M. Popsy Formulation and Evaluation of Novel Solid Lipid Microparticles for the Sustained Release of Ofloxacin. Pharm. Nanotechnol. 2018, 5, 329–341. [Google Scholar] [CrossRef]
- Patel, M.M.; Vora, Z.M. Formulation Development and Optimization of Transungual Drug Delivery System of Terbinafine Hydrochloride for the Treatment of Onychomycosis. Drug Deliv. Transl. Res. 2016, 6, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Verma, D.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Extraction of a water soluble bioactive hypoxoside and its development into an ethosomal system for deep dermal delivery. Int. J. Pharm. Pharm. Sci. 2015, 7, 211–215. [Google Scholar]
- Patel, J.; Patel, A.; Raval, M.; Sheth, N. Formulation and Development of a Self-Nanoemulsifying Drug Delivery System of Irbesartan. J. Adv. Pharm. Technol. Res. 2011, 2, 9–16. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ahmad, S.; Mallick, M.N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a Novel Synergistic Thermosensitive Gel for Vaginal Candidiasis: An In Vitro, In Vivo Evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef]
- Chan, M.Y.; Dowling, Q.M.; Sivananthan, S.J.; Kramer, R.M. Particle Sizing of Nanoparticle Adjuvant Formulations by Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA). Methods Mol. Biol. 2017, 1494, 239–252. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-Concentration Zeta Potential Measurements Using Light-Scattering Techniques. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- Pathan, I.; Mangle, M.; Bairagi, S. Design and Characterization of Nanoemulsion for Transdermal Delivery of Meloxicam. Anal. Chem. Lett. 2016, 6, 286–295. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, X.; Xu, B.; Shen, Y.; Ye, Z.; Chaurasiya, B.; Liu, L.; Li, Y.; Xing, X.; Chen, D. Eprinomectin Nanoemulgel for Transdermal Delivery against Endoparasites and Ectoparasites: Preparation, In Vitro and In Vivo Evaluation. Drug Deliv. 2019, 26, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Taleuzzaman, M.; Sartaj, A.; Kumar, G.D.; Gilani, S.J.; Mirza, M.A. Phytosomal Gel of Manjistha Extract (MJE) Formulated and Optimized with Central Composite Design of Quality by Design (QbD). J. Dispers. Sci. Technol. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Khuroo, T.; Verma, D.; Khuroo, A.; Ali, A.; Iqbal, Z. Simultaneous Delivery of Paclitaxel and Erlotinib from Dual Drug Loaded PLGA Nanoparticles: Formulation Development, Thorough Optimization and in Vitro Release. J. Mol. Liq. 2018, 257, 52–68. [Google Scholar] [CrossRef]
- Palliyil, B.B.; Li, C.; Owaisat, S.; Lebo, D.B. Lateral Drug Diffusion in Human Nails. AAPS PharmSciTech 2014, 15, 1429–1438. [Google Scholar] [CrossRef] [PubMed][Green Version]





| S. No. | Trade Name | Composition | Manufacturing Authorization Holder & Product Category | Indication | NDA No/Weblink |
|---|---|---|---|---|---|
| 1 | Loceryl (Nail Lacquer) | 5% w/v Amorolfine in HCl | Galderma (UK), PoM | Onychomycoses caused by dermatophytes, yeasts and moulds. | https://www.medicines.org.uk/emc/product/1411 (accessed on 11 July 2021) |
| 2 | Penlac (Nail Lacquer) | Ciclopirox 8% | Valeant Bermuda (USA), PoM | Immunocompetent patients with mild to moderate onychomycosis due to Trichophyton rubrum | NDA-021022 |
| 3 | Kerydin (Nail solution) | Tavaborole 5% | Anacor Pharmaceuticals Inc., (USA), PoM | Onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes | NDA-204427 |
| 4 | Trosyl (Nail solution) | Tioconazole 283 mg/mL | Pfizer (UK), PoM | Nail infections due to susceptible fungi (dermatophytes and yeasts) and bacteria. | https://www.medicines.org.uk/emc/product/1071/smpc (accessed 11 July 2021) |
| 5 | JUBLIA (Nail solution) | Efinaconazole 10% | DOW PHARM (USA), PoM | Onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes | NDA 203567 |
| 6 | Danipro (Nail polish) | Undecylenic Acid, B7, Vit E, Vit A | Danipro (OTC) | Several benefits owing to the presence of different components | https://danipronails.com/ (accessed on 11 July 2021) |
| 7 | Myco Nail (Nail Lacquer) | Undecylenic acid 25% | Kramer Novis (OTC) | Athlete’s foot (Tinea pedis), ringworm (tinea corporis). For effective relief of itching, irritation, and burning feet. | www.drugs.com/otc/129363/myco-nail-a-antifungal-solution.html (accessed on 11 July 2021) |
| Compounds name | G-Score | Lipophilic E vdw | H-bond | Electro | Protein Ligands Interaction |
|---|---|---|---|---|---|
| Amorolfine HCl | −7.325 | −5.29 | −0.45 | −0.14 | Tyr A:449, Leu A:452, Leu A:453, Glu A:456, Tyr B:482, Leu B:485, Leu B:486, Glu B:487, Gly B:488, Glu B:489 |
| Ciclopirox | −6.668 | −4.65 | −0.65 | 0.22 | Tyr A:449, Leu A:452, Leu A:453, Gly A:455, Leu B:485, Leu B:486 |
| Efinaconazole | −5.033 | −5.29 | −0.53 | −0.11 | Tyr A:449, Leu A:452, Leu A:453, Glu A:456 |
| Tioconazole | −4.200 | −5.30 | 0 | 0.03 | Tyr A:449, Leu A:452, Gly A:455, Glu A:456 |
| Tavaborole | v3.769 | −4.74 | 0 | 0.02 | Tyr A:449, Leu A:452, Leu B:485 |
| Factors | Response | ||||
|---|---|---|---|---|---|
| Drug Free Formulation (DFF) | HPC (% w/v) | Eudragit RS (% w/v) | Salicylic Acid (% w/v) | Drying Time (s) ± SD (n = 3) | Non-Volatile Content (%) ± SD (n = 3) |
| DFF 1 | 5 | 5 | 2 | 220 ± 0.89 | 27 ± 0.09 |
| DFF 2 | 1 | 1 | 2 | 160 ± 0.65 | 26 ± 0.08 |
| DFF 3 | 5 | 9 | 5 | 340 ± 0.32 | 34 ± 0.18 |
| DFF 4 | 5 | 5 | 2 | 220 ± 0.58 | 27 ± 0.12 |
| DFF 5 | 9 | 1 | 2 | 240 ± 0.84 | 23 ± 0.11 |
| DFF 6 | 9 | 5 | 5 | 280 ± 0.89 | 24 ± 0.05 |
| DFF 7 | 5 | 1 | 5 | 260 ± 0.64 | 22 ± 0.06 |
| DFF 8 | 1 | 5 | 1 | 220 ± 0.69 | 28 ± 0.13 |
| DFF 9 | 1 | 5 | 5 | 280 ± 0.75 | 30 ± 0.09 |
| DFF 10 | 1 | 9 | 2 | 340 ± 0.95 | 33 ± 0.11 |
| DFF 11 | 4 | 7 | 2 | 200 ± 0.83 | 29 ± 0.09 |
| DFF 12 | 9 | 5 | 1 | 190 ± 0.51 | 19 ± 0.11 |
| DFF 13 | 9 | 9 | 2 | 280 ± 0.63 | 27 ± 0.05 |
| DFF 14 | 5 | 5 | 2 | 200 ± 0.93 | 29 ± 0.06 |
| DFF 15 | 5 | 5 | 3 | 180 ± 0.72 | 21 ± 0.08 |
| DFF 16 | 5 | 4 | 1 | 160 ± 0.37 | 23 ± 0.10 |
| DFF 17 | 5 | 9 | 1 | 300 ± 0.96 | 32 ± 0.04 |
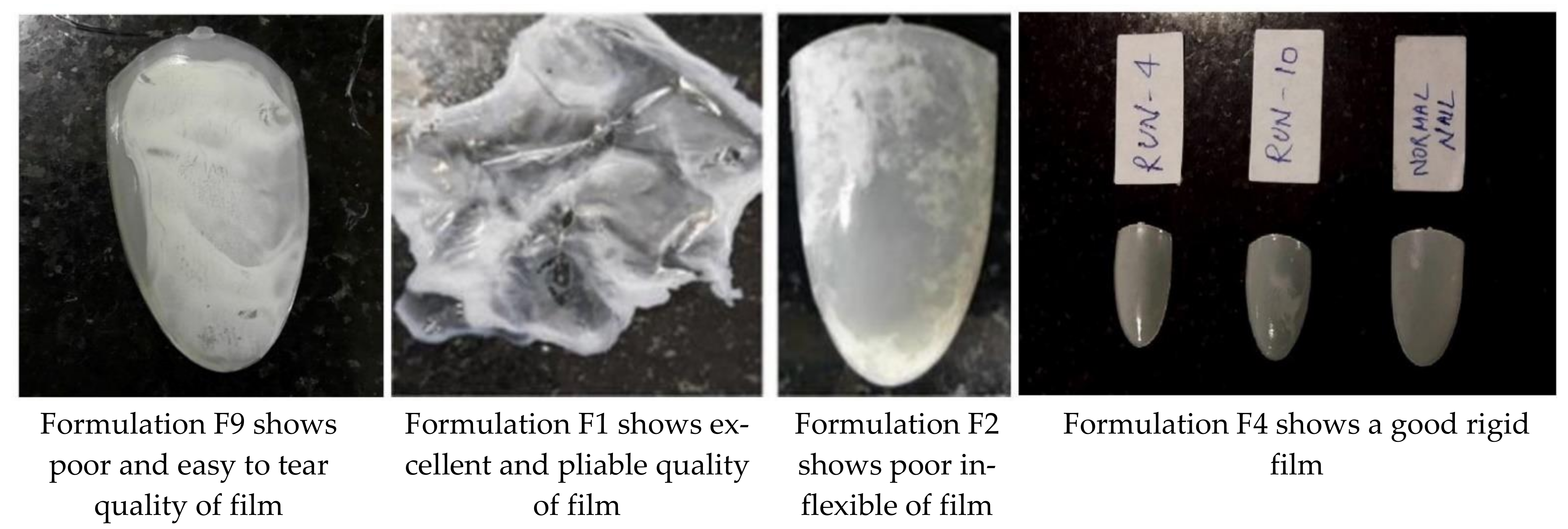
| Formulation Code (F) | HPC Conc. (% w/v) | Eudragit RS 100 (% w/v) | Ethanol:Water (9:1) mL | Quality of Film |
|---|---|---|---|---|
| F1 | 5 | 5 | 5 | Excellent pliable |
| F2 | 9 | 5 | 5 | Poor inflexible |
| F4 | 1 | 5 | 5 | Good rigid |
| F6 | 9 | 9 | 5 | Very bad |
| F9 | 9 | 1 | 5 | Very easy to tear |
| F11 | 1 | 9 | 5 | Hard |
| Smix | Formulation No. (F) | Turbidity | After 24 h Turbidity | Heating-Cooling Cycle | Centrifugation | Freeze Thaw Cycle |
|---|---|---|---|---|---|---|
| 1:1 | F1(1:9) | No | No | Fail | - | - |
| 2:1 | F2(1:9) | No | No | Fail | - | - |
| 2:1 | F3(1:8) | Yes | Yes | Pass | Pass | Fail |
| 3:1 | F4(1:9) | No | No | Pass | Pass | Pass |
| 3:1 | F5(2:8) | No | Yes | - | - | - |
| 3:1 | F6(3:7) | No | Yes | - | - | - |
| 3:1 | F7(1:5) | No | No | Pass | Pass | Pass |
| 3:1 | F8(1:6) | No | No | Pass | Pass | Pass |
| 3:1 | F9(1:7) | No | No | Pass | Pass | Fail |
| 3:1 | F10(1:3.5) | No | Yes | - | - | - |
| 4:1 | F11(1:9) | No | No | Fail | - | - |
| 4:1 | F12(1:8) | No | No | Pass | Pass | Pass |
| 4:1 | F13(1:7) | Yes | Yes | Pass | Pass | Pass |
| Formulation (Nanoemulgel = NEG) | Carbopol (% w/v) | Thioglycolic Acid % (v/v) | pH | Homogeneity | Spread Ability |
|---|---|---|---|---|---|
| NEG 0.5 | 0.5 | 1 | 6.3 ± 0.29 | Good | Watery |
| NEG 0.75 | 0.75 | 1.5 | 6.4 ± 0.21 | Better | Good |
| NEG 1.0 | 1 | 1.75 | 6.4 ± 0.18 | Coarse | Hard |
| NEG 1.5 | 1.5 | 2 | 6.4 ± 0.32 | Hard | Hard |
| S. No. | Parameters (Mean ± SD) (n = 3) | Values |
|---|---|---|
| 1. | Spreadability (cm) | 6.8 ± 0.127 (Easily spreadable) |
| 2. | Homogeneity | Smooth texture (no grittiness was found) |
| 3. | Extrudability (gm/cm2) | 1.7 ± 0.32 |
| 4. | pH | 6.4 ± 0.324 |
| 5. | Drug Content (%) | 94.65 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Monawwar, S.; Mohapatra, S.; Alex, T.S.; Ahmed, A.; Taleuzzaman, M.; Ali, A.; Ansari, M.J.; Mirza, M.A.; Iqbal, Z. In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management. Gels 2021, 7, 221. https://doi.org/10.3390/gels7040221
Fatima M, Monawwar S, Mohapatra S, Alex TS, Ahmed A, Taleuzzaman M, Ali A, Ansari MJ, Mirza MA, Iqbal Z. In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management. Gels. 2021; 7(4):221. https://doi.org/10.3390/gels7040221
Chicago/Turabian StyleFatima, Mahak, Sadia Monawwar, Sradhanjali Mohapatra, Thomson Santosh Alex, Abdulrahman Ahmed, Mohamad Taleuzzaman, Asgar Ali, Mohammad Javed Ansari, Mohd. Aamir Mirza, and Zeenat Iqbal. 2021. "In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management" Gels 7, no. 4: 221. https://doi.org/10.3390/gels7040221
APA StyleFatima, M., Monawwar, S., Mohapatra, S., Alex, T. S., Ahmed, A., Taleuzzaman, M., Ali, A., Ansari, M. J., Mirza, M. A., & Iqbal, Z. (2021). In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management. Gels, 7(4), 221. https://doi.org/10.3390/gels7040221