A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels
Abstract
:1. Introduction
2. N,N-Dimethylacrylamide-Based Hydrogels for Dye Removal
3. The Removal of Hazardous Heavy Metal Ions
4. N,N-Dimethylacrylamide Hydrogels for Self-Healing Materials
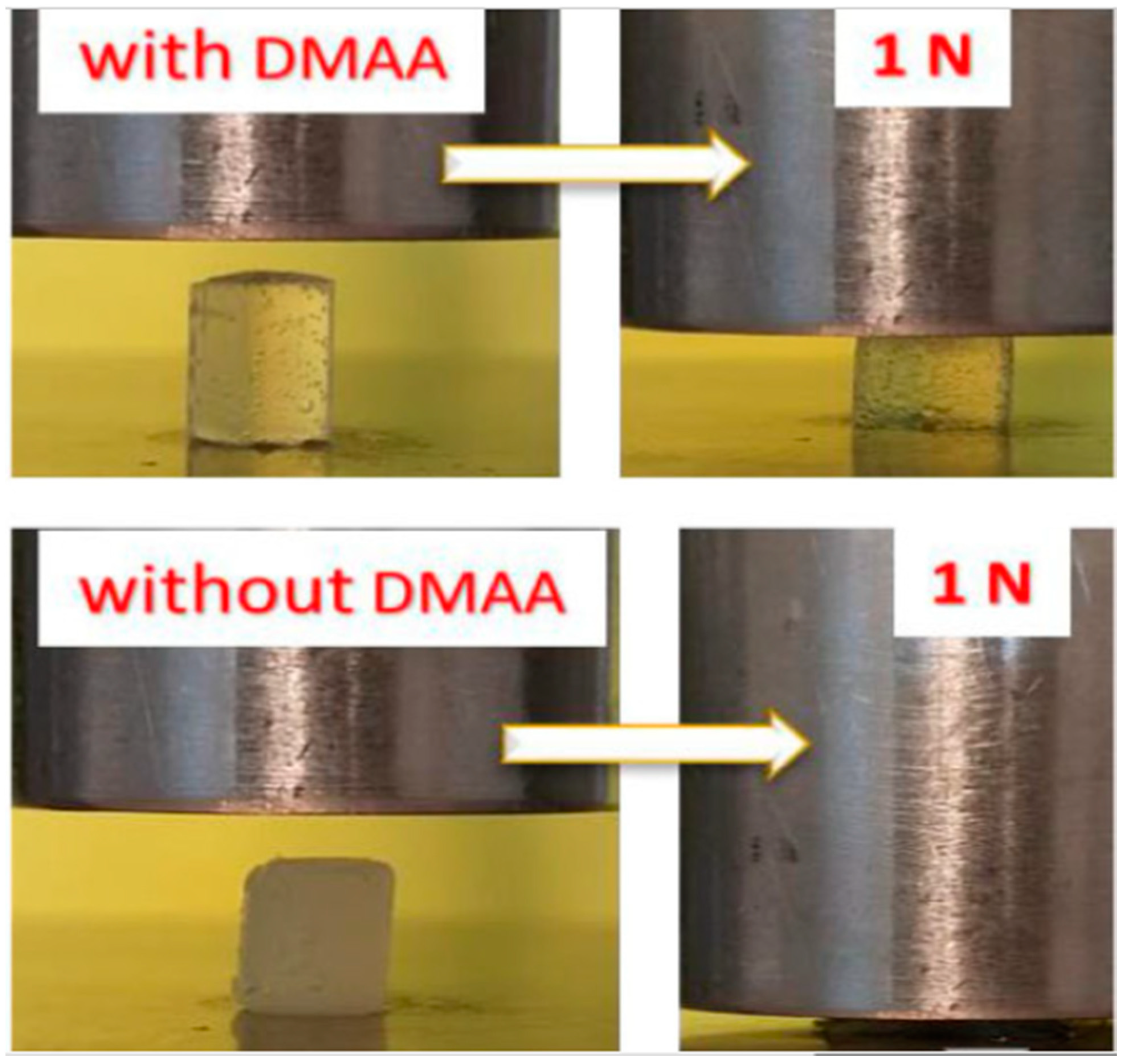
5. N,N-Dimethylacrylamide Hydrogels for Enhancing Mechanical Properties of the Materials
6. Synthesis of DMAA-Based Hydrogels
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dauletov, Y.; Nuraje, N.; Abdiyev, K.; Toktarbay, Z.; Zhursumbaeva, M. Copolymers of diallyldimethylammonium chloride and vinyl ether of monoethanolamine: Synthesis, flocculating, and antimicrobial properties. J. Surfact. Deterg. 2019, 22, 1129–1137. [Google Scholar] [CrossRef]
- Dauletov, Y.; Abdiyev, K.; Toktarbay, Z.; Nuraje, N.; Zhursumbaeva, M.; Kenzhaliyev, M. Radical polymerization and kinetics of N,N-diallyl-N,N-dimethylammonium chloride and vinyl ether of monoethanolamine. Fibers Polym. 2018, 19, 2023–2029. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Toktarbay, Z.; Zhenissova, A.Z.; Zhursumbaeva, M.B.; Kainazarova, R.N.; Nuraje, N. The new effective flocculants copolymers of N,N-dimethyl-N,N-diallyl-ammonium chloride and N,N-dimethylacrylamide. Colloids Surf. A 2015, 480, 228–235. [Google Scholar] [CrossRef]
- Liu, T.; Ding, E.; Xue, F. Polyacrylamide and poly(N,N-dimethylacrylamide) grafted cellulose nanocrystals as efficient flocculants for kaolin suspension. Int. J. Biol. Macromol. 2017, 103, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.S.; Verma, S.K.; Yadav, M.; Behari, K. Studies on graft copolymerization of gellan gum with N,N-dimethylacrylamide by the redox system. Int. J. Biol. Macromol. 2014, 70, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.S.; Verma, S.K.; Yadav, M.; Behari, K. Guar gum-g-N,N′-dimethylacrylamide: Synthesis, characterization and applications. Carbohydr. Polym. 2014, 99, 284–290. [Google Scholar] [CrossRef]
- Tripathy, J.; Mishra, D.K.; Yadav, M.; Behari, K. Synthesis, characterization and applications of graft copolymer (Chitosan-g-N,N-dimethylacrylamide). Carbohydr. Polym. 2010, 79, 40–46. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Biodegradable flocculants based on polyacrylamide and poly(N,N-dimethylacrylamide) grafted amylopectin. Int. J. Biol. Macromol. 2014, 70, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Du, X. Enhanced dispersion of carbon nanotubes in water by plasma induced graft poly(N,N-dimethylacrylamide) and its application in humic acid capture. J. Mol. Liq. 2019, 277, 380–387. [Google Scholar] [CrossRef]
- Bashir, S.; Omar, F.S.; Hina, M.; Numan, A.; Iqbal, J.; Ramesh, S.; Ramesh, K. Synthesis and characterization of hybrid poly (N,N-dimethylacrylamide) composite hydrogel electrolytes and their performance in supercapacitor. Electrochim. Acta 2019, 332, 135438. [Google Scholar] [CrossRef]
- Hu, X.; Feng, L.; Wei, W.; Xie, A.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel semi-IPN hydrogel based on Salecan and poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Carbohydr. Polym. 2014, 105, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.I.; Farag, A.H.; Hamada, A.W.; Hamza, M. Synthesis, characterization, swelling studies and dye removal of chemically crosslinked acrylic acid/acrylamide/N,N-dimethyl acrylamide hydrogels. Am. J. Appl. Chem. 2016, 4, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Razzaque, M.S. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Marquès, M. The effects of some essential and toxic metals/metalloids in COVID-19: A review. Food Chem. Toxicol. 2021, 152, 112161. [Google Scholar] [CrossRef]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Shumoy, H.; Raes, K. Dissecting the facts about the impact of contaminant iron in human nutrition: A review. Trends Food Sci. Technol. 2021, 116, 918–927. [Google Scholar] [CrossRef]
- Culbertson, E.M.; Culotta, V.C. Copper in infectious disease: Using both sides of the penny. Semin. Cell Dev. Biol. 2021. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.; Azat, S.; Nurgozhin, T.; Inglezakis, V. Removal of Cd2+ from water by use of super-macroporous cryogels and comparison to commercial adsorbents. Polymers 2020, 12, 2405. [Google Scholar] [CrossRef] [PubMed]
- Azat, S.; Busquets, R.; Pavlenko, V.V.; Kermikulova, A.R.; Whitby, R.L.D.; Mansurov, Z.A. Applications of activated carbon sorbents based on greek walnut. Appl. Mech. Mater. 2014, 467, 49–51. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Toktarbay, Z.; Zhenissova, A.Z.; Zhursumbaeva, M.B.; Kainazarova, R.N. Copolymerization of N,N-dimethyll-N,N-diallylammonium chloride with N,N-dimethylacrylamide. Polym. Sci. Ser. B 2015, 57, 217–223. [Google Scholar] [CrossRef]
- Ayatzhan, A.; Tashenov, A.; Nurgeldi, A.; Zhanar, O.; Zhexenbek, T.; Kaldibek, A.; Nuraje, N. P(DADMAAC-co-DMAA): Synthesis, thermal stability, and kinetics. Polym. Adv. Technol. 2020, 32, 2669–2675. [Google Scholar] [CrossRef]
- Akhmetzhan, A.; Abeu, N.; Longinos, S.N.; Tashenov, A.; Myrzakhmetova, N.; Amangeldi, N.; Kuanyshova, Z.; Ospanova, Z. Toktarbay, Z. Synthesis and heavy-metal sorption studies of N,N-dimethylacrylamide based hydrogels. Polymers 2021, 13, 3084. [Google Scholar] [CrossRef]
- Zehm, D.; Lieske, A.; Stoll, A. On the thermoresponsivity and scalability of N,N-dimethylacrylamide modified NIPAM microgels. Macromol. Chem. Phys. 2020, 221, 2000018. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Biotechnol. 2015, 14, 73–92. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Kinetic analysis of CO2 hydrate formation by the use of different impellers. Reac. Kinet. Mech. Cat. 2021, 133, 85–100. [Google Scholar] [CrossRef]
- Hossain, S.; Shahruzzaman, M.; Kabir, S.F.; Rahman, M.S.; Sultana, S.; Mallik, A.K.; Haque, P.; Takafuji, M.; Rahman, M.M. Jute cellulose nanocrystal/poly(N,N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) hybrid hydrogels for removing methylene blue dye from aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 254–263. [Google Scholar] [CrossRef]
- Preetha, B.K.; Vishalakshi, B. Karaya gum-grafted-poly(N,N′-dimethylacrylamide) gel: A pH responsive potential adsorbent for sequestration of cationic dyes. J. Environ. Chem. Eng. 2019, 8, 103608. [Google Scholar] [CrossRef]
- Bao, S.; Wu, D.; Wang, Q.; Su, T. Functional elastic hydrogel as recyclable membrane for the adsorption and degradation of methylene blue. PLoS ONE 2014, 9, e88802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subinoy, J.; Jagabandhu, R.; Barun, M.; Tridib, T. Efficient and selective removal of cationic organic dyes from their aqueous solutions by a nanocomposite hydrogel, katira gum-cl-poly(acrylic acid-co-N,N-dimethylacrylamide)@bentonite. Appl. Clay Sci. 2019, 173, 46–64. [Google Scholar] [CrossRef]
- Sadik, W.A.A.; El-Demerdash, A.G.M.; Abbas, R.; Gabre, H.A. Fast synthesis of an eco-friendly starch-grafted poly(N,N-dimethyl acrylamide) hydrogel for the removal of Acid Red 8 dye from aqueous solutions. Polym. Bull. 2020, 77, 4445–4468. [Google Scholar] [CrossRef]
- Jana, S.; Pradhan, S.S.; Tripathy, T. Poly(N,N-dimethylacrylamide-co-acrylamide) grafted hydroxyethyl cellulose hydrogel: A useful Congo red dye remover. J. Polym. Environ. 2018, 26, 2730–2747. [Google Scholar] [CrossRef]
- Hernandez-Martínez, A.R.; Lujan-Montelongo, J.A.; Silva-Cuevas, C.; Mota-Morales, J.D.; Cortez-Valadez, M.; de Jesus Ruíz-Baltazar, A.; Cruz, M.; Herrera-Ordonez, J. Swelling and methylene blue adsorption of poly(N,N-dimethylacrylamideco-2-hydroxyethyl methacrylate) hydrogel. React. Funct. Polym 2018, 122, 75–84. [Google Scholar] [CrossRef]
- Bekiari, V.; Sotiropoulou, M.; Bokias, G.; Lianos, P. Use of poly(N,N-dimethylacrylamide-co-sodium acrylate) hydrogel to extract cationic dyes and metals from water. Colloids Surf. A Physicochem. Eng. 2008, 312, 214–218. [Google Scholar] [CrossRef]
- Kozhabekov, S.S.; Zhubanov, A.A.; Toktarbay, Z. Study the rheological properties of waxy oil with modified pour point depressants for the South Turgai oil field in Kazakhstan. Oil Gas Sci. Technol 2019, 74, 28. [Google Scholar] [CrossRef]
- Tokuyama, H.; Kitamura, E.; Seida, Y. Detection of AU(III) ions using a poly(N,N-dimethylacrylamide)-coated QCM sensor. Talanta 2016, 146, 507–509. [Google Scholar] [CrossRef]
- Tokuyama, H.; Kanehara, A. Temperature swing adsorption of gold (III) ions on poly (N-isopropylacrylamide) gel. React. Funct. Polym 2007, 67, 136–143. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Kinetic analysis of arginine, glycine and valine on methane (95%)–propane (5%) hydrate formation. React. Kinet. Mech. Catal. 2021, 133, 741–751. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Are the amino acids inhibitors or promoters on methane (95%)–propane (5%) hydrate formation? Reac. Kinet. Mech. Cat. 2021, 132, 795–809. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Trache, D.; Yazdani Nezhad, H.; Thakur, V.K. Microwave-assisted rapid synthesis of reduced graphene oxide-based gum tragacanth hydrogel nanocomposite for heavy metal ions adsorption. Nanomaterials 2020, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Dirani, A.; Glinel, K.; Jonas, A.M. Temperature dependence of the surface and volume hydrophilicity of hydrophilic polymer brushes. Langmuir 2016, 32, 3433–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Jacques, A.L.; Lujan-Montelongo, J.A.; Silva-Cuevas, C.; Cortez-Valadez, M.; Estevez, M.; Hernandez-Martínez, A.R. Lead (II) removal by poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Eur. Polym. J. 2018, 101, 262–272. [Google Scholar] [CrossRef]
- Tokuyama, H.; Kitamura, E.; Seida, Y. Development of zirconia nanoparticle-loaded hydrogel for arsenic adsorption and sensing. React. Funct. Polym 2020, 146, 104427. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Kang, M.; Fosso-Kanke, E. Microwave assisted synthesis of xanthan gum-cl-Dimethyl acrylamide hydrogel based silica hydrogel as adsorbent for cadmium (II) removal. In Proceedings of the International Conference on Science, Engineering, Technology and Waste Management (SETWM-19), Johannesburg, South Africa, 18–19 November 2019. [Google Scholar]
- Patiño, Z.; Ortega, A.; Burillo, G. Removal of Cr(VI) ions using a binary grafting of N-vinylcaprolactam and N,N-dimethylacrylamide onto crosslinked chitosan, synthesized by gamma radiation. J. Mex. Chem. Soc. 2019, 63, 155–163. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Preparation, investigation of metal ion removal and flocculation performances of grafted hydroxyethyl starch. Int. J. Biol. Macromol. 2013, 62, 557–564. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, C.; Tao, Z.; Fan, Y.; Tang, X.; Yang, H. Zwitterionic copolymer-based and hydrogen bonding-strengthened self-healing hydrogel. RSC Adv. 2015, 5, 33083–33088. [Google Scholar] [CrossRef]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Du, W.; Deng, A.; Guo, J.; Chen, J.; Li, H.; Gao, Y. An injectable self-healing hydrogel-cellulose nanocrystals conjugate with excellent mechanical strength and good biocompatibility. Carbohydr. Polym. 2019, 223, 115084. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Xi, B.; Wang, X.; Yang, Y.; Zhao, H.; Li, W.; Qin, J.; He, Y. Self-healing hydrogel with cross-linking induced thermo-response regulated light emission property. Colloids Surf. B: Biointerfaces 2019, 183, 110441. [Google Scholar] [CrossRef] [PubMed]
- Algi, M.P.; Okay, O. Highly stretchable self-healing poly(N,N-dimethylacrylamide) hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Huang, C.; Wei, J. Self-healing graphene oxidebased nanocomposite hydrogels serve as near-infrared light-driven valves. Sens. Actuators B Chem. 2019, 298, 126908. [Google Scholar] [CrossRef]
- Weng, L.; Gouldstone, A.; Wu, Y.; Chen, W. Mechanically strong double network photocrosslinked hydrogels from N,N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterials 2008, 29, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, J.; Lin, Q.; Zhu, Y.; Wang, L.; Li, Y. Movable-crosslinking tough hydrogels with lithium ion as sensitive and durable compressive sensor. Polymer 2021, 214, 123257. [Google Scholar] [CrossRef]
- Yessimova, O.; Kumargaliyeva, S.; Kerimkulova, M.; Mussabekov, K.; Toktarbay, Z. Wetting ability of a phytopreparation and their associates with polyelectrolytes. Rasayan J. Chem 2020, 13, 481–487. [Google Scholar] [CrossRef]
- Oral, C.B.; Yetiskin, B.; Okay, O. Stretchable silk fibroin hydrogels. Int. J. Biol. Macromol. 2020, 161, 1371–1380. [Google Scholar] [CrossRef]
- Toktabayeva, A.; Rakhmetullayeva, R.; Abutalip, M.; Mamutova, A.; Batyrbayeva, A.; Mun, G. The new hybrid copolymers based on N-isopropylacrylamide. J. Chem. Technol. Metall. 2019, 54, 475–482. [Google Scholar]
- Rakhmetullayeva, R.K.; Azhkeyeva, A.N.; Yeligbayeva, G.Z.; Shaikhutdynov, Y.M.; Mun, G.A.; Abutalip, M. New thermo-sensitive hydrogel based on copolymer of 2-hydroxyethyl acrylate and ethyl acrylate. Eurasian Chem.-Technol. J. 2017, 19, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Tavsanli, B.; Okay, O. Mechanically robust and stretchable silk/hyaluronic acid hydrogels. Carbohydr. Polym. 2019, 208, 413–420. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Examination of behavior of lysine on methane (95%)–propane (5%) hydrate formation by the use of different impellers. J. Pet. Explor. Prod. Technol. 2021, 11, 1823–1831. [Google Scholar]
- Nakan, U.; Mun, G.A.; Shaikhutdinov, Y.M.; Yeligbayeva, G.Z.; Bieerkehazhi, S.; Negim, E.S.; Samatovna, B.S.; Nauryzova, S.Z. Hydrogels based on N-isopropylacrylamide and 2-hydroxyethylacrylate: Synthesis, characterization and investigation its antibacterial activity. Polym. Int. 2020, 69, 1220–1226. [Google Scholar] [CrossRef]
- Nakan, U.; Mun, G.; Rakhmetullayeva, R.; Tolkyn, B.; Bieerkehazhi, S.; Yeligbayeva, G.; Negim, E. Thermosensitive N-isopropylacrylamide-CO-2-hydroxyethyl acrylate hydrogels interactions with poly(acrylic acid) and surfactants. Polym. Adv. Technol. 2021, 32, 2676–2681. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Examination of methane hydrate formation by the use of dual impeller combinations. React. Kinet. Mech. Catal. 2021, 133, 729–740. [Google Scholar] [CrossRef]
- Merey, Ş.; Longinos, S.N. The gas hydrate potential of the Eastern Mediterranean basin. Bull. Miner. Res. Explor 2019, 160, 117–134. [Google Scholar] [CrossRef] [Green Version]
- Longinos, S.N.; Longinou, D.D.; Achinas, S. Natural gas hydrates: Possible environmental issues. In Contemporary Environmental Issues and Challenges in Era of Climate Change; Springer: Singapore, 2020; pp. 277–293. [Google Scholar]
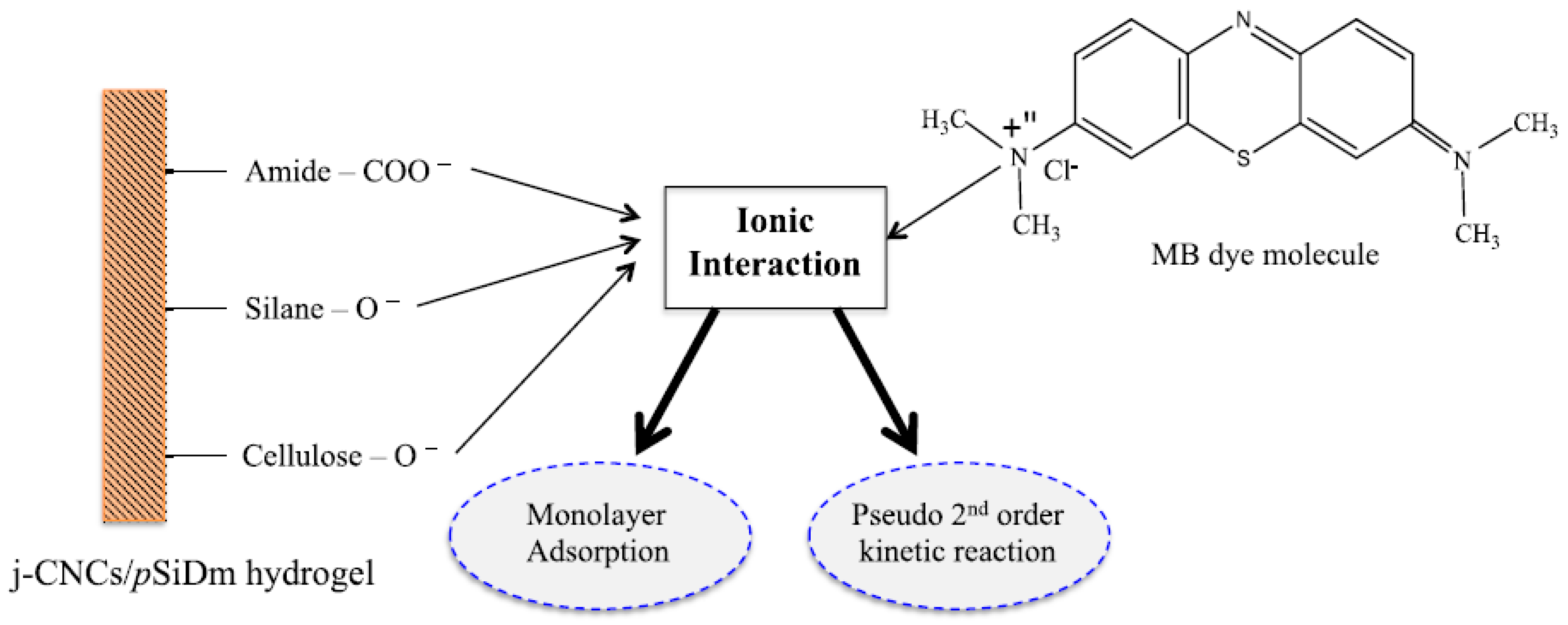
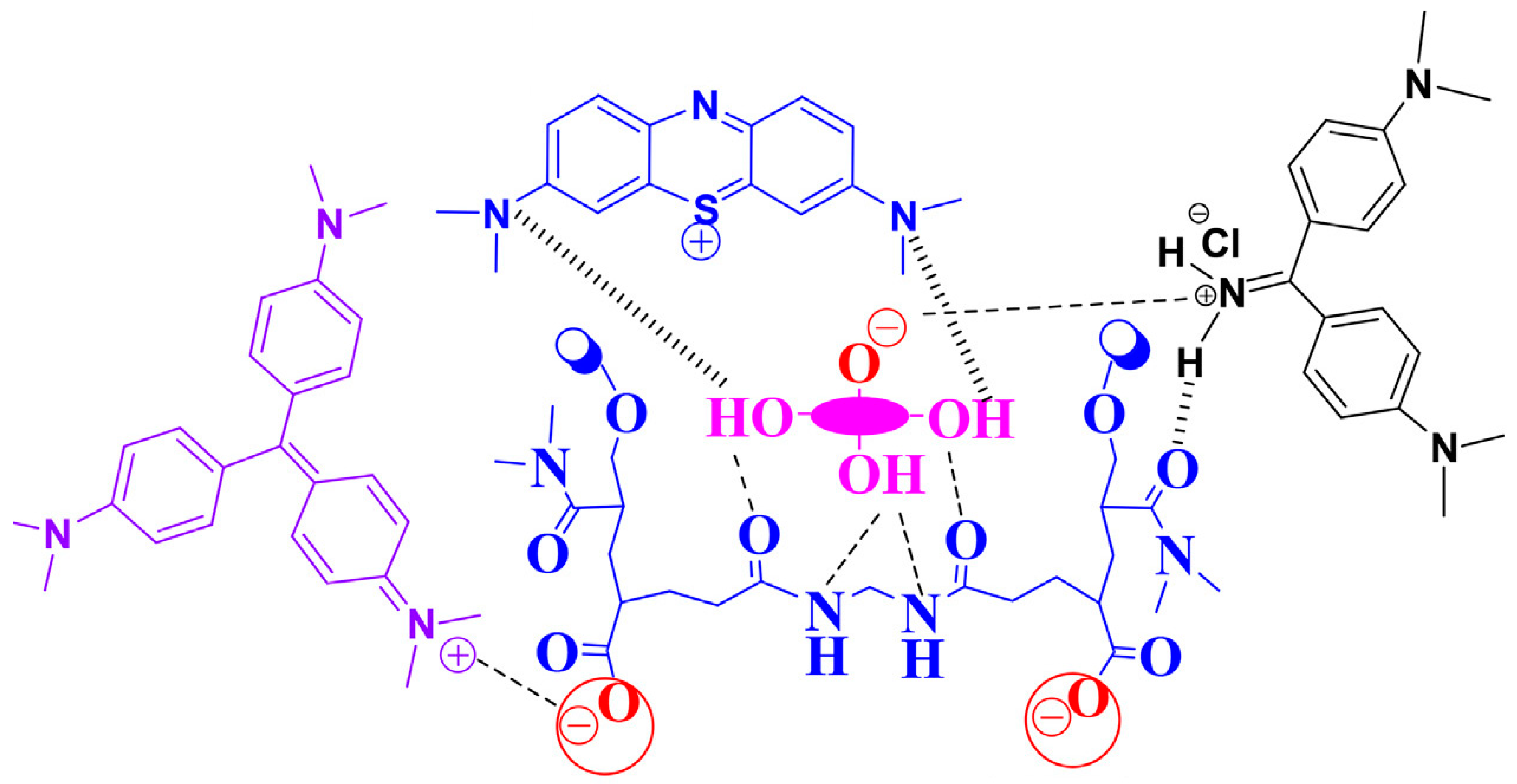
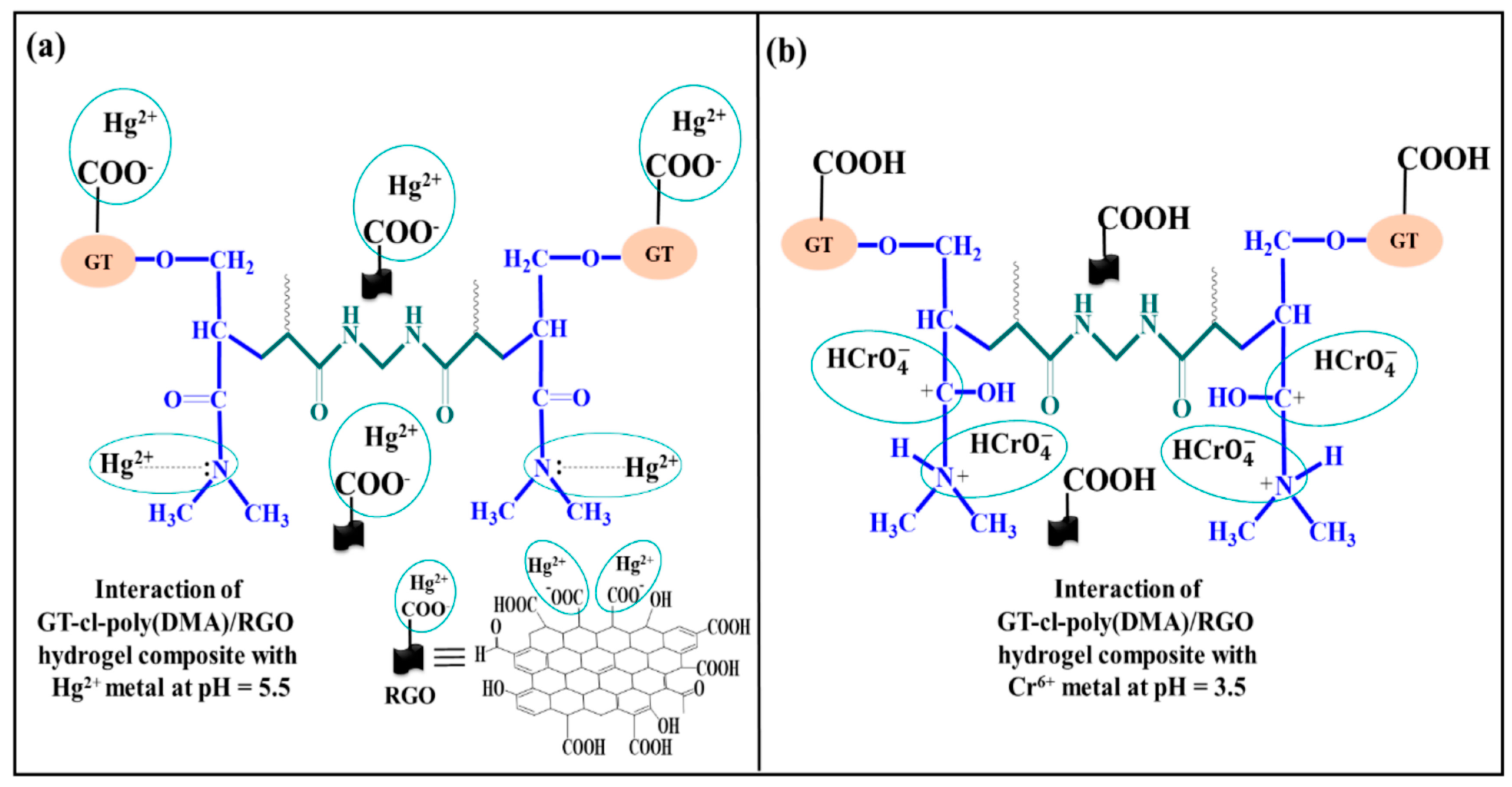
| № | Name of the Hydrogel | The Adsorption Capacity, Qe (mg/g) | References | |
|---|---|---|---|---|
| Methylene Blue | Crystal Violet | |||
| 1 | poly(N,N-dimethylacrylamide-co-sodium acrylate) | 800 | 320 | [38] |
| 2 | katira gum-cl-poly(acrylic acid-co-N,N-dimethylacrylamide)@bentonite | 165.28 | 158.73 | [34] |
| 3 | poly(N,N-dimethylacrylamideco- 2-hydroxyethyl methacrylate) | 80.27 | - | [37] |
| 4 | poly(N,N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) | 131.58 | - | [31] |
| 5 | karaya gum-grafted-poly( N,N-dimethylacrylamide) gel | 11.93 | 41.84 | [32] |
| № | Name of the Hydrogel | Metal Ions | The Adsorption Capacity, Qe (mg/g) | References |
|---|---|---|---|---|
| 1 2 3 | N,N-dimethylacrylamide-co-sodium acrylate | Cr(III) | 450 | [38] |
| Co(II) | 300 | |||
| Ni(II) | 298 | |||
| 4 5 | graphene oxide incorporated gum tragacanth-cl-N,N-dimethylacrylamide (GT-cl-poly(DMAA)/RGO) | Hg(II) | 636.94 | [44] |
| Cr(VI) | 416.66 | |||
| 6 | N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate | Pb(II) | 70.52 | [46] |
| 7 | xanthan gum-cl-Dimethyl acrylamide hydrogel containing silica | Cd(II) | 150.7 | [48] |
| 8 | 50.35 | |||
| 9 | N,N-dimethylacrylamide- N-vinylcaprolactam-g- Chitosan | Cr(VI) | 142.86 | [49] |
| 10 | N,N-dimethyl acrylamide-g- Hydroxyethyl starch | Hg(II) | 300 | [50] |
| Cu(II) | 80.6 | |||
| Zn(II) | 64 | |||
| Pb(II) | 51.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetzhan, A.; Myrzakhmetova, N.; Amangeldi, N.; Kuanyshova, Z.; Akimbayeva, N.; Dosmaganbetova, S.; Toktarbay, Z.; Longinos, S.N. A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels. Gels 2021, 7, 234. https://doi.org/10.3390/gels7040234
Akhmetzhan A, Myrzakhmetova N, Amangeldi N, Kuanyshova Z, Akimbayeva N, Dosmaganbetova S, Toktarbay Z, Longinos SN. A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels. Gels. 2021; 7(4):234. https://doi.org/10.3390/gels7040234
Chicago/Turabian StyleAkhmetzhan, Ayatzhan, Nurbala Myrzakhmetova, Nurgul Amangeldi, Zhanar Kuanyshova, Nazgul Akimbayeva, Saule Dosmaganbetova, Zhexenbek Toktarbay, and Sotirios Nik. Longinos. 2021. "A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels" Gels 7, no. 4: 234. https://doi.org/10.3390/gels7040234







