Ambroxol Hydrochloride Loaded Gastro-Retentive Nanosuspension Gels Potentiate Anticancer Activity in Lung Cancer (A549) Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. ABX-CRGK Characterization
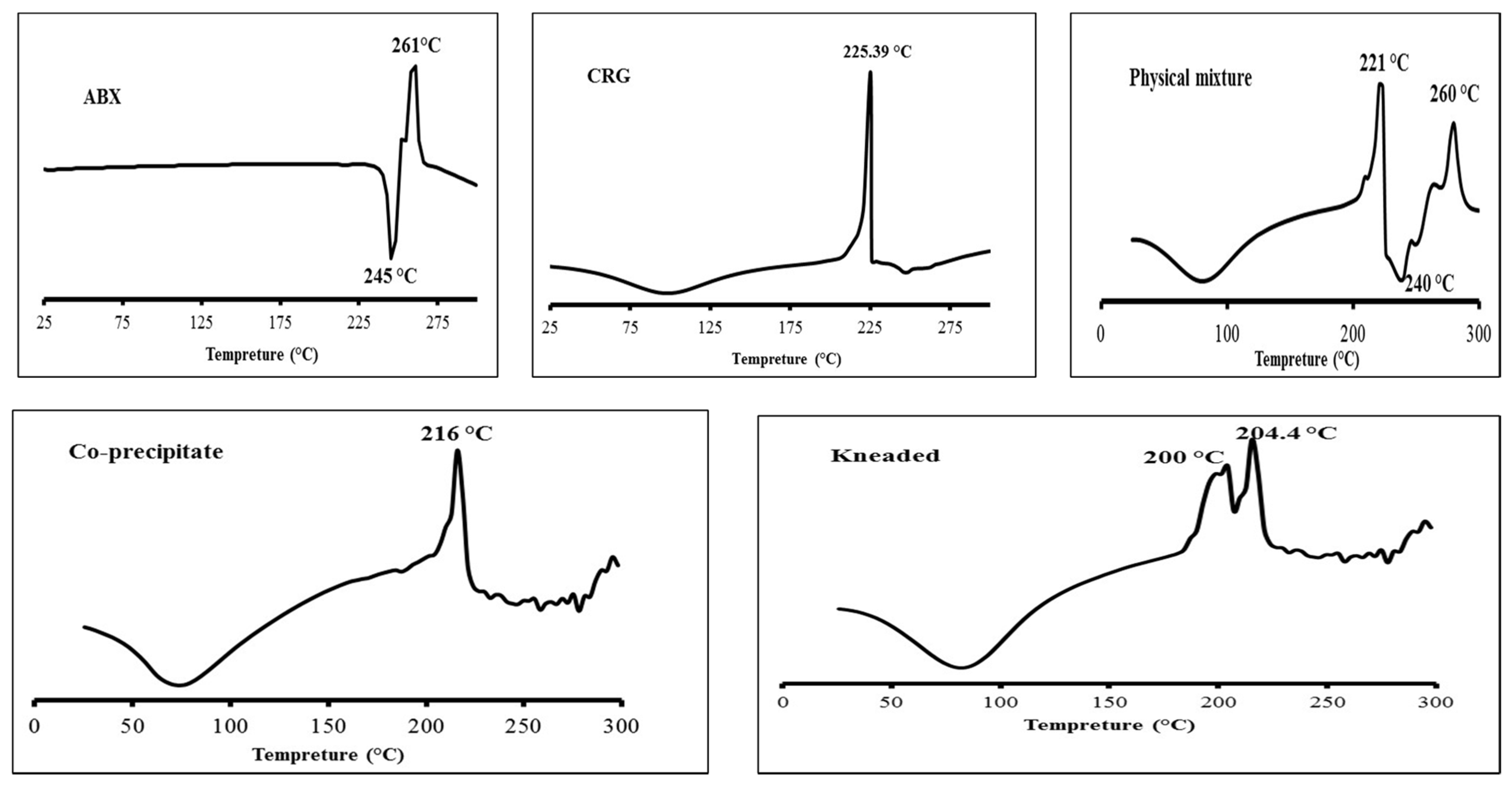
2.1.1. Differential Scanning Calorimetry
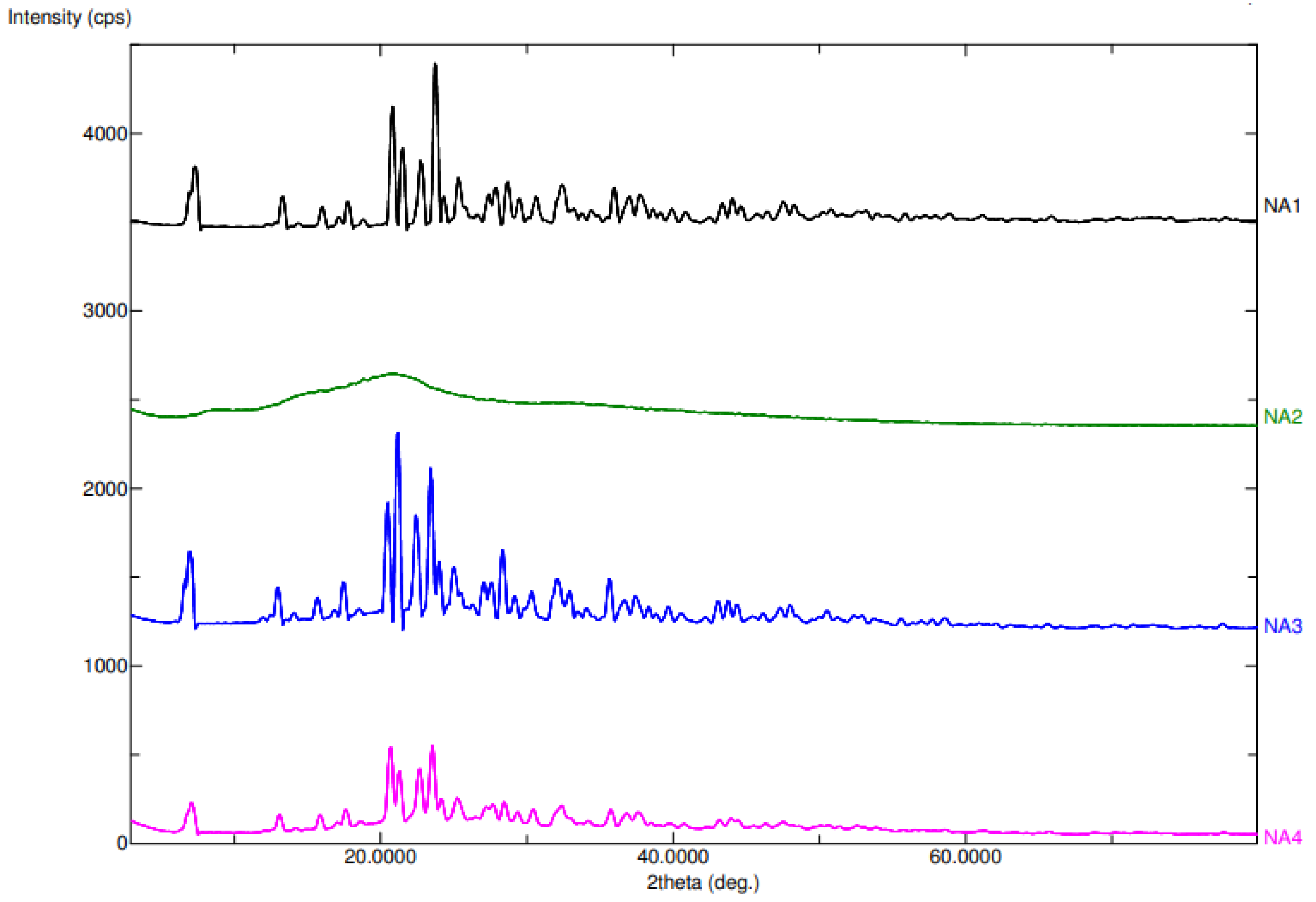
2.1.2. Powder X-ray Diffraction (PXRD)
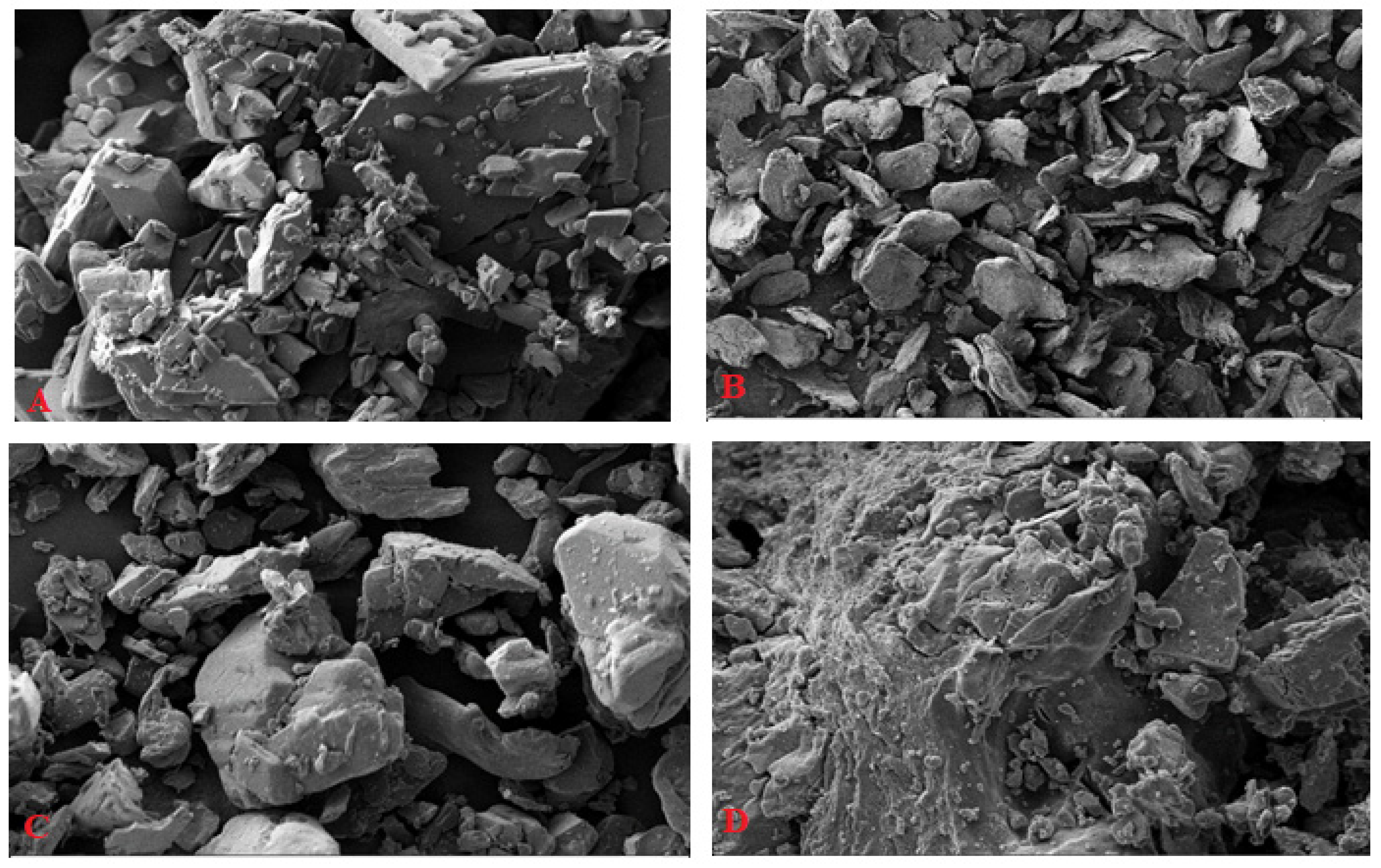
2.1.3. Scanning Electron Microscopy (SEM)
2.2. Nanosuspension Formulation
2.3. Drug-Release Study and Gel Floatation
2.4. Aging Effect on the Drug Release
2.5. ABX Nanosuspension Characterization
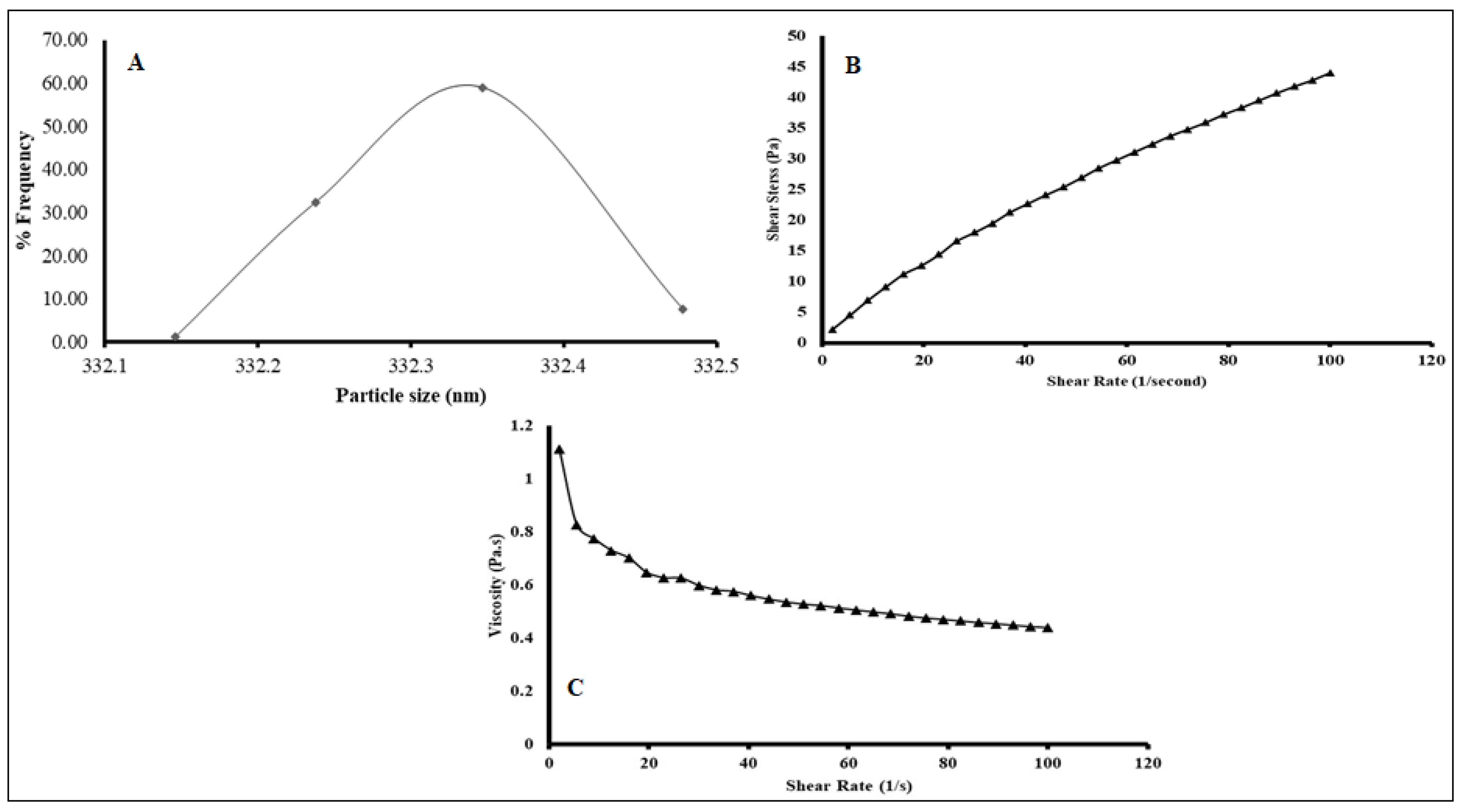
2.5.1. Particle Size Analysis (PSA)
2.5.2. Viscosity Measurement
2.6. In Vitro Cell Line Study in A549 Cells
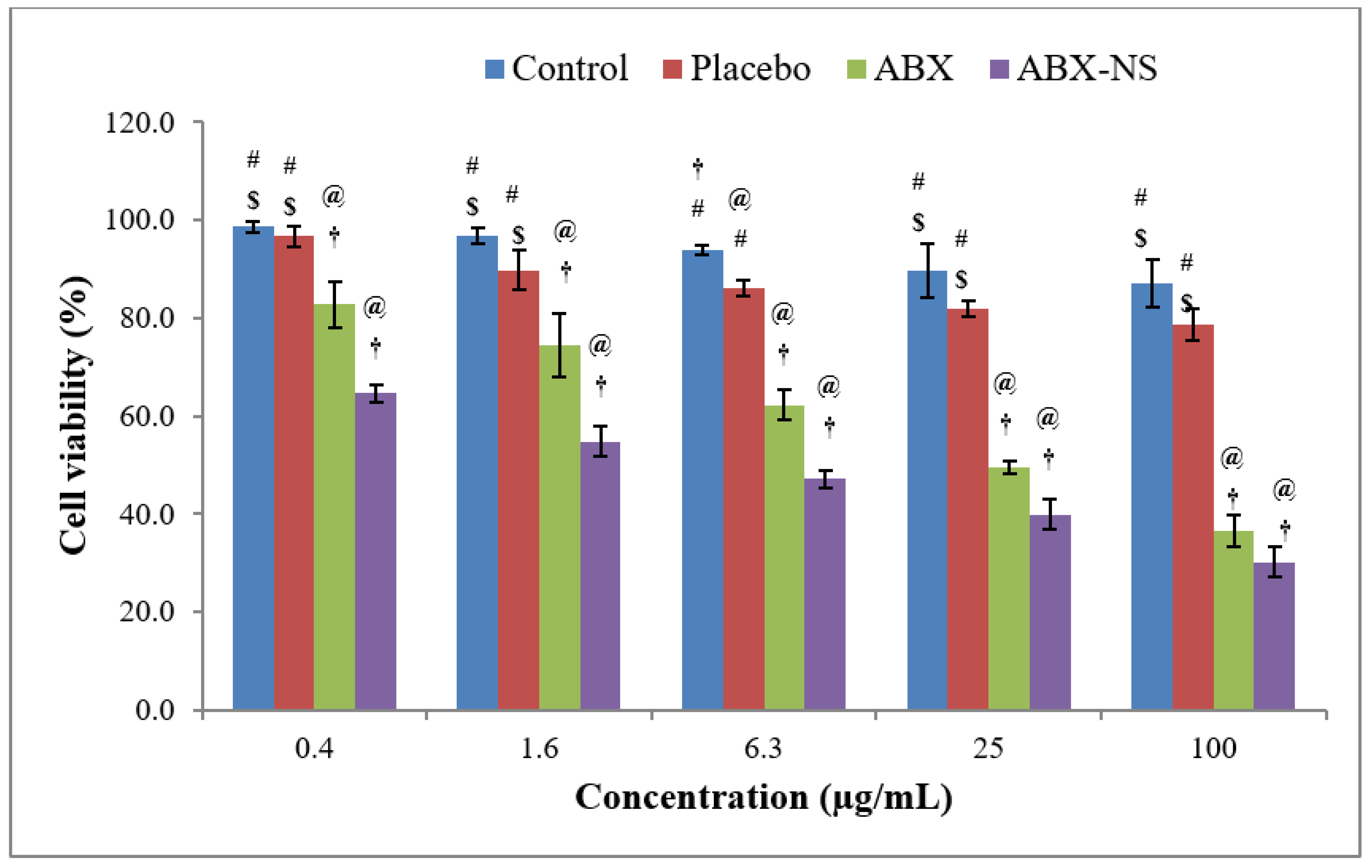
2.6.1. Cell Viability Using MTT Assay
2.6.2. Apoptotic Activity by Flow Cytometry
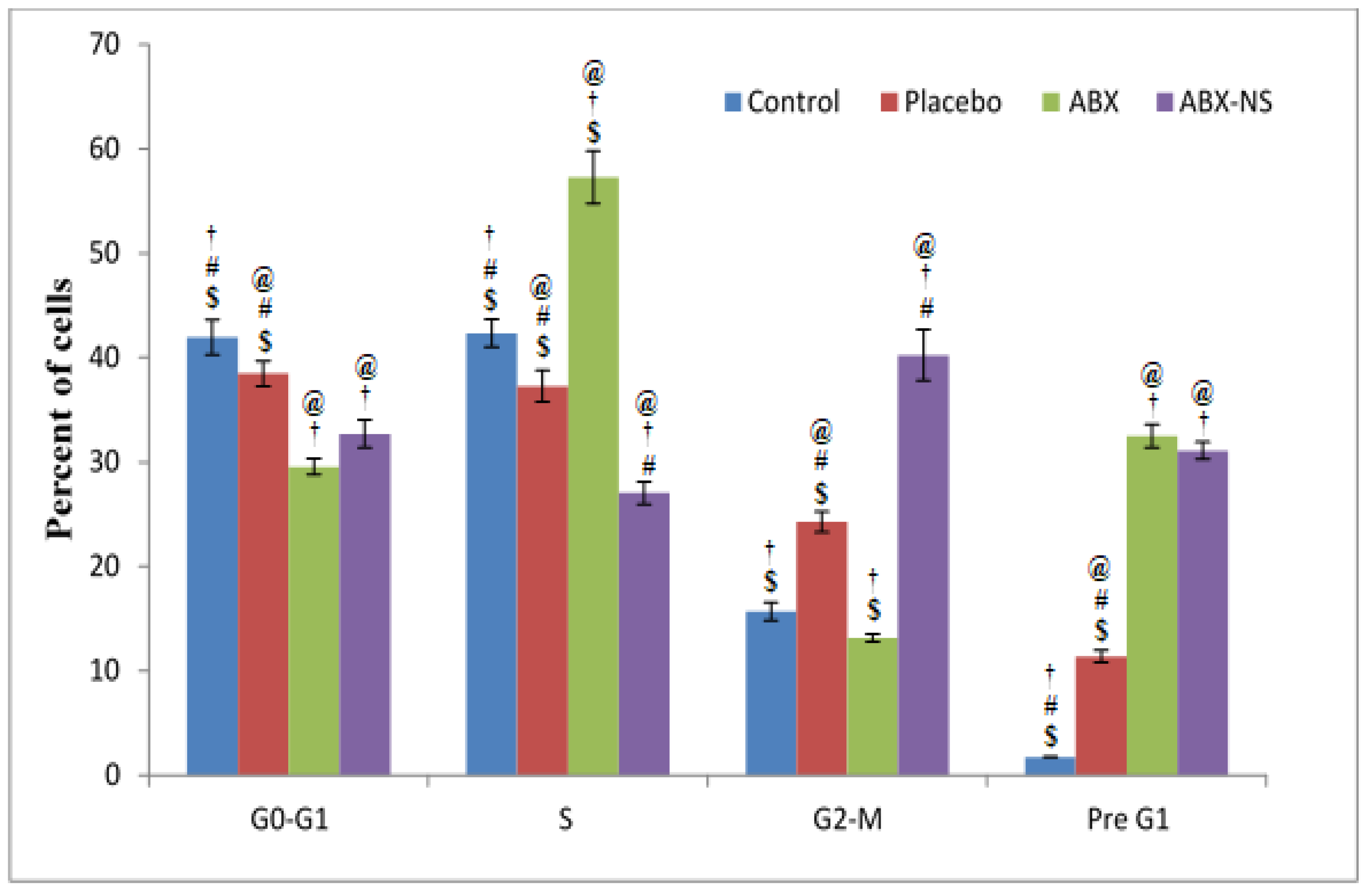
2.6.3. Cell Cycle Analysis by Flow Cytometry
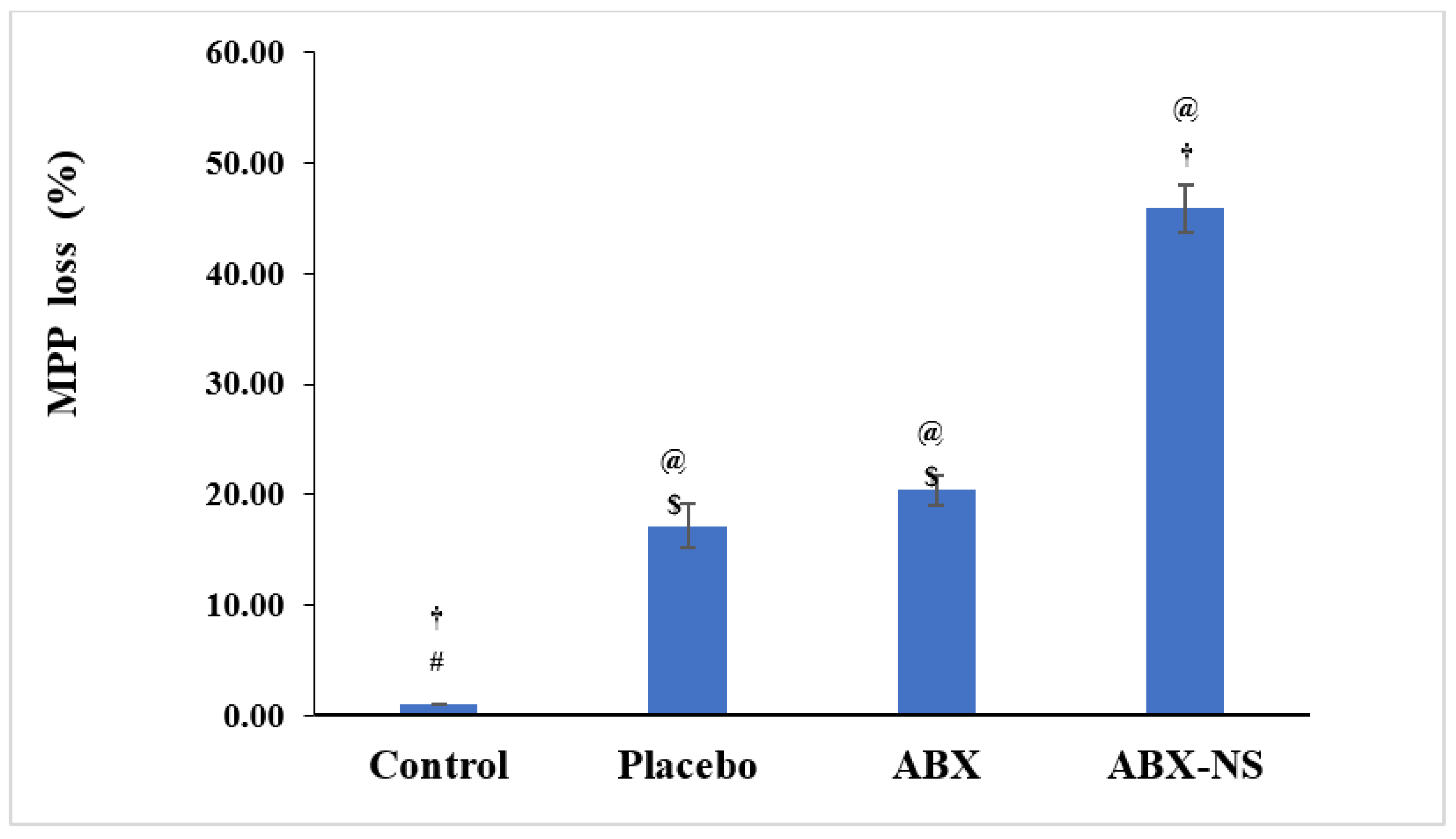
2.6.4. Mitochondrial Membrane Potential Activity (MMP)
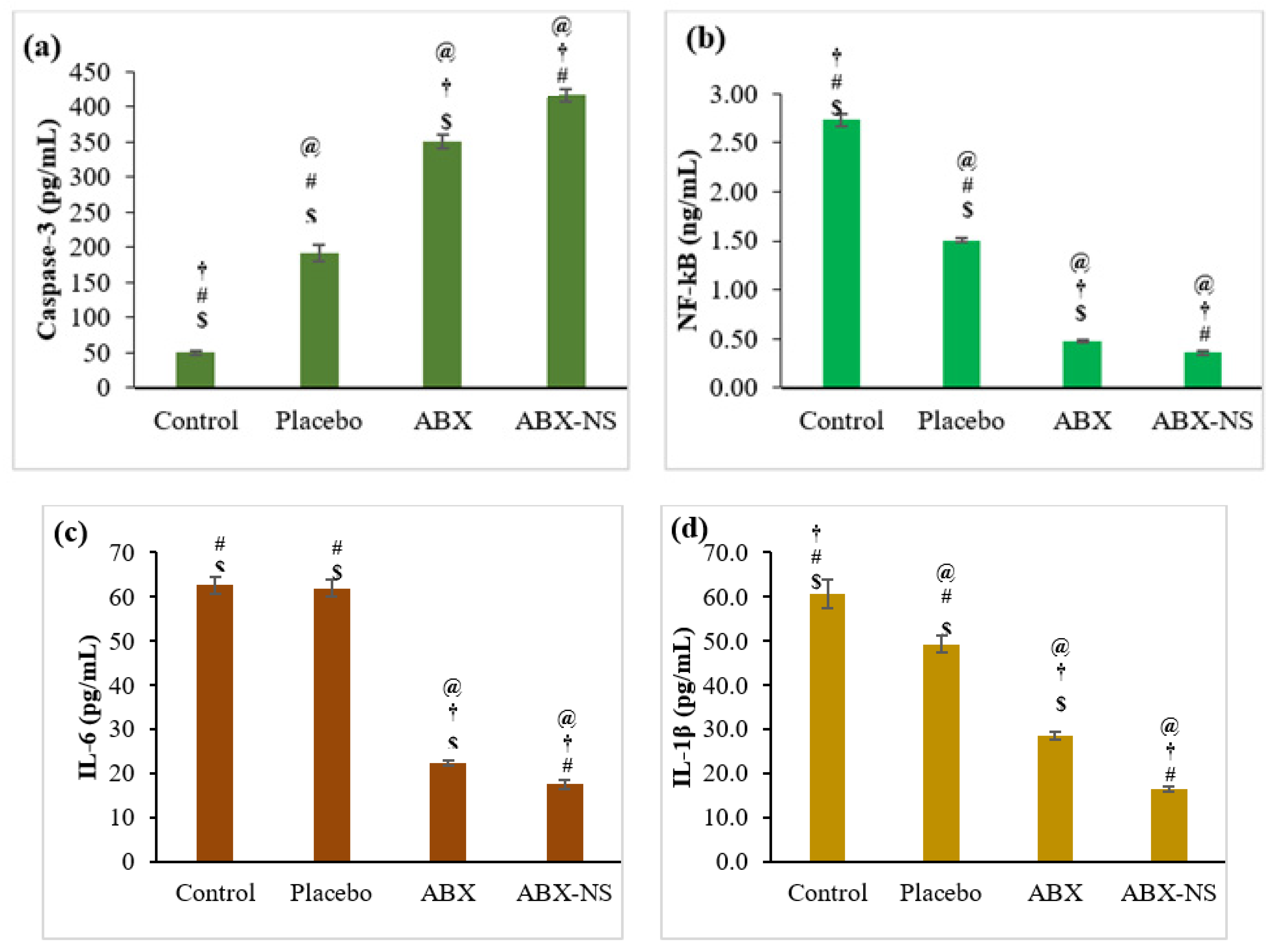
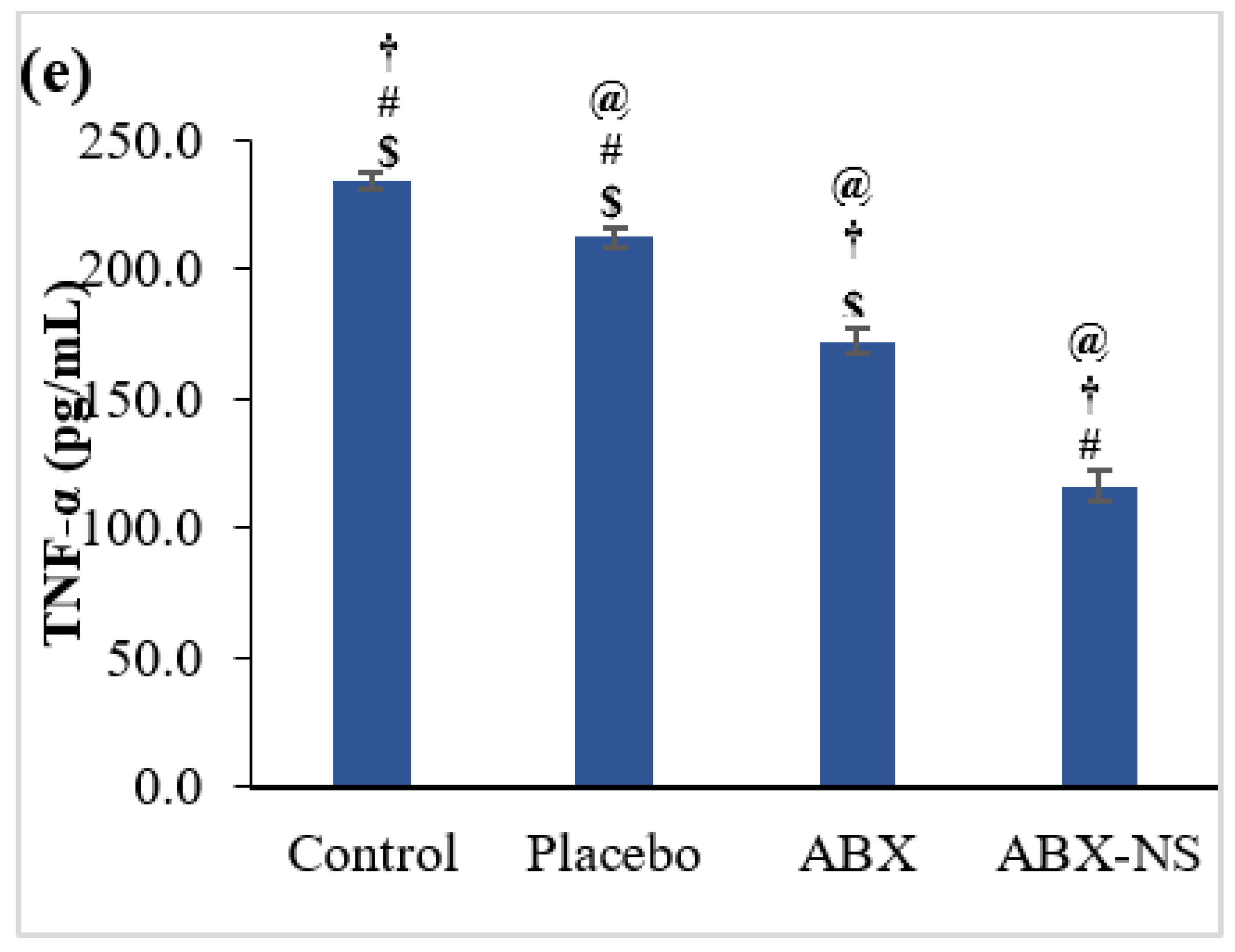
2.6.5. Effect of ABX-NS on Caspase-3, NF-kB, IL-6, IL-1β, and TNF-α Activities by ELISA Method
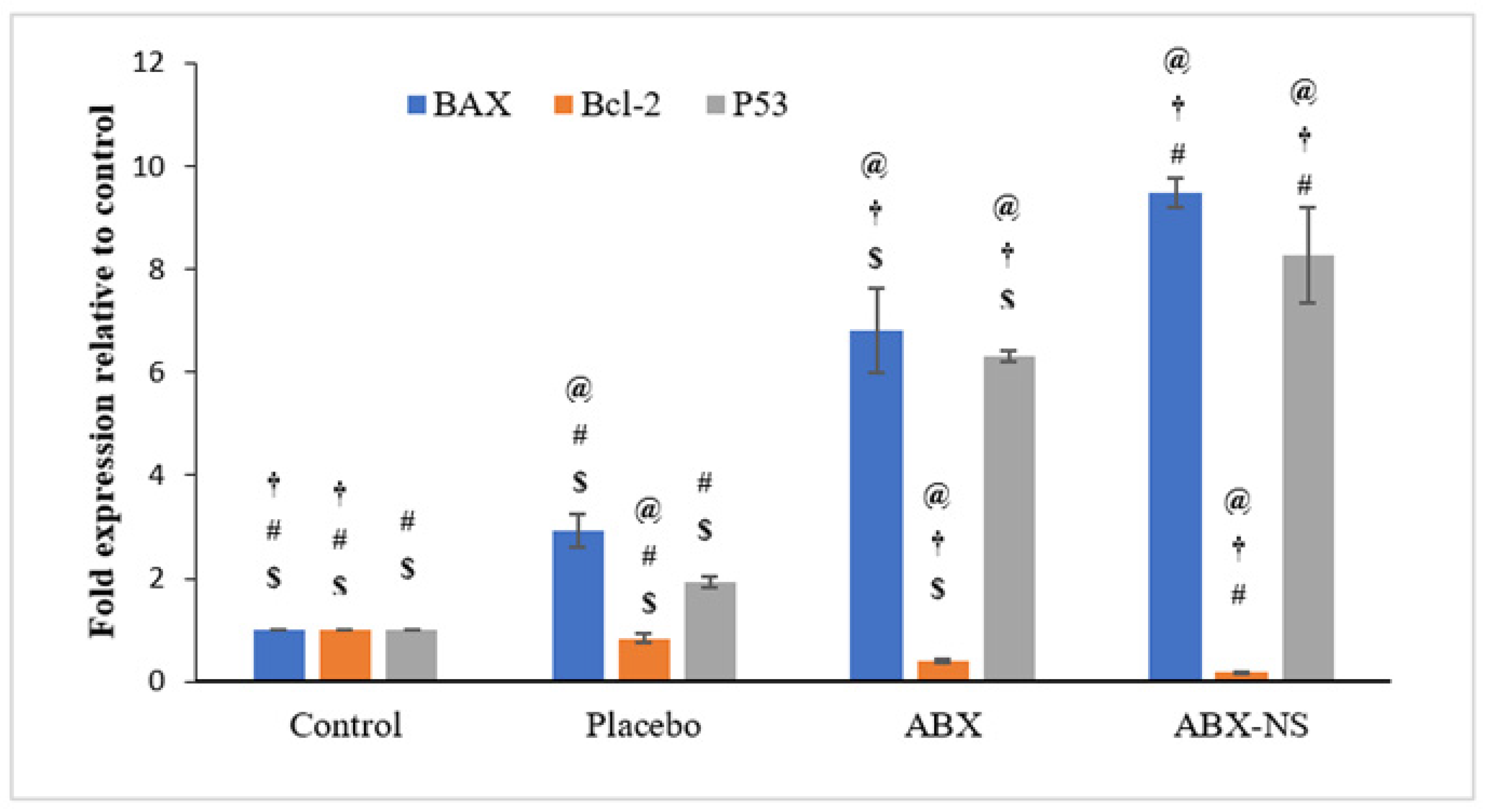
2.6.6. Effect of ABX-NS on Molecular Markers (Bax, Bcl-2, and p53) Using RT-PCR
3. Conclusions
4. Materials and Methods
4.1. ABX-CRGK Complex Formation
4.1.1. Co-Precipitation
4.1.2. Kneading
4.2. ABX-CRGK Characterization
4.2.1. Differential Scanning Calorimetry (DSC)
4.2.2. Powder X-ray Diffraction (PXRD)
4.2.3. Scanning Electron Microscopy (SEM)
4.3. Nanosuspension Formulation
4.4. In Vitro Drug Release Study
4.5. Aging Effect on the Drug Release
4.6. ABX Nanosuspension Characterization
Particle Size Analysis (PSA) and Viscosity Measurement
4.7. In Vitro Cell Line Study in A549 Cells
4.7.1. Cell Viability Assay
4.7.2. Apoptotic Activity by Flow Cytometry
4.7.3. Cell Cycle Analysis by Flow Cytometry
4.7.4. Mitochondrial Membrane Potential Activity (MMP)
4.7.5. Effect of ABX-NS on Caspase-3, NF-kB, IL-6, IL-1β, and TNF-α Activities as Measured by the ELISA Method
4.7.6. Effect of ABX-NS on Molecular Markers (Bax, Bcl-2, and p53) Using RT-PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirker, R. Conquering lung cancer: Current status and prospects for the future. Pulmonology 2020, 26, 283–290. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Ray, M.R.; Jablons, D.; He, B. Lung cancer therapeutics that target signaling pathways: An update. Expert Rev. Respir. Med. 2010, 4, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yi, W.; Jiang, P.; Sun, R.; Li, T. Effects of ambroxol hydrochloride on concentrations of paclitaxel and carboplatin in lung cancer patients at different administration times. Cell Mol. Biol. 2016, 62, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, Q.; Chen, M.; Ren, X.; Wang, X.; Qian, J.; Sun, Y.; Sha, X. Ambroxol enhances anti-cancer effect of microtubule-stabilizing drug to lung carcinoma through blocking autophagic flux in lysosome-dependent way. Am. J. Cancer Res. 2017, 7, 2406–2421. [Google Scholar] [PubMed]
- Wang, X.; Wang, L.; Wang, H.; Zhang, H. Perioperative Lung Protection Provided by High-Dose Ambroxol in Patients with Lung Cancer. Cell Biochem. Biophys. 2015, 73, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, S.; Thatikonda, S.; Pooladanda, V.; Challa, V.S.; Godugu, C. Protective effects of ambroxol in psoriasis like skin inflammation: Exploration of possible mechanisms. Int. Immunopharmacol. 2019, 71, 301–312. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xiao, W.; Zhang, X.; Sun, Y.; Chen, Y.; Chen, Q.; Fang, X.; Du, S.; Sha, X. Pulmonary-Affinity Paclitaxel Polymer Micelles in Response to Biological Functions of Ambroxol Enhance Therapeutic Effect on Lung Cancer. Int. J. Nanomed. 2020, 15, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Ollier, C.; Sent, U.; Mesquita, M.; Michel, M.C. Pharmacokinetics of Ambroxol Sustained Release (Mucosolvan® Retard) Compared with Other Formulations in Healthy Volunteers. Pulm. Ther. 2020, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, M.; Gao, Y.; Cao, F.; Zhai, G. Design and evaluation of osmotic pump-based controlled release system of Ambroxol Hydrochloride. Pharm. Dev. Technol. 2011, 16, 392–399. [Google Scholar] [CrossRef]
- Cho, C.-W.; Choi, J.-S.; Shin, S.-C. Development of the Ambroxol Gels for Enhanced Transdermal Delivery. Drug Dev. Ind. Pharm. 2008, 34, 330–335. [Google Scholar] [CrossRef]
- Dabhi, M.; Gohel, M.; Parikh, R.; Sheth, N.; Nagori, S. Formulation development of ambroxol hydrochloride soft gel with application of statistical experimental design and response surface methodology. PDA J. Pharm. Sci. Technol. 2011, 65, 20–31. [Google Scholar] [PubMed]
- Okunlola, A.; Odeniyi, M.A.; Arhewoh, M.I. Microsphere formulations of ambroxol hydrochloride: Influence of Okra (Abelmoschus esculentus) mucilage as a sustained release polymer. Prog. Biomater. 2020, 9, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Gangurde, H.H.; Chavan, N.V.; Mundada, A.S.; Derle, D.V.; Tamizharasi, S. Biodegradable Chitosan-Based Ambroxol Hydrochloride Microspheres: Effect of Cross-Linking Agents. J. Young Pharm. 2011, 3, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, W.; Miyazaki, S.; Dairaku, M.; Togashi, M.; Mikami, R.; Attwood, D. Oral sustained delivery of ambroxol from in situ-gelling pectin formulations. Int. J. Pharm. 2004, 271, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Bani-Jaber, A.; Abdullah, S. Development and characterization of novel ambroxol sustained-release oral suspensions based on drug-polymeric complexation and polymeric raft formation. Pharm. Dev. Technol. 2020, 25, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Shadab Ahuja, A.; Khar, R.K.; Baboota, S.; Chuttani, K.; Mishra, A.K.; Ali, J. Gastroretentive drug delivery system of acyclovir-loaded alginate mucoadhesive microspheres: Formulation and evaluation. Drug Deliv. 2011, 18, 255–264. [Google Scholar]
- Dorożyński, P.; Kulinowski, P.; Mendyk, A.; Jachowicz, R. Gastroretentive drug delivery systems with l-dopa based on carrageenans and hydroxypropylmethylcellulose. Int. J. Pharm. 2011, 404, 169–175. [Google Scholar] [CrossRef]
- Walter, F.; Winter, E.; Rahn, S.; Heidland, J.; Meier, S.; Struzek, A.-M.; Lettau, M.; Philipp, L.-M.; Beckinger, S.; Otto, L.; et al. Chitosan nanoparticles as antigen vehicles to induce effective tumor specific T cell responses. PLoS ONE 2020, 15, e0239369. [Google Scholar] [CrossRef]
- Ahuja, M.; Verma, P.; Bhatia, M. Preparation and evaluation of chitosan–itraconazole co-precipitated nanosuspension for ocular delivery. J. Exp. Nanosci. 2015, 10, 209–221. [Google Scholar] [CrossRef]
- Calvo, G.H.; Cosenza, V.A.; Sáenz, D.A.; Navarro, D.A.; Stortz, C.A.; Céspedes, M.A.; Mamone, L.A.; Casas, A.G.; Di Venosa, G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019, 9, 6654. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Differential scanning calorimetry and large deformation behaviour of kappa-carrageenan gels containing alkali metal ions. Colloid Polym. Sci. 1985, 263, 744–748. [Google Scholar] [CrossRef]
- Abdullah, S.; El Hadad, S.; Aldahlawi, A. In vitro optimization, characterization and antitumor evaluation against colorectal cancer of a novel 5Fluorouracil fluorouracil oral nanosuspension using soy protein, polysaccharides-protein complexation, and in-situ gel formation. J. Drug Deliv. Sci. Technol. 2021, 102857. [Google Scholar] [CrossRef]
- Bettini, R.; Bonferoni, M.C.; Colombo, P.; Zanelotti, L.; Caramella, C. Drug release kinetics and front movement in matrix tablets containing diltiazem or metoprolol/lambda-carrageenan complexes. Biomed. Res. Int. 2014, 14, 671532. [Google Scholar]
- Dalei, G.; Swain, S.; Das, S.; Das, S.P. Controlled Release of 5-Fluorouracil from Alginate Hydrogels by Cold HMDSO−Plasma Surface Engineering. ChemistrySelect 2020, 5, 2168–2178. [Google Scholar] [CrossRef]
- Hens, B.; Bermejo, M.; Cristofoletti, R.; Amidon, G.E.; Amidon, G.L. Application of the Gastrointestinal Simulator (GIS) Coupled with In Silico Modeling to Measure the Impact of Coca-Cola® on the Luminal and Systemic Behavior of Loratadine (BCS Class 2b). Pharmaceutics 2020, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Shishu Gupta, N.; Aggarwal, N. Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS PharmSciTech 2007, 8, E143–E148. [Google Scholar] [CrossRef] [Green Version]
- Sentein, C.; Guizard, B.; Giraud, S.; Yé, C.; Ténégal, F. Dispersion and stability of TiO2 nanoparticles synthesized by laser pyrolysis in aqueous suspensions. J. Phys. Conf. Ser. 2009, 170, 012013. [Google Scholar] [CrossRef] [Green Version]
- Vallar, S.; Houivet, D.; El Fallah, J.; Kervadec, D.; Haussonne, J.M. Oxide slurries stability and powders dispersion: Optimization with zeta potential and rheological measurements. J. Eur. Ceram. Soc. 1999, 19, 1017–1021. [Google Scholar] [CrossRef]
- Power, R.M.; Simpson, S.H.; Reid, J.P.; Hudson, A.J. The transition from liquid to solid-like behaviour in ultrahigh viscosity aerosol particles. Chem. Sci. 2013, 4, 2597–2604. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Shazly, G.A.; Aleanizy, F.S.; Alqahtani, F.Y.; Elosaily, G.M. Formulation and evaluation of docetaxel nanosuspensions: In-vitro evaluation and cytotoxicity. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 49–55. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.; Fahmy, U.A.; Md, S. Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer. Polymers 2021, 13, 884. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene 2004, 23, 6702–6711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, S.E.; Keating, N.; Belz, G.T. Natural killer cells and anti-tumor immunity. Mol. Immunol. 2019, 110, 40–47. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.-X.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.; Deng, X.; et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef]
- Su, X.; Wang, L.; Song, Y.; Bai, C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004, 30, 133–140. [Google Scholar] [CrossRef]
- Xia, D.-H.; Xi, L.; Xv, C.; Mao, W.-D.; Shen, W.-S.; Shu, Z.-Q.; Yang, H.; Dai, M. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor β1 and tumor necrosis factor α. Med. Oncol. 2010, 27, 697–701. [Google Scholar] [CrossRef]
- Khanzadeh, T.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Azimi, A.; Hossein Pour Feizi, A.A.; Baradaran, B. Investigation of BAX and BCL2 expression and apoptosis in a resveratrol- and prednisolone-treated human T-ALL cell line, CCRF-CEM. Blood Res. 2018, 53, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, U.; Schilli, M.; Haag, U.; Neumann, K.; Kreipe, H.; Kogan, E.; Havemann, K. Expression of bcl-2--protein in small cell lung cancer. Lung Cancer 1996, 15, 31–40. [Google Scholar] [CrossRef]
- Laudanski, J.; Chyczewski, L.; Niklińska, W.E.; Kretowska, M.; Furman, M.; Sawicki, B.; Nikliński, J. Expression of bcl-2 protein in non-small cell lung cancer: Correlation with clinicopathology and patient survival. Neoplasma 1999, 46, 25–30. [Google Scholar]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.J.; Mane, V.B. Raft-Forming Agents: Antireflux Formulations. Drug Dev. Ind. Pharm. 2007, 33, 1350–1361. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; AFahmy, U.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Wu, Y.-J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer Effects of Sinulariolide-Conjugated Hyaluronan Nanoparticles on Lung Adenocarcinoma Cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| # | SA-Level | CS-Level (mg/mL) | Free ABX or ABX-CRGK Complex | Physical Status |
|---|---|---|---|---|
| F1 | 18 mg/mL | - | ABX-CRGK complex | Liquid Suspension |
| F2 | 18 mg/mL | 10 mg/mL | Free ABX | Liquid Suspension |
| F3 * | 0.42 mg/mg | 0.23 mg/mg | ABX-CRGK complex | Dry physical mixture |
| F4 | 18 mg/mL | 10 mg/mL | ABX-CRGK complex | Liquid Suspension |
| Formulation | IDR (mg/min) | MDTI (h) | MDTSR (h) |
|---|---|---|---|
| F1 | 1.668 ± 0.005 | 0.149 ± 0.002 | 1.847 ± 0.001 |
| F2 | 0.983 ± 0.002 | 0.176 ± 0.004 | 2.965 ± 0.003 |
| F3 | 0.995 ± 0.004 | 0.161 ± 0.003 | 2.976 ± 0.004 |
| F4 | 0.320 ± 0.003 | 0.204 ± 0.001 | 3.157 ± 0.005 |
| Formula Number | IDR * (mg/min) | MDTInitial * (h) | MDTSR * (h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 Weeks | 4 Weeks | 0 | 2 Weeks | 4 Weeks | 0 | 2 Weeks | 4 Weeks | |
| 4 | 0.320 ± 0.041 | 0.686 ± 0.013 | 0.707 ± 0.013 | 0.204 ± 0.043 | 0.144 ± 0.013 | 0.147 ± 0.013 | 3.157 ± 0.03 | 2.481 ± 0.012 | 2.471 ± 0.012 |
| Nano-Suspension | Mean/Average 1,2 | Median 2 | Mode 2 | Minimum 2 | Maximum 2 | Range 2 | Zeta Potential (mV) 1 | PDI |
|---|---|---|---|---|---|---|---|---|
| F4 | 332.3 ± 0.14 | 332.5 | 332 | 332.14 | 332.47 | 0.33 | −42 ± 1.48 | 0.41 |
| Gene | Primer Sequence | |
|---|---|---|
| Bax | F | 5′-TGGCAGCTGACATGTTTTCTGAC-3′ |
| R | 5′-TCACCCAACCACCCTGGTCTT-3′ | |
| Bcl-2 | F | 5′-TCGCCCTGTGGATGACTGA-3′ |
| R | 5′-CAGAGACAGCCAGGAGAAATCA-3′ | |
| p53 | F | 5′-GACGGTGACACGCTTCCCTGGATT-3′ |
| R | 5′-GGGAACAAGAAGTGGAGAATGTCA-3′ | |
| GAPDH | F | 5′-AATGCATCCTGCACCACCAA-3′ |
| R | 5′-GATGCCATATTCATTGTCATA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md, S.; Abdullah, S.T.; Alhakamy, N.A.; Bani-Jaber, A.; Radhakrishnan, A.K.; Karim, S.; Shahzad, N.; Gabr, G.A.; Alamoudi, A.J.; Rizg, W.Y. Ambroxol Hydrochloride Loaded Gastro-Retentive Nanosuspension Gels Potentiate Anticancer Activity in Lung Cancer (A549) Cells. Gels 2021, 7, 243. https://doi.org/10.3390/gels7040243
Md S, Abdullah ST, Alhakamy NA, Bani-Jaber A, Radhakrishnan AK, Karim S, Shahzad N, Gabr GA, Alamoudi AJ, Rizg WY. Ambroxol Hydrochloride Loaded Gastro-Retentive Nanosuspension Gels Potentiate Anticancer Activity in Lung Cancer (A549) Cells. Gels. 2021; 7(4):243. https://doi.org/10.3390/gels7040243
Chicago/Turabian StyleMd, Shadab, Samaa T. Abdullah, Nabil A. Alhakamy, Ahmad Bani-Jaber, Ammu Kutty Radhakrishnan, Shahid Karim, Naiyer Shahzad, Gamal A. Gabr, Abdulmohsin J. Alamoudi, and Waleed Y. Rizg. 2021. "Ambroxol Hydrochloride Loaded Gastro-Retentive Nanosuspension Gels Potentiate Anticancer Activity in Lung Cancer (A549) Cells" Gels 7, no. 4: 243. https://doi.org/10.3390/gels7040243







